Tsoulfanidis N. Measurement and detection of radiation
Подождите немного. Документ загружается.


148
MEASUREMENT
AND
DETECTION
OF
RADIATION
Equations giving the value of
q
have been reported by many
investigator^.'^-'^
The most recent equation reported by Forster et al.17 valid for
8
I
Z,
I
20
and
for
v/v,
>
2 is
q
=
1
-
A(Zl) exp
with
The proton stopping power is known.lg Brown19 has developed an equation of
the form
by least squares fitting the data of Northcliffe and
chilling."
The most recent
data are those of Janni4
The experimental determination of dE/h is achieved by passing ions of
known initial energy through a thin layer of a stopping material and measuring
the energy loss. The thickness Ax of the material should be small enough that
dE/h
=
AE/Ax. Unfortunately, such a value of Ax is so small, especially for
very heavy ions, that the precision of measuring Ax is questionable and the
uniformity of the layer has an effect on the measurement. Typical experimental
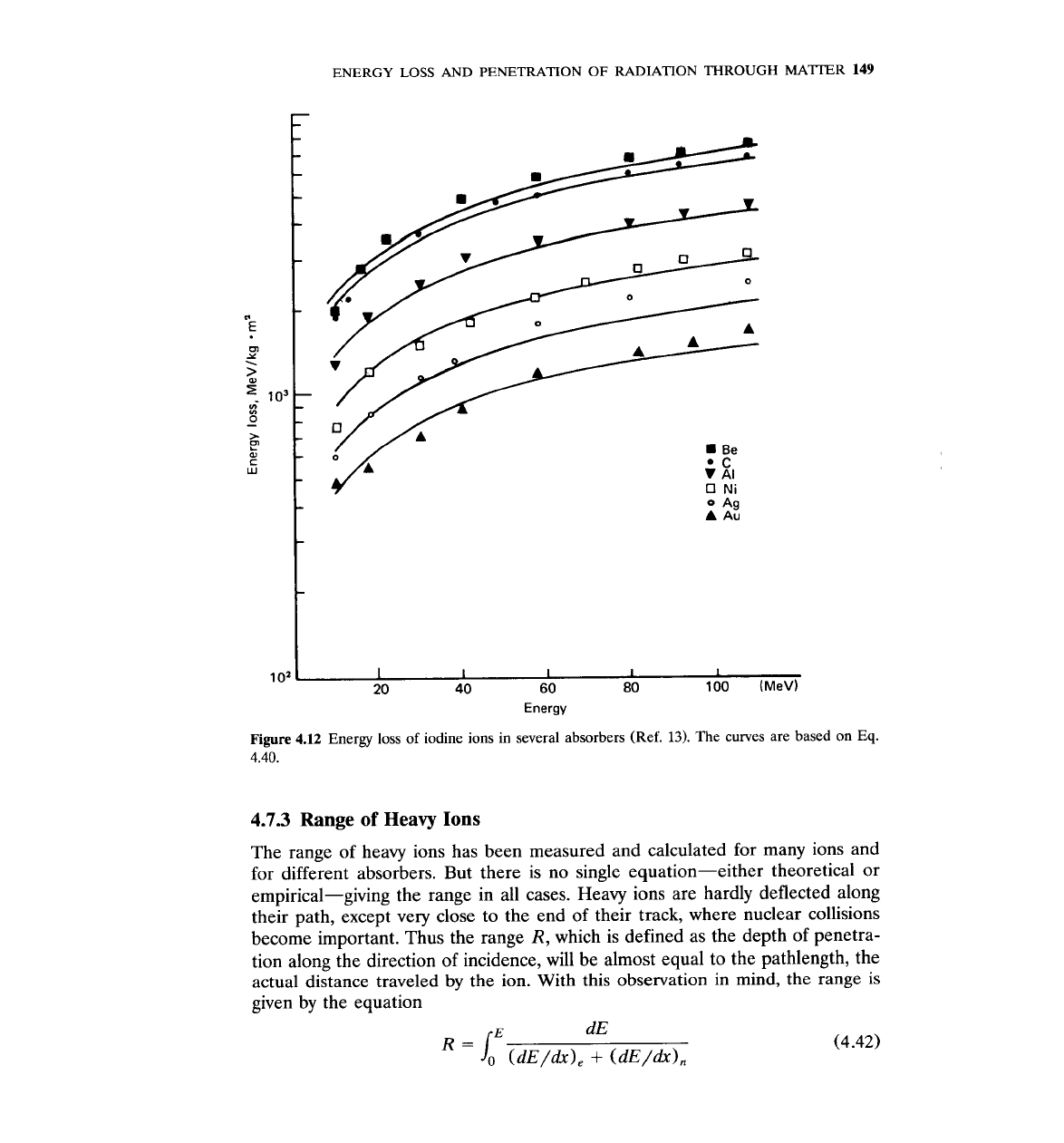
results of stopping power are presented in Fig. 4.12. The data of Fig. 4.12 come
from Ref. 13. The solid line is based on the following empirical equation
proposed by Bridwell and
BUC~"
and Bridwell and Moak2':
where
T
is the kinetic energy of the ion in MeV.
For a compound or mixture, dE/h can be obtained by using Eq. 4.12 with
(dE/d~)~ obtained from Eq. 4.36 or Eq. 4.40.
