Tsoulfanidis N. Measurement and detection of radiation
Подождите немного. Документ загружается.

98
MEASUREMENT
AND DETECTlON
OF
RADIATION
Energy
130
u
0.23%
(MeV)
Energy (MeV)
4
1
23%
77%
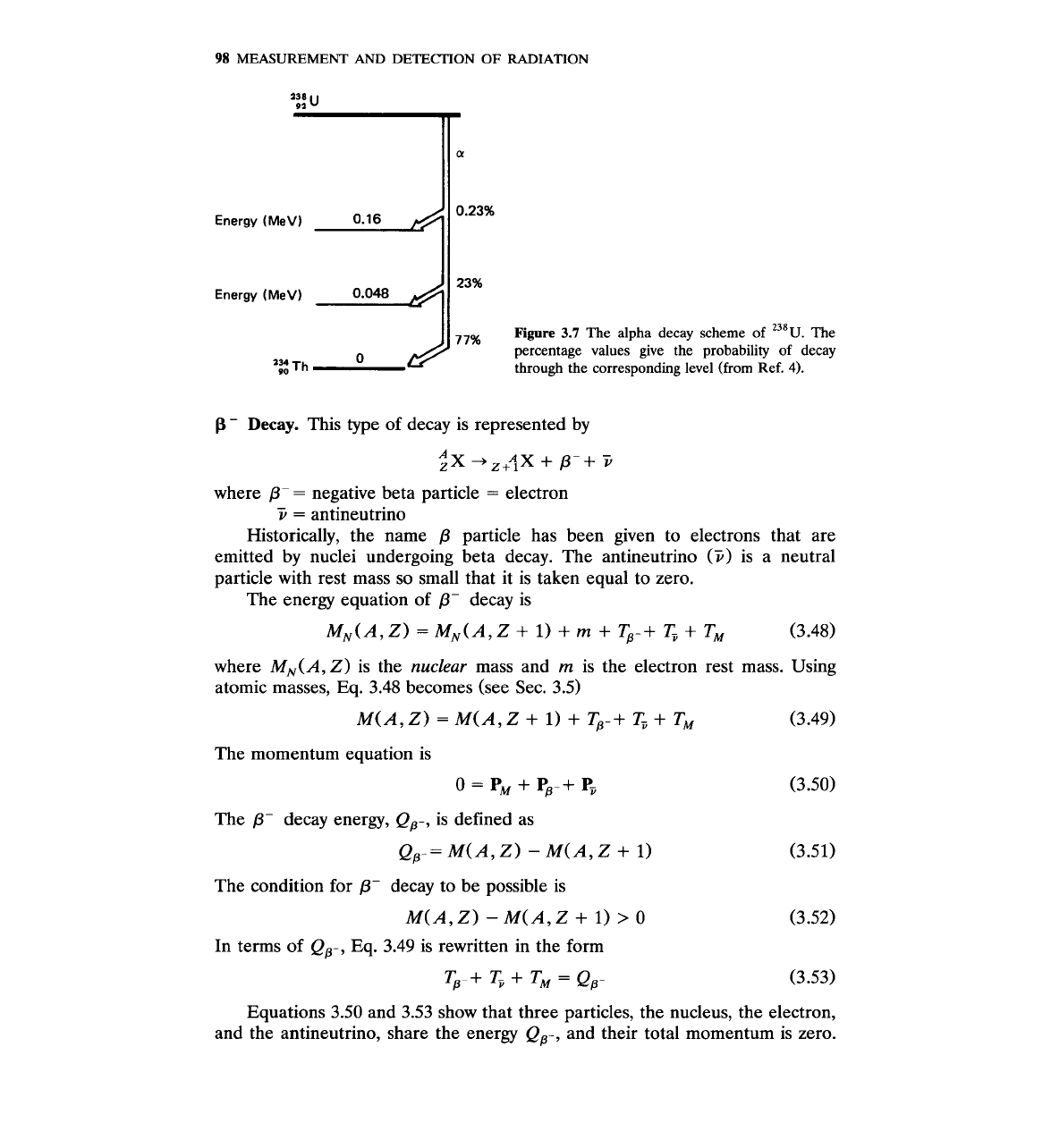
Figure
3.7
The
alpha decay scheme of
238~.
