Tsoulfanidis N. Measurement and detection of radiation
Подождите немного. Документ загружается.

138
MEASUREMENT
AND
DETECTION
OF
RADIATION
The range of the 5-MeV deuteron (in air) is then (Eq. 4.20)
Example
4.10
What is the range of a 5-MeV deuteron in aluminum?
Answer
Using the Bragg-Kleeman rule (Eq. 4.17) and the result of the
previous example,
4.6.2
Range
of Electrons and Positrons
Electrons and positrons behave in essentially the same way with regard to
energy loss, slowing down, and penetration through matter. Small differences
exist; one was indicated when
dE/h
was discussed in Sec. 4.3. Small differences
in the values of the range between electrons and positrons should also be
expected, and indeed this is the case. Most of the range measurements have
been performed with electrons because electrons are used much more fre-
quently than positrons in radiation measurements. For this reason, from this
point on, only electrons will be discussed. The reader should be aware that the
results are equally applicable for positrons, to a first approximation, but for very
accurate results the references listed at the end of this chapter should be
consulted.
If the experiment shown in Fig. 4.5 and discussed in Sec. 4.6.1 is repeated
with the incident beam consisting of monoenergetic electrons, the result will
look as shown in Fig. 4.8. For electrons, the transmission curve does not have a
flat part. It decreases gradually to a level which is the background. The ranget is