At velocities v
<
v,z,~/~, the energy loss through nuclear elastic collisions
becomes important. The so-called
nuclear stoppingpower
is given by the follow-
ing approximate expressionlo
:
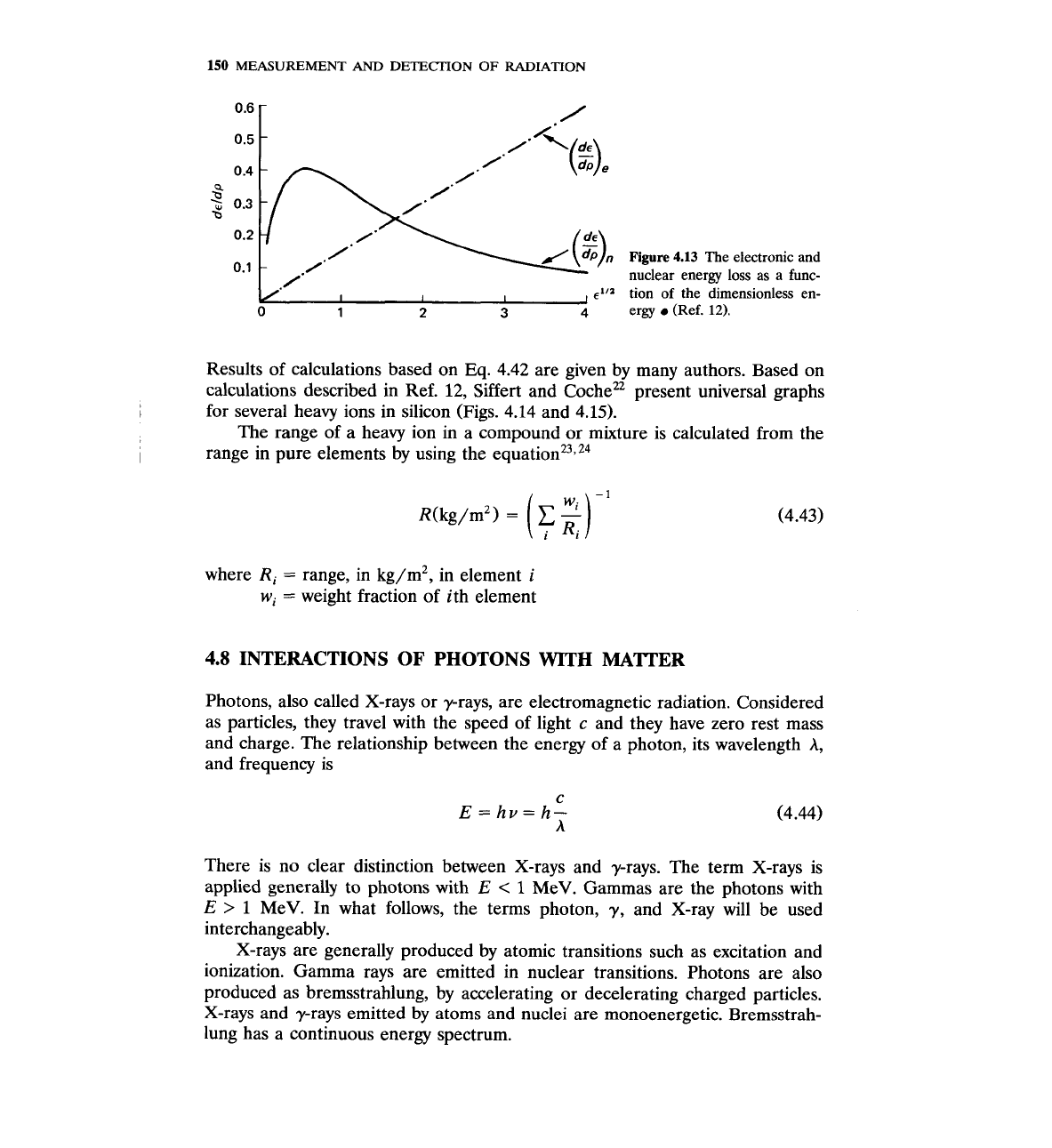
While the electronic stopping power
(d~/dp),
continuously decreases as the ion
speed
v
decreases, the nuclear stopping power increases as
v
decreases, goes
through a maximum, and then decreases again (Fig. 4.13).
ENERGY
LOSS
AND
PENETRATION
OF
RADIATION
THROUGH
MA'ITER
149
F
lo2
-
I
I
I
I
1
20 40 60 80 100
(MeV)
Energy
Figure
4.12
Energy loss of iodine ions in several absorbers (Ref.
13).
The curves are based on Eq.
4.40.
4.7.3 Range of Heavy Ions
The range of heavy ions has been measured and calculated for many ions and
for different absorbers. But there is no single equation-either theoretical or
empirical-giving the range in all cases. Heavy ions are hardly deflected along
their path, except very close to the end of their track, where nuclear collisions
become important. Thus the range R, which is defined as the depth of penetra-
tion along the direction of incidence, will be almost equal to the pathlength, the
actual distance traveled by the ion. With this observation in mind, the range is
given by the equation
150
MEASUREMENT
AND
DETECTION
OF
RADIATION
0.6
r
/
Figure
4.13
The electronic and
nuclear energy loss as
a
func-
.-
I
I I
&1
tion of the dimensionless en-
0
1
2
3
4
ergy
(Ref.
12).
Results of calculations based on
Eq.