The
0
percentage values give the probability of decay
'2
Th
through the corresponding level (from Ref.
4).
p
-
Decay.
This type of decay is represented by
where
p
=
negative beta particle
=
electron
-
v
=
antineutrino
Historically, the name
p
particle has been given to electrons that are
emitted by nuclei undergoing beta decay. The antineutrino
(F)
is a neutral
particle with rest mass so small that it is taken equal to zero.
The energy equation of
p-
decay is
MN(A, Z)
=
MN(A,Z
+
1)
+
m
+
Tp-+
+
TM
(3.48)
where MN(A,
Z)
is the
nuclear
mass and
m
is the electron rest mass. Using
atomic masses, Eq. 3.48 becomes (see Sec. 3.5)
The momentum equation is
0
=
PM
+
Pp-+ PF
(3.50)
The
p-
decay energy,
Qp-,
is defined as
Qp-=
M(A, Z)
-
M(A, Z
+
1)
(3.51)
The condition for
p-
decay to be possible is
M(A,Z)
-
M(A,Z
+
1)
>
0
In terms of Qp-, Eq. 3.49 is rewritten in the form
Tp-+
q,
+
TM
=
Qp-
Equations 3.50 and 3.53 show that three particles, the nucleus, the electron,
and the antineutrino, share the energy Q,-, and their total momentum is zero.

REVIEW
OF ATOMIC
AND
NUCLEAR
PHYSICS
99
There is an infinite number of combinations of kinetic energies and momenta
that satisfy these two equations and as a result, the energy spectrum of the betas
is continuous.
In
Eq.
3.53, the energy of the nucleus,
TM,
is much smaller than either T,-
or
T,
because the nuclear mass is huge compared to that of the electron or the
antineutrino. For all practical purposes, TM can be neglected and
Eq.
3.53 takes
the form
As
in the case of
a
decay, the daughter nucleus may be left in an excited
state after the emission of the
p-
particle. Then, the energy available to
become kinetic energy of the emitted particles is less. If the nucleus is left in the
ith excited state
Ei,
Eq.
3.54
takes the form
According to
Eq.
3.54, the electron and the antineutrino share the energy Qp-
(or
Emax)
and there is a certain probability that either particle may have an
energy within the limits
which means that the beta particles have a continuous energy spectrum. Let
P(T)
dT
be the number of beta particles with kinetic energy between T and
T
+
dT.
The function B(T) has the general shape shown in Fig. 3.8. The energy
spectrum of the antineutrinos is the complement of that shown in Fig. 3.8,
consistent with
Eq.
3.57. The continuous energy spectrum of
P
particles
should be contrasted with the energy spectrum of internal conversion electrons
shown by Fig. 3.6.
As stated earlier, beta particles are electrons. The practical difference
between the terms
electrons
and
betas
is this: A beam of electrons of energy
T
consists of electrons each of which has the kinetic energy
T.
A beam of beta
particles with energy
Emax
consists of electrons that have a continuous energy
spectrum (Fig 3.8) ranging from zero up to a maximum kinetic energy
Em,,.