equal to the thickness of the material, which is defined
by
the point where the
linear extrapolation of the transmission curve meets the background.
'1n many texts, this is called the "extrapolated" range. Since only one type of range is used,
there is no need to carry along the world "extrapolated."
Figure
4.8
The number of elec-
trons transmitted throub thick-
-
ness t. Experiment setup shown
in
Fig.
4.5.
ENERGY
LOSS
AND
PENETRATION
OF
RADIATION
THROUGH
MA'ITER
139
The semiempirical equation giving the range of electrons for the energy
range 0.3 keV to 30 MeV has been developed by Tabata, Ito, and Okabe,1°
based on the experimental results available until 1972. This equation, indicated
from now on as the TI0 equation, has the following form:
where
A,
Z,
and
y
have been defined in Sec. 4.3.
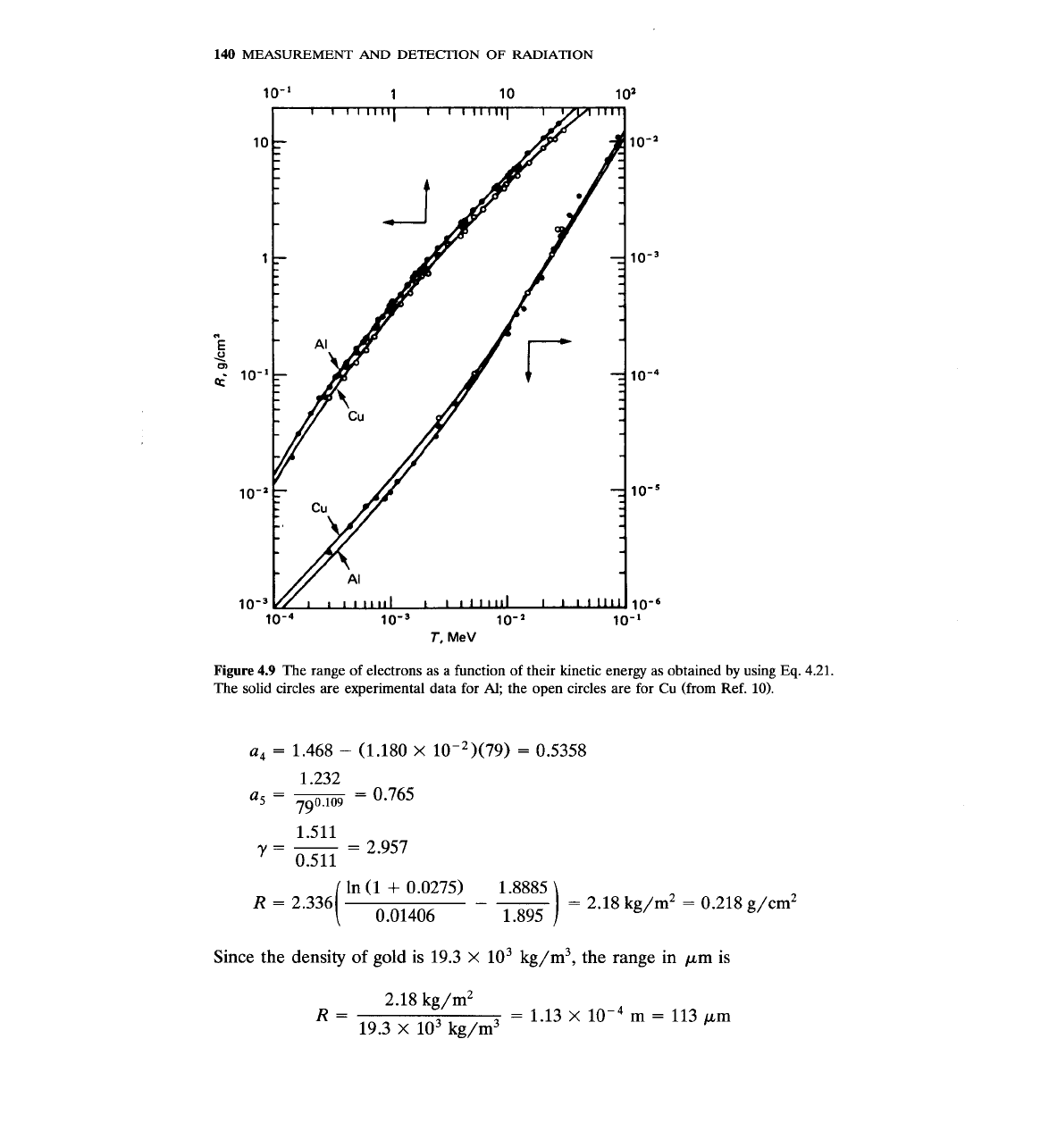
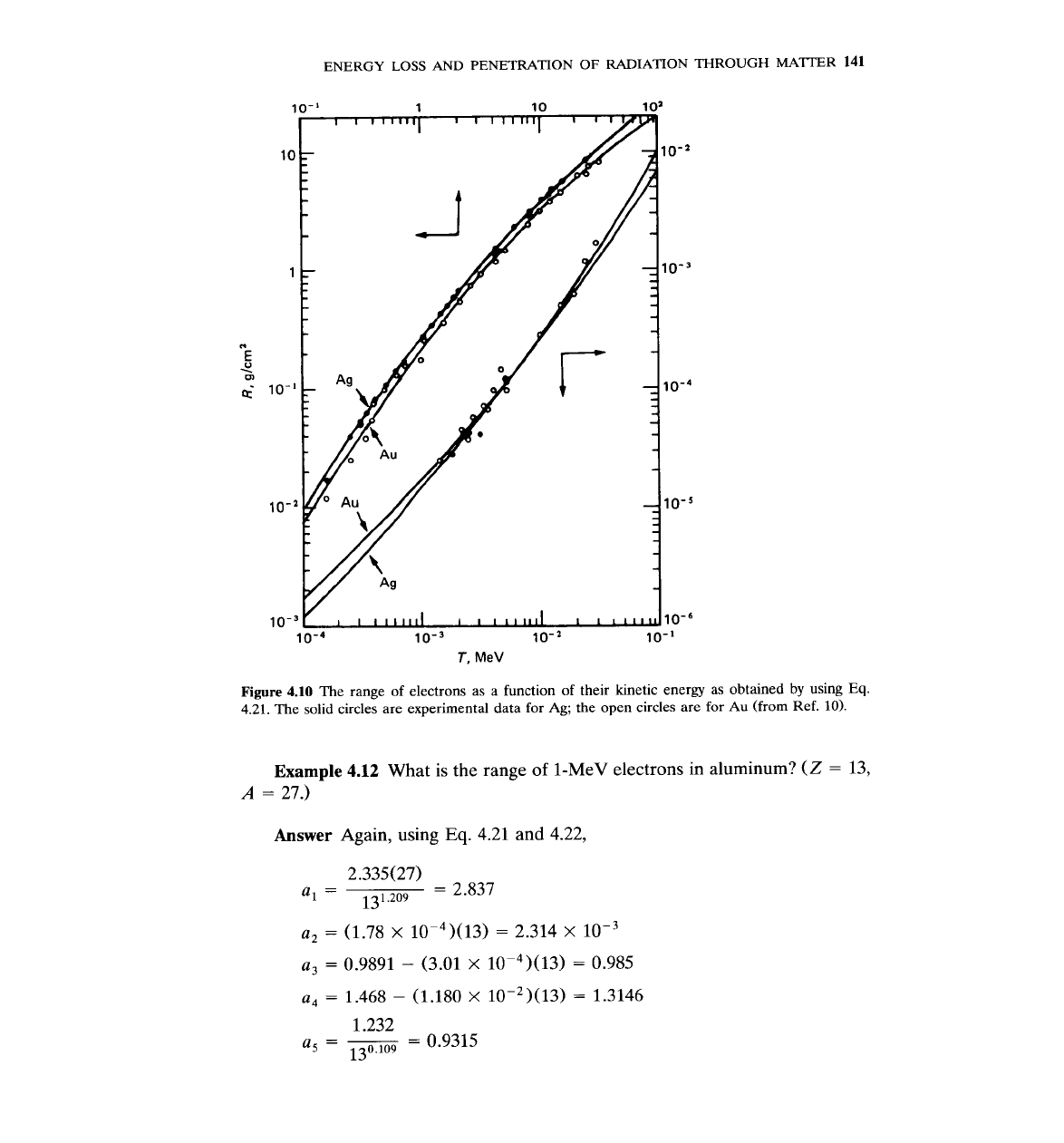
Figures 4.9 and 4.10 show results based on
Eq.
4.21, as well as experimental
points.
In the case of absorbers that are mixtures or compounds, the atomic
number
Z
and atomic weight
A
to be used in
Eq.
4.22 are given
by
L
z,,
=
C
wizi
i
where
wi
is the weight fraction of element with atomic number
Zi
and atomic
weight
Ai.
Example
4.11
What is the range of 1-MeV electrons in gold?
(Z
=
79,
A
=
197.)
Answer
Using Eqs. 4.21 and 4.22,
140
MEASUREMENT
AND
DETECTION
OF
RADIATION
lo-'
1
10 10'
T.
MeV
Figure
4.9
The range of electrons as a function of their kinetic energy as obtained by using