4.42 are given by many authors. Based on
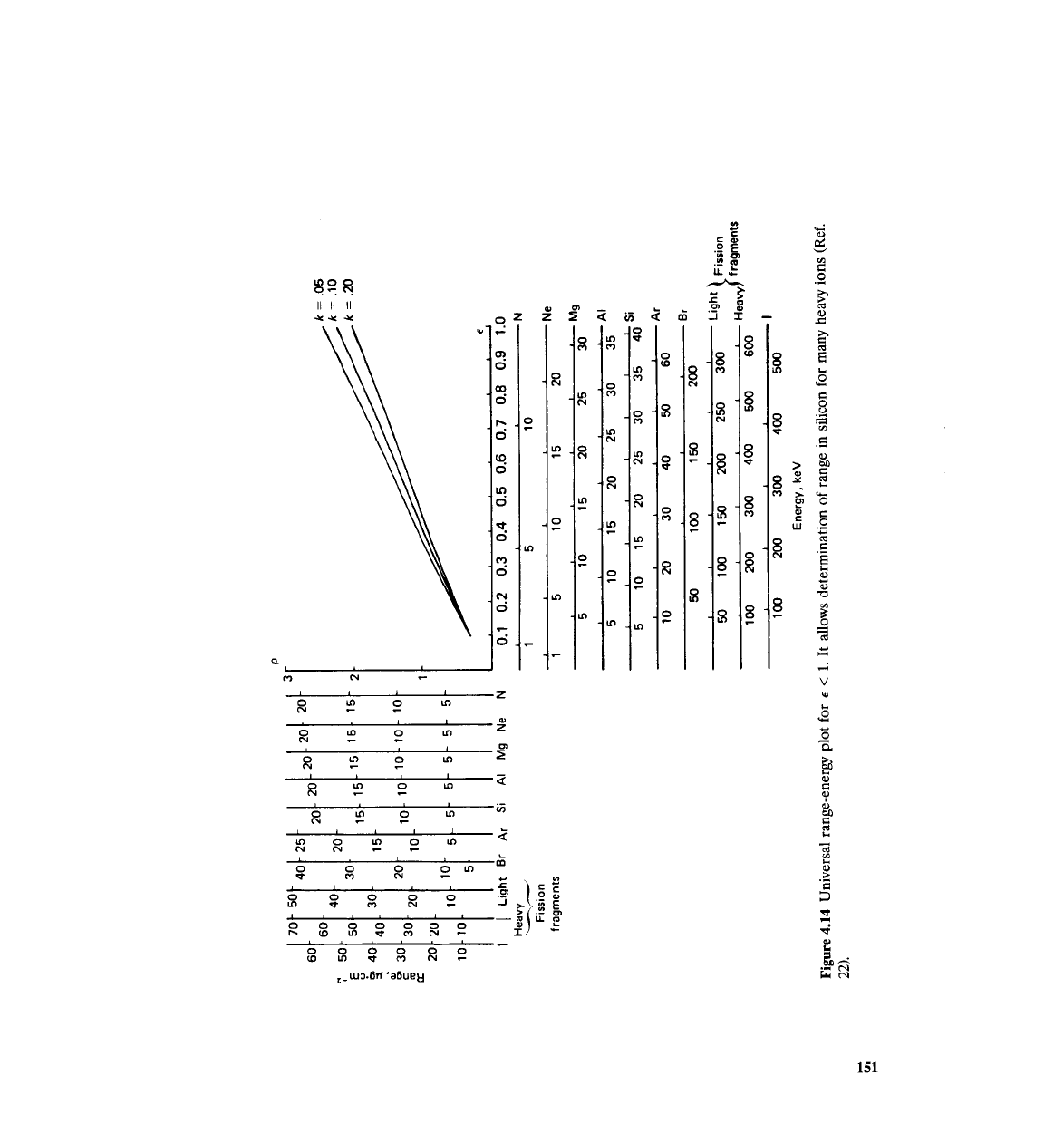
calculations described in Ref. 12, Siffert and ~oche" present universal graphs
for several heavy ions in silicon (Figs. 4.14 and 4.15).
The range of a heavy ion in a compound or mixture is calculated from the
range in pure elements by using the equati~n~~,~~
where
R,
=
range, in kg/m2, in element
i
wi
=
weight fraction of ith element
4.8
INTERACTIONS OF PHOTONS WITH MATI'ER
Photons, also called X-rays or y-rays, are electromagnetic radiation. Considered
as particles, they travel with the speed of light
c
and they have zero rest mass
and charge. The relationship between the energy of a photon, its wavelength A,
and frequency is
There is no clear distinction between X-rays and y-rays. The term X-rays is
applied generally to photons with
E
<
1
MeV. Gammas are the photons with
E
>
1
MeV. In what follows, the terms photon, y, and X-ray will be used
interchangeably.
X-rays are generally produced by atomic transitions such as excitation and
ionization. Gamma rays are emitted in nuclear transitions. Photons are also
produced as bremsstrahlung, by accelerating or decelerating charged particles.
X-rays and y-rays emitted by atoms and nuclei are monoenergetic.
Bremsstrah-
lung has a continuous energy spectrum.