Figure 3.9 shows the
P-
decay scheme of the isotope '::CS. For an example
of a
Qp-
calculation, consider the decay of '35:~s:
=
0.0012625(931.478 MeV)
=
1.1760 MeV
=
1.36
X
10-13
J
If
the ':;~a is left in the 0.6616-MeV state (which happens 93.5 percent of the
time), the available energy is
Emax
=
1
.I760
-
0.6616
=
0.5144 MeV
100
MEASUREMENT AND DETECTION OF RADIATION
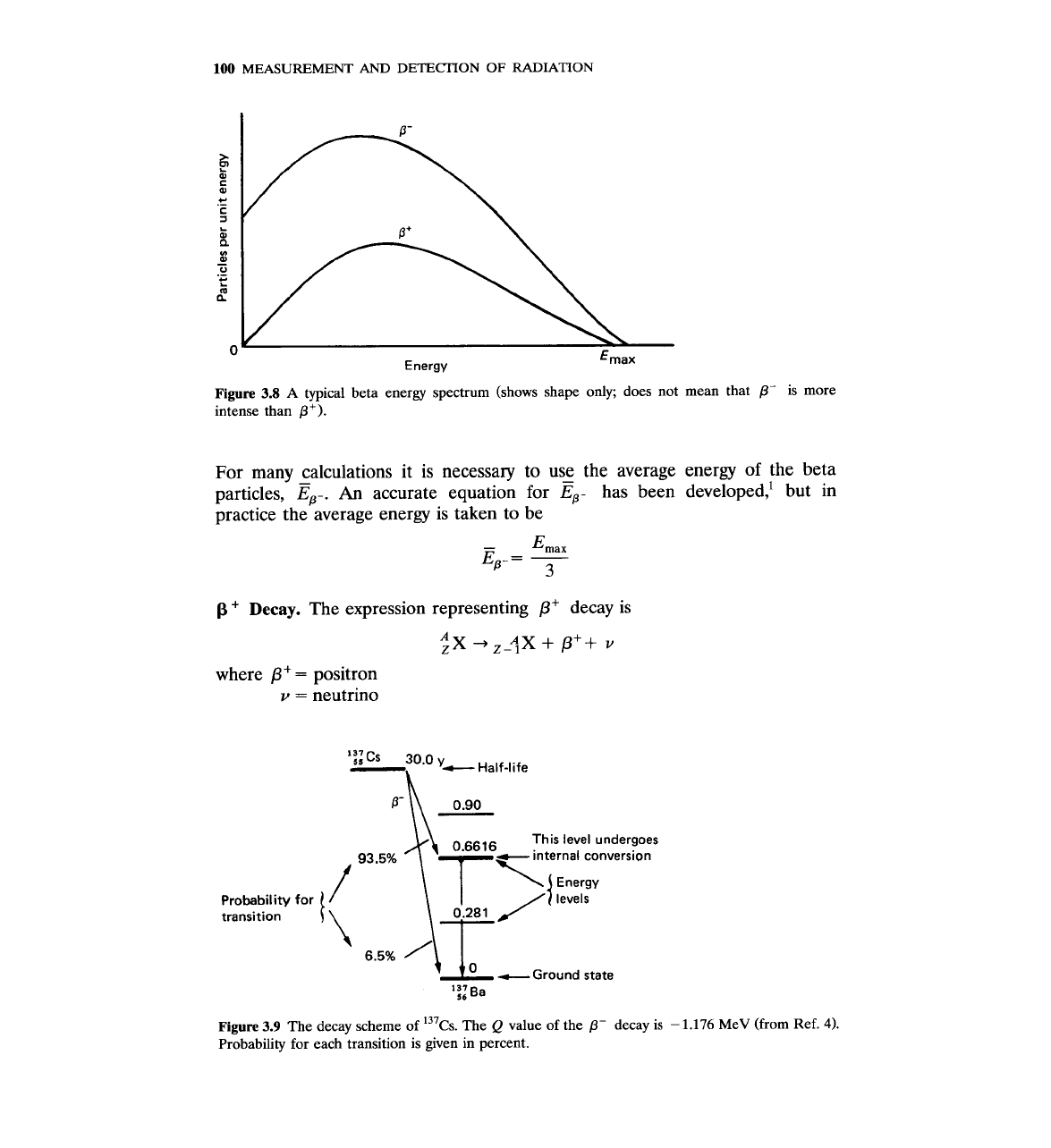
Figure
3.8
A
typical beta energy spectrum (shows shape only; does not mean that
P-
is more
intense than
P').
For many calculations it is necessary to use the average energy of the beta
particles,
&-.
An
accurate equation for
q-
has been developed: but in
practice the average energy is taken to be
-
Em,,
Ep-=
-
3
p
+
Decay.
The expression representing
/3+
decay is
where
p+
=
positron
v
=
neutrino
Probability for
transition
Figure
3.9
The decay scheme of 137~s. The
Q
value of the
P-
decay is
-
1.176
MeV (from Ref.
4).
Probability for each transition is given in percent.