Eq.
4.21.
The solid circles are experimental data for
Al;
the open circles are for Cu (from Ref.
10).
a4
=
a5
=
Y=
R
=
Since the density of gold is 19.3
X
lo3
kg/m3, the range in
pm
is
ENERGY LOSS
AND
PENETRATION OF RADIATION THROUGH MATTER
141
T,
MeV
Figure
4.10
The range of electrons as a function of their kinetic energy as obtained by using
Eq.
4.21.
The solid circles are experimental data for Ag; the open circles are for
Au
(from Ref.
10).
Example
4.12
What is the range
of
1-MeV electrons in aluminum?
(Z
=
13,
A
=
27.)
Answer
Again, using
Eq.
4.21 and 4.22,
142
MEASUREMENT
AND DETECTION OF
RADIATION
Since the density of aluminum is 2.7
X
lo3
kg/m3, the range in pm is
4.6.3 Transmission of Beta Particles
Beta particles have a continuous energy spectrum extending from zero energy
up to maximum kinetic energy
Em,,
(see Sec. 3.7.3). If the transmission experi-
ment shown in Fig. 4.5 is repeated with an incident beam of
P
particles, the
result will look as shown in Fig.
4.11.
The number of betas N(t) transmitted
through a thickness
t
is very closely represented by
N(t)
=
N(0)e-Wt (4.25)
where
p
is called the mass absorption coefficient.
The value of
p
has been determined experimentally as a function of the
maximum beta energy and is given by
\'
,
\
',
Electron beam
\
,(monoenergetic)
\
Beta beam
\,
Absorber thickness (relative units)
Figure
4.11
Transmission
of
betas.
A
corresponding curve for monoener-
getic electrons is also shown with
Emono
=
Emax.