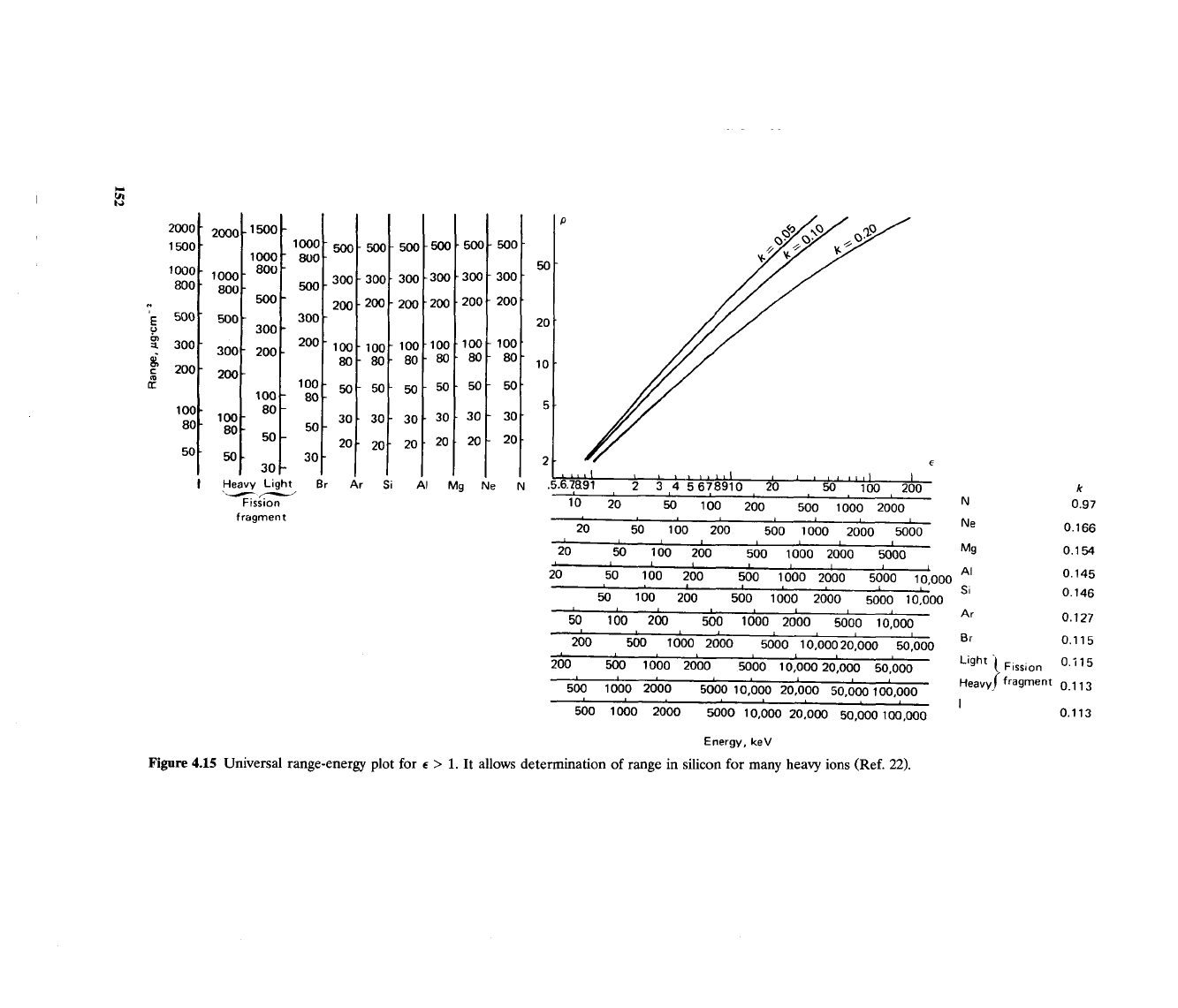
Figure
4.1
Heavy Light 8r
Ar
Si
'-
yV
Fission
fragment
.5
Universal range-energy plot
for
E
>
1.
It allows
de
Energy,
keV
:termination of range in silicon for many heavy ions (Ref.
22).
Br
0.115
Light
\
Flss,on
0.i 15
~eavy! fragment 0.1 13
I
0.113
ENERGY
LOSS
AND
PENETRATION
OF
RADIATION
THROUGH
MATTER
153
There is a long list of possible interactions of photons, but only the three
most important ones will be discussed here: the photoelectric effect, Compton
scattering, and pair production.