REVIEW
OF
ATOMIC
AND
NUCLEAR
PHYSICS
101
The energy equation of
P+
decay is
Using atomic masses, Eq.
3.58
becomes
The momentum equation is
The
P+
decay energy is
The condition for
P+
decay to be possible is
A
comparison of Eqs.
3.52
and
3.62
shows that
P-
decay is possible if the
mass of the parent is just bigger than the mass of the daughter nucleus, while
p+
decay is possible only if the parent and daughter nuclear masses differ by at
least
2mc2
=
1.022
MeV.
The energy spectrum of
P+
particles is continuous, for the same reasons
the
p-
spectrum is, and similar to that of
p-
decay (Fig.
3.8).
The average
energy of the positrons from
pf
decay,
&+,
is also taken to be equal to
E,,,/3
unless extremely accurate values are needed, in which case the equation given in
Ref.
1
should be used.
A
typical
P+
decay scheme is shown in Fig.
3.10.
Electron Capture.
In some cases, an atomic electron is captured by the nucleus
and a neutrino is emitted according to the equation
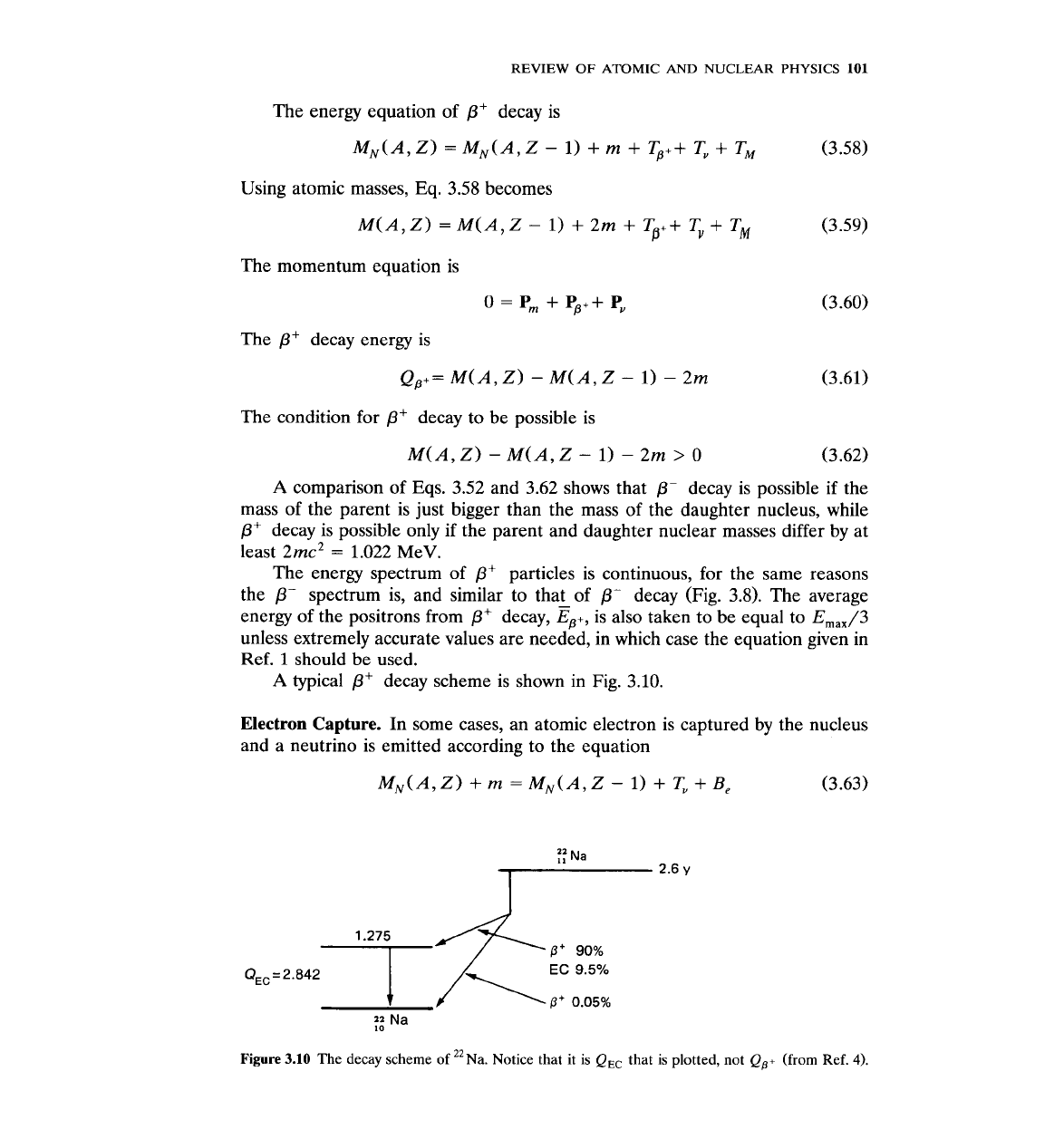
Figure
3.10
The decay scheme of "~a. Notice that it is
Q,,
that is plotted, not
QS+
(from Ref.