ENERGY
LOSS
AND
PENETRATION OF RADIATION THROUGH
MATI'ER
143
where
Em,,
is in MeV. Notice that
p
is given in units of m2/kg; therefore the
thickness
t
in the exponent of Eq. 4.25 should be in kg/m2. The exponential
transmission law represented by
Eq.
4.25 is the result of experimental observa-
tion. There is no theory predicting it. The range of
P
particles is calculated
using Eq. 4.21 for kinetic energy equal to Em,,.
Example
4.13
What fraction of 2-MeV betas will go through a single
A1
foil
of thickness 0.1 mm?
Answer
The mass absorption coefficient is, using Eq.
4.26.
The fraction transmitted is, using
Eq.
4.25,
N(t)
-
=
e-p'
=
exp [-0.7714 m2/kg(0.1
X
ml(2.7
x
lo3
kg/m3)1
N(0)
Therefore, 81 percent of the betas will go through this foil.
4.6.4
Energy Loss after Traversing a Material of Thickness
t
<
R
One is often required to calculate the energy loss of a charged particle after it
traverses a material of thickness
t.
The first step in solving such a problem is to
calculate the range of the particle in that medium. If the range is
R
<
t,
the
particle stopped in the medium and the total energy lost is equal to the initial
energy of the particle. If
R
>
t,
the energy loss
AE
is given by
where dE/& is the total stopping power (ionization-excitation plus radiation
loss). If t
t
R,
one may take dE/& as constant and obtain
where (dE/dr), is the stopping power calculated for the initial energy of the
particle.
If the thickness
t
is a considerable fraction of the range, dE/& cannot be
considered constant. Then, Eq. 4.27 should be integrated using the appropriate
form of dE/&. Since the stopping power is a complicated expression, the
integration cannot be carried out by hand.
A
numerical integration can be
performed by a computer. In most cases, however, the following approach gives
adequate results.
144
MEASUREMENT AND DETECTION OF RADIATION
The thickness
t
is divided into
N
segments of length Axi, where
N
CAX~
=t
i=
1
Equation 4.27 takes the form
where (dE/d~)~ is the stopping power calculated for the kinetic energy of the
particle at the beginning of the segment Axi.
There is no general rule as to the best value of the number of segments
N.
Obviously,
N
should be such that (dE/d~)~ changes by a small but acceptable
amount as the particle travels the segment Axi.
Example
4.14
What is the energy loss of a 10-MeV electron going through
15 mm of aluminum?
Answer
Using
Eq.
4.21 or Fig. 4.9, the range of a 10-MeV electron in
aluminum is
R
=
20.4 mm. The particle will emerge, but the thickness of the
absorber is a considerable fraction of the range. Therefore, one should use Eq.
4.29.
If
one chooses
N
=
5 and equal segments, Eq. 4.29 takes the form
The table below shows how the calculation proceeds.
Total energy loss is 7.958 MeV. Using
(dE/cix),, the energy loss would have
been equal to 0.605 MeV/mm
x
15mm
=
9.075 MeV, which is overestimated
by about 14%.
4.7 STOPPING POWER
AND
RANGE OF
HEAVY
IONS
(Z
>
2,
A
>
4)
4.7.1
Introduction
The equations presented in Secs. 4.3-4.6 for energy loss and range of charged
particles were derived with the assumption that the charge of the particle does
not change as the particle traverses the medium. This assumption is certainly
ENERGY
LOSS
AND
PENETRATION
OF
RADIATION THROUGH
MATTER
145
valid for electrons, positrons, protons, and deuterons
(Z
=
1).
It holds well for
alphas too
(Z
=
2).
However, for
Z
>
2,
the charge of the particle cannot be
assumed constant, and for this reason the energy loss and range calculations
require special treatment.
Consider an atom or an ion with speed greater than the orbital velocity of
its own electrons. If this particle enters a certain medium, the atomic electrons
will be quickly removed from the atom or ion, leaving behind a bare nucleus.
The nucleus will keep moving through the medium, continuously losing energy
in collisions with the electrons of the
medium.+ It is probable that the ion will
capture an electron in one of these collisions. It is also probable that the
electron will be lost in another collision. As the ion slows down and its speed
becomes of the same order of magnitude as the orbital speeds of the atomic
electrons, the probability for electron capture increases, while the probability for
electron loss decreases. When the ion slows down even farther and is slower
than the orbiting electrons, the probability of losing an electron becomes
essentially zero, while the probability of capturing one becomes significant.