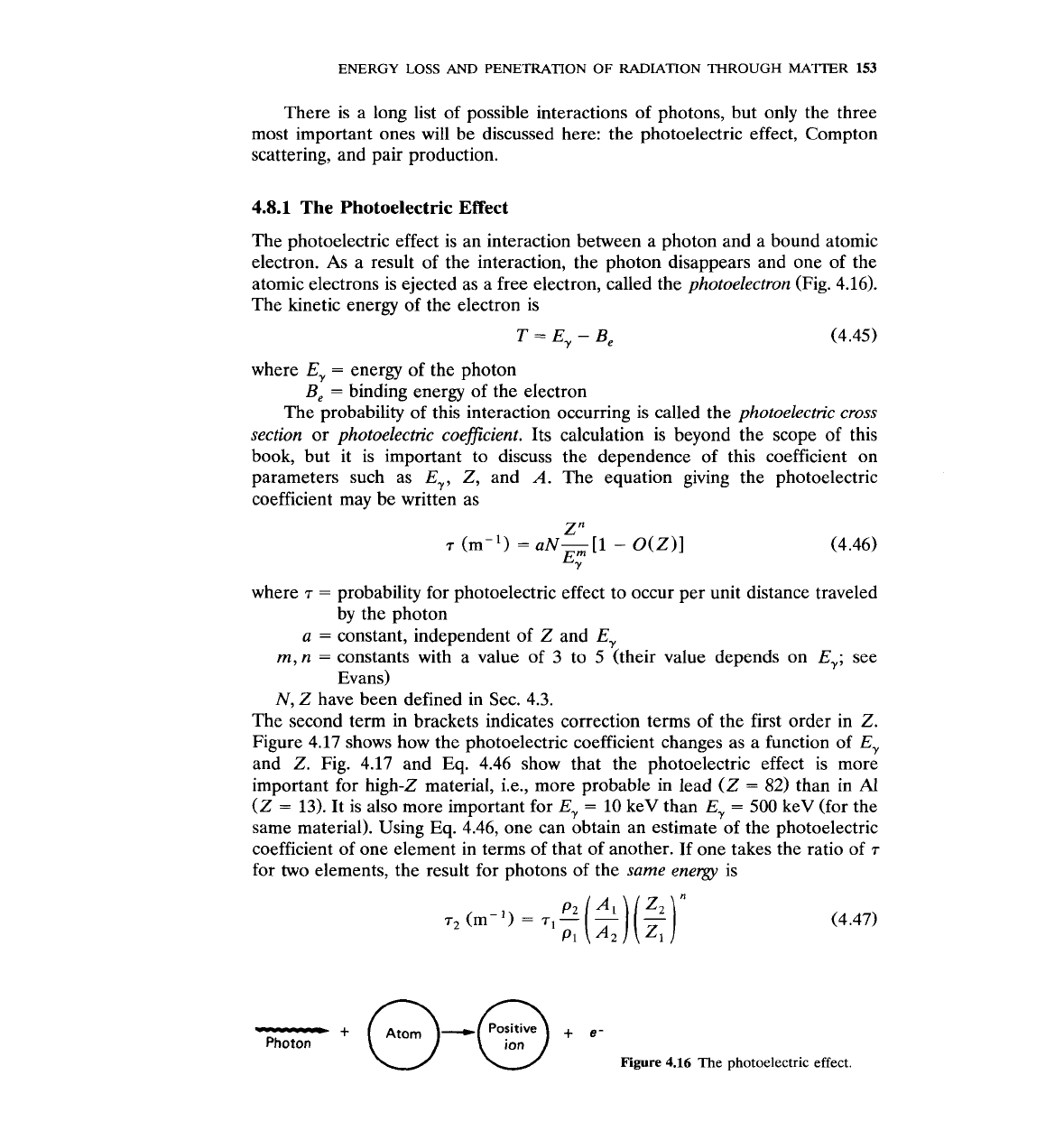
4.8.1
The Photoelectric Effect
The photoelectric effect is an interaction between a photon and a bound atomic
electron. As a result of the interaction, the photon disappears and one of the
atomic electrons is ejected as a free electron, called the
photoelectron
(Fig. 4.16).
The kinetic energy of the electron is
T
=
E,
-
Be
(4.45)
where E,
=
energy of the photon
Be
=
binding energy of the electron
The probability of this interaction occurring is called the
photoelectric cross
section
or
photoelectric coeficient.
Its calculation is beyond the scope of this
book, but it is important to discuss the dependence of this coefficient on
parameters such as E,, Z, and
A.
The equation giving the photoelectric
coefficient may be written as
zn
T
(m-')
=
aN-[I
-
O(Z)] (4.46)
E,"
where
T
=
probability for photoelectric effect to occur per unit distance traveled
by the photon
a
=
constant, independent of Z and E,
m, n
=
constants with a value of 3 to 5 (their value depends on E,; see
Evans)
N,
Z
have been defined in Sec. 4.3.
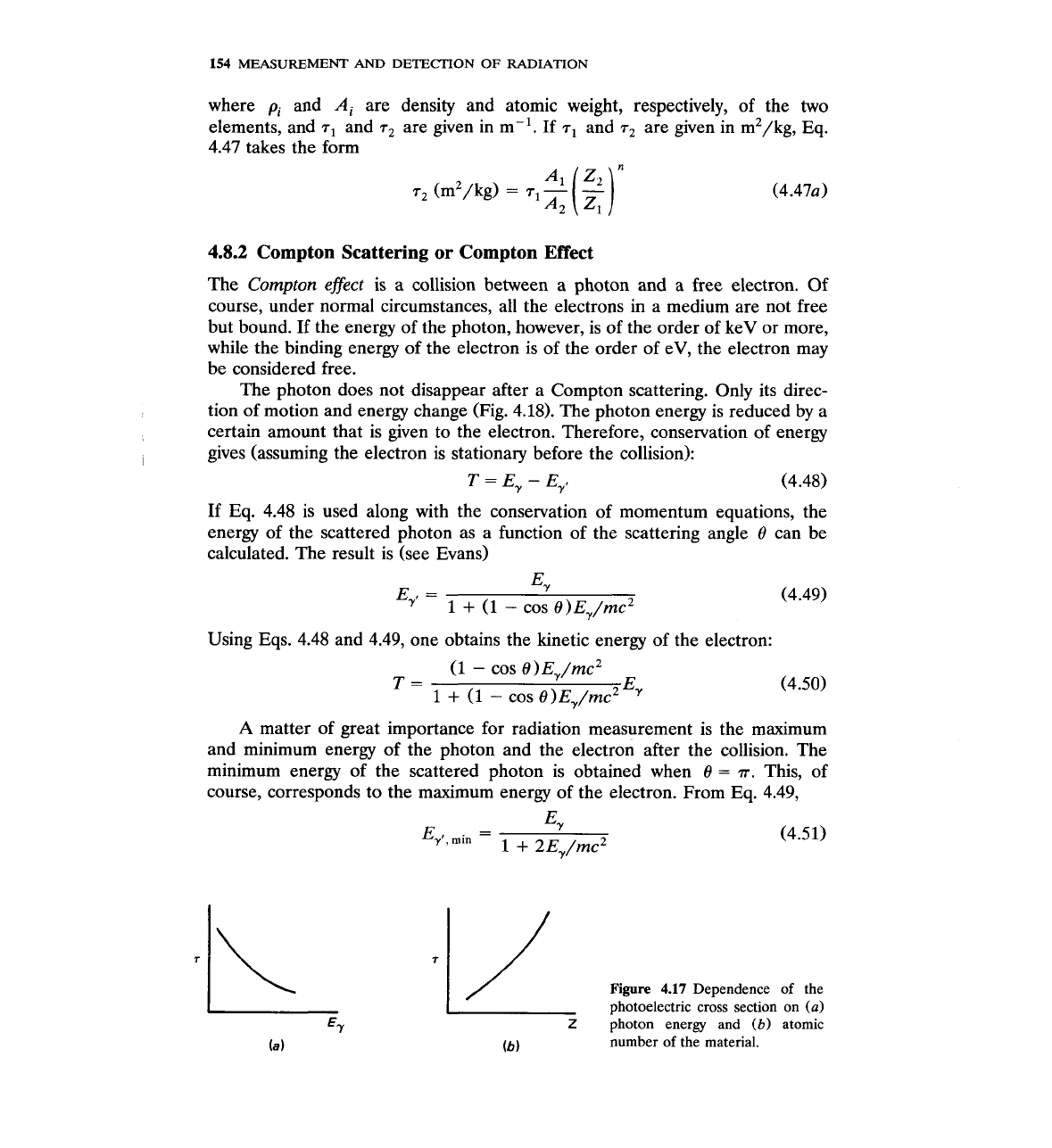
The second term in brackets indicates correction terms of the first order in
Z.
Figure 4.17 shows how the photoelectric coefficient changes as a function of E,
and Z. Fig. 4.17 and Eq. 4.46 show that the photoelectric effect is more
important for
high-Z material, i.e., more probable in lead
(Z
=
82)
than in
Al
(2
=
13). It is also more important for
E,
=
10 keV than E,
=
500 keV (for the
same material). Using Eq. 4.46, one can obtain an estimate of the photoelectric