4).

102
MEASUREMENT
AND
DETECTION
OF
RADIATION
In Eq. 3.63 all the symbols have been defined before except Be, binding energy
of the electron captured by the nucleus. This transformation is called electron
capture
(EC). In terms of atomic masses, Eq. 3.63 takes the form
The energy
QEc
released during EC is
The condition for EC to be possible is
Electron capture is an alternative to
/3+
decay. Comparison of Eqs. 3.61 and
3.66 shows that nuclei that cannot experience
/3+
decay can undergo EC, since
a smaller mass difference is required for the latter process. Of course, EC is
always possible if
/3+
decay is. For example, "~a (Fig. 3.10) decays both by
/3+
and EC.
After EC, there is a vacancy left behind that is filled by an electron falling
in from a higher orbit. Assuming that a
K
electron was captured, an
L
electron
may fill the empty state left behind. When this happens, an energy approxi-
mately equal to
B,
-
B, becomes available (where B, and BL are the binding
energy of a
K
or
L
electron, respectively). The energy BK
-
BL may be emitted
as a
K
X-ray called fluorescent radiation, or it may be given to another atomic
electron. If this energy is given to an
L
electron, that particle will be emitted
with kinetic energy equal to (B,
-
B,)
-
BL
=
B,
-
2BL.
Atomic electrons
emitted in this way are called Auger electrons.
Whenever an atomic electron is removed and the vacancy left behind is
filled by an electron from a higher orbit, there is a competition between the
emission of Auger electrons and fluorescent radiation. The number of X-rays
emitted per vacancy in a given shell is the fluorescent yield. The fluorescent yield
increases with atomic number.
3.7.4
Particles, Antiparticles, and Electron-Positron Annihilation
Every known subatomic particle has a counterpart called the antiparticle.
A
charged particle and an antiparticle have the same mass, and opposite charge. If
a particle is neutral-for example, the neutron-its antiparticle is still neutral.
Then their difference is due to some other property, such as magnetic moment.
Some particles, like the photon, are identical with their own antiparticles.
An
antiparticle cannot exist together with the corresponding particle: when an
antiparticle meets a particle, the two react and new particles appear.
Consider the example of the electron and the "antielectron," which is the
positron. The electron and the positron are identical particles except for their
charge, which is equal to e but negative and positive, respectively. The rest mass
of either particle is equal to 0.511 MeV. A positron moving in a medium loses

REVIEW
OF
ATOMIC
AND NUCLEAR
PHYSICS
103
energy continuously, as a result of collisions with atomic electrons (see Chap.
4).
Close to the end of its track, the positron combines with an atomic electron, the
two annihilate, and photons appear with a total energy equal to 2mc2. At least
two photons should be emitted for
conservation of energy and momentum to be
satisfied (Fig.
3.11).
Most of the time, two photons, each with energy
0.511
MeV,
are emitted.
As
a result, every positron emitter is also a source of 0.511-MeV
annihilation gammas.