As
the speed of the ion continues to decrease, a third electron is captured, then a
fourth, and so on.
At
the end, the ion is slower than the least bound electron. By
that time, it is a neutral atom. What is left of its kinetic energy is exchanged
through nuclear and not electronic collisions. The neutral atom is considered as
stopped when it either combines chemically with one of the atoms of the
material or is in thermal equilibrium with the medium.
4.7.2
The
dE
/
dr
Calculation
The qualitative discussion of Sec.
4.7.1
showed how the charge of a heavy ion
changes as the ion slows down in the medium. It is this variation of the charge
that makes the energy loss calculation very difficult. There is no single equation
given
dE/&
for all heavy ions and for all stopping materials. Instead,
dE/&
is
calculated differently, depending on the speed of the ion relative to the speed of
the orbital electrons.
The stopping power is written, in general, as the sum of two terms:
where
(dE/cix),
=
electronic energy loss
(dE/dx),
=
nuclear energy loss
An
excellent review of the subject is presented by Northcliffe" and Lindhard,
Scharff, and Schiott." The results are usually presented as universal curves in
terms of two dimensionless quantities, the distance
s
and the energy
E,
first
t~ollisions with nuclei are not important if the particle moves much faster than the atomic
electrons.
146
MEASUREMENT
AND
DETECTION OF RADIATION
introduced by Lindhard et al.1° and defined as follows:
wheret a
=
0.8853a,(~:/~
+
~,2/~)-'/~
x
=
actual distance traveled
a,
=
h2/me2
=
Bohr radius
=
5.29
X
lo-''
m
Z,, MI
=
charge and mass of incident particle
Z2,
M2
=
charge and mass of stopping material
The parameters N, r,, and mc2 have been defined in Sec. 4.3.
At high ion velocities,
u
*
V,Z?/~, where
v,
=
e2/fi
=
orbital velocity of
the electron in the hydrogen atom, the nuclear energy loss is negligible. The
particle has an effective charge equal to Z,, and the energy loss is given by an
equation of the form
which is similar to
Eq.
4.2.
At velocities of the order of
u
=
u,z:/~, the ion starts picking up electrons
and its charge keeps decreasing. The energy loss through nuclear collisions is
still negligible.
In the velocity region
v
<
U,Z?/~, the electronic energy loss equation takes
the formlo
where
1
12.13
and
n
has a value very close to
T.
The constant
k
depends on Z and
A
only,
not on energy, and its value is less than
1.
Some typical values are given in Table
4.2.
Table 4.3 shows the kinetic energy per unit atomic mass, as well as the
kinetic energy, of several ions for
u
=
v,Z:/~.
The electronic stopping power for different ions and stopping materials is
obtained by using the following semiempirical approach.
se he
number
0.8853
=
(9.rr2)1/3/27/3 is called the Thomas-Fermi constant.

ENERGY LOSS
AND
PENETRATION OF RADIATION THROUGH MATIER
147
Table
4.2
Values of
k
Used in Eq.
4.34
The ratio of stopping power for two ions having the same velocity and
traveling in the same medium is given
by
(using
Eq.
4.33):
The application of
Eq.
4.35 to heavy ions should take into account the change of
the charge Z, as the ion slows down. This is accomplished
by
replacing Z, with
an effective charge,
Zeff
=
7721
where
77
is a parameter that depends on energy. The second particle in
Eq.
4.35
is taken to be the proton (Z,
=
A,
=
I),
thus leading to the form14-l6
where the effective proton charge
77,
is given
by
Eq.
4.37, reported
by
Booth
&
rant'^,
and
T,
is the proton kinetic energy in MeV:
77;
=
[I
-
exp
(-
150~,)]
exp (-0.835e-'~.~'~)
(4.37)
Table
4.3
The Kinetic Energy of Heavy Ions for Several
Values of
v
=
U,,Z~/~
~-
voZ:'=
(X
lo-')
P
Ion
z1
(4s)
(X
10')
T/A
T (MeV)