coefficient of one element in terms of that of another. If one takes the ratio of
T
for two elements, the result for photons of the
same energy
is
Figure
4.16 The photoelectric effect.
154
MEASUREMENT
AND
DETECTION
OF
RADIATION
where
pi
and
A,
are density and atomic weight, respectively, of the two
elements, and
T~
and
7,
are given in m-'. If
7,
and
7,
are given in m2/kg, Eq.
4.47 takes the form
4.8.2
Compton
Scattering
or
Compton Effect
The Compton eflect is a collision between a photon and a free electron. Of
course, under normal circumstances, all the electrons in a medium are not free
but bound. If the energy of the photon, however, is of the order of keV or more,
while the binding energy of the electron is of the order of eV, the electron may
be considered free.
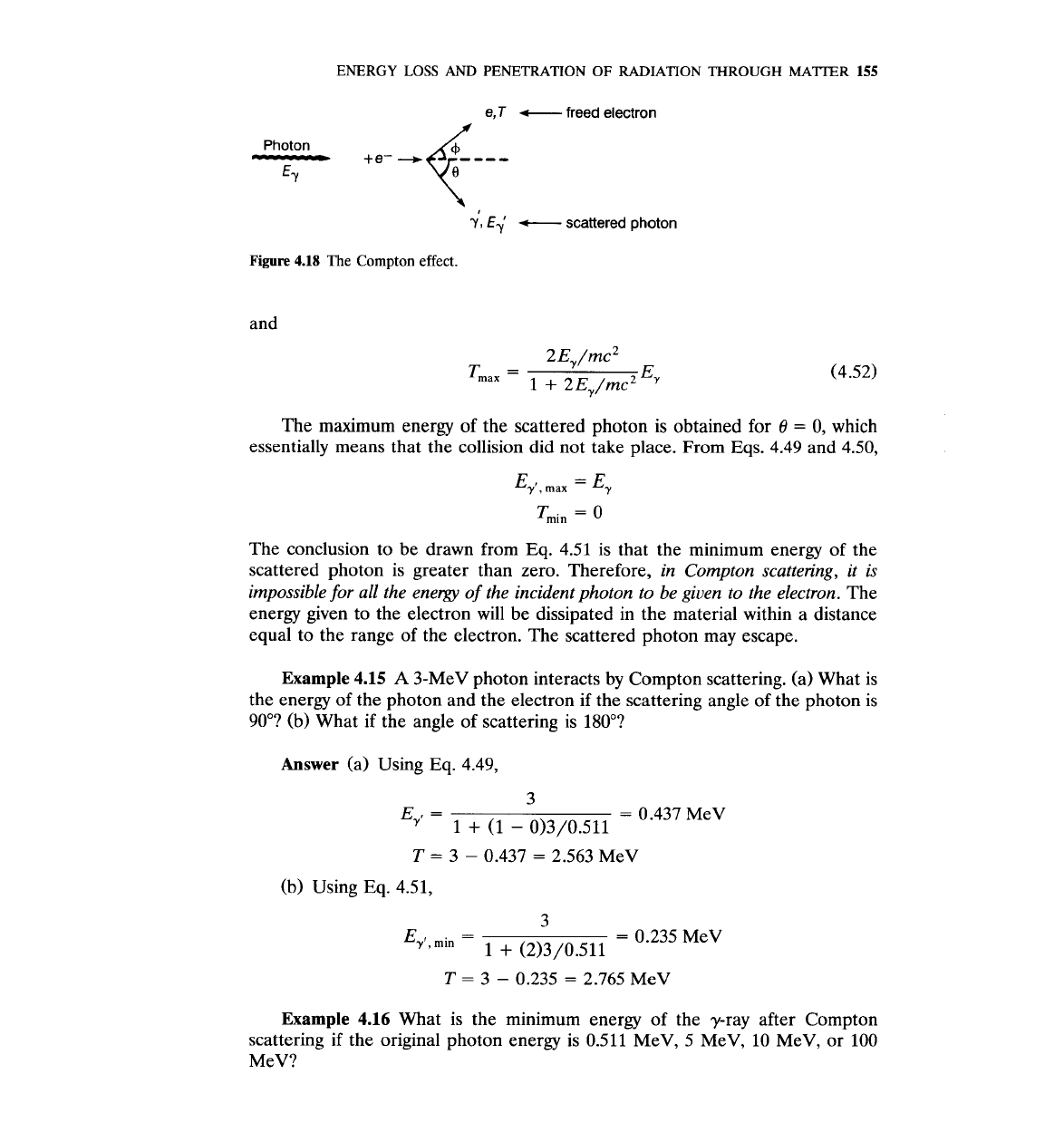
The photon does not disappear after a Compton scattering. Only its direc-
tion of motion and energy change (Fig. 4.18). The photon energy is reduced by a
certain amount that is given to the electron. Therefore, conservation of energy
gives (assuming the electron is stationary before the collision):
If Eq. 4.48 is used along with the conservation of momentum equations, the
energy of the scattered photon as a function of the scattering angle
0
can be
calculated. The result is (see Evans)
Using Eqs. 4.48 and 4.49, one obtains the kinetic energy of the electron:
(1
-
cos 6)Ey/mc2
T=
E
1
+
(1 -cos~)~~/mc~
A
matter of great importance for radiation measurement is the maximum
and minimum energy of the photon and the electron after the collision. The
minimum energy of the scattered photon is obtained when
8
=
T.
This, of
course, corresponds to the maximum energy of the electron. From Eq. 4.49,
Ey',
min
=
EY
1
+
2E,/mc2
Z
photon energy and
(b)
atomic
(b)
number of the material.
ENERGY LOSS
AND
PENETRATION OF RADIATION THROUGH MATIER
155
e,T
-
freed
electron
Photon
EY
-
+e--B---
Y, Ej
-
scattered photon
Figure
4.18
The
Compton effect.
and
The
maximum
energy of the scattered photon is obtained for
8
=
0, which
essentially means that the collision did not take place. From Eqs.
4.49
and 4.50,
The conclusion to be drawn from
Eq.
4.51 is that the minimum energy of the
scattered photon is greater than zero. Therefore,
in Compton scattering, it is
impossible for all the eneqy of the incident photon to be given to the electron.
The
energy given to the electron will be dissipated in the material within a distance
equal to the range of the electron. The scattered photon may escape.
Example
4.15
A
3-MeV photon interacts by Compton scattering. (a) What is
the energy of the photon and the electron if the scattering angle of the photon is
90"? (b) What if the angle of scattering is 180"?
Answer
(a) Using
Eq.
4.49,
E,=
=
0.437 MeV
1
+
(1
-
0)3/0.511
T
=
3
-
0.437
=
2.563 MeV
(b) Using Eq. 4.51,
J
Eyl,
min
=
=
0.235 MeV
1
+
(2)3/0.511
T
=
3
-
0.235
=
2.765 MeV
Example
4.16
What is the minimum energy of the y-ray after Compton
scattering if the original photon energy is 0.511 MeV, 5 MeV, 10 MeV, or 100
MeV?
156
MEASUREMENT
AND
DETECTION
OF
RADIATION
Answer
The results are shown in the table below (Eq. 4.51 has been used).
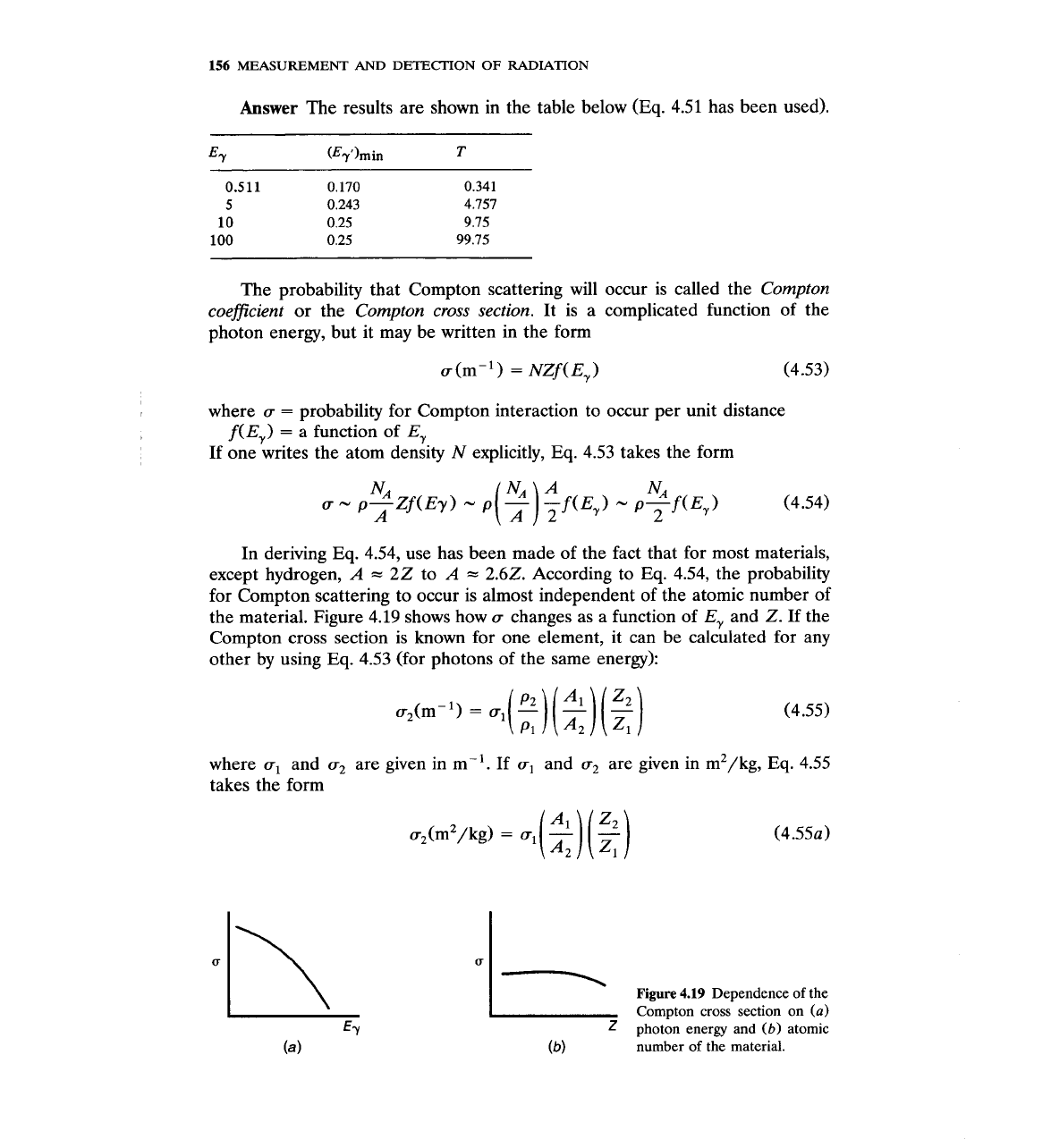
The probability that Compton scattering will occur is called the
Compton
coeficient
or the
Compton cross section.
It is a complicated function of the
photon energy, but it may be written in the form
where
u
=
probability for Compton interaction to occur per unit distance
f(EJ
=
a function of
E,
If one writes the atom density
N
explicitly, Eq. 4.53 takes the form
In deriving Eq. 4.54, use has been made of the fact that for most materials,
except hydrogen,
A
.=
22 to
A
=
2.62. According to Eq. 4.54, the probability
for Compton scattering to occur is almost independent of the atomic number of
the material. Figure 4.19 shows how
u
changes as a function of
E,
and
Z.
If the
Compton cross section is known for one element, it can be calculated for any
other by using Eq. 4.53 (for photons of the same energy):
where
ul
and
a,
are given in m-'. If
a,
and
a,
are given in m2/kg, Eq. 4.55
takes the form
Figure
4.19
Dependence of the
Compton cross section on
(a)
photon energy and
(b)
atomic
(b)
number of the material.

ENERGY
LOSS
AND
PENETRATION
OF
RADIATION
THROUGH
MATTER
157
4.8.3
Pair Production
Pair production is an interaction between a photon and a nucleus. As a result of
the interaction, the photon disappears and an electron-positron pair appears
(Fig. 4.20). Although the nucleus does not undergo any change as a result of this
interaction, its presence is necessary for pair production to occur.
A
y-ray will
not disappear in empty space by producing an electron-positron pair.+
Conservation of energy gives the following equation for the kinetic energy
of the electron and the positron:
Te+ T,+= E,
-
(mc21e- -(mc21e+
=
E,
-
1.022 MeV (4.56)
The available kinetic energy is equal to the energy of the photon minus 1.022
MeV, which is necessary for the production of the two rest masses. Electron and
positron share, for all practical purposes, the available kinetic energy, i.e.,
Te-= Te+= +(E,
-
1.022 MeV)
(4.57)
Pair production eliminates the original photon, but two photons are created
when the positron annihilates (see
Sec. 3.7.4). These annihilation gammas are
important in constructing a shield for a positron source as well as for the
detection of gammas (see Chap. 12).
The probability for pair production to occur, called the pair production
coeficient or cross section is a complicated function of E, and
Z
(see Evans and
Roy
&
Reed). It may be written in the form
where
K
is the probability for pair production to occur per unit distance traveled
and f( E,, Z) is a function that changes slightly with
Z
and increases with E,.
Figure 4.21 shows how
K
changes with
E,
and
2.
It is important to note
that
K
has a threshold at 1.022 MeV and increases with E, and
Z.
Of the three
coefficients
(r
and
a
being the other two),
K
is the only one increasing with the
energy of the photon.
'pair production may take place in the field of an electron. The probability for that to happen
is much smaller and the threshold for the gamma energy is
4mc2
=
2.04 MeV.
E7
=
0.51
1
MeV
Figure
4.20
Pair production. The gamma disappears and a positron-electron pair is created. Two
0.511-MeV photons are produced when the positron annihilates.