3.7.5 Complex Decay Schemes
For many nuclei, more than one mode of decay is positive. Users of radioiso-
topic sources need information about particles emitted, energies, and probabili-
ties of emission. Many books on atomic and nuclear physics contain such
information, and the most comprehensive collection of data on this subject can
be found in the Table
of
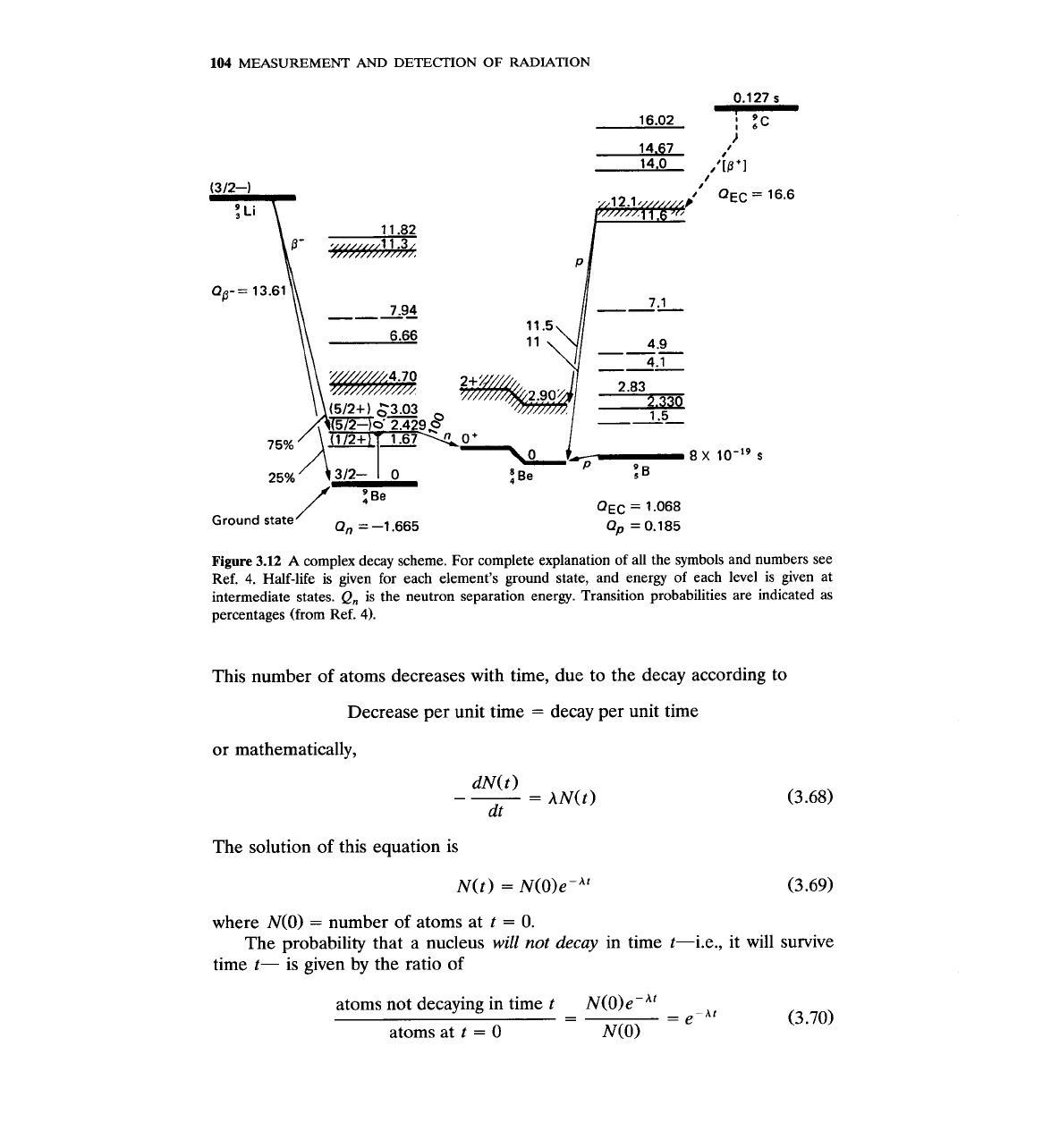
Isotopes by Lederer and ~hirley.~ Figure 3.12 shows an
example of a complex decay scheme taken from that book.
3.8
THE RADIOACTIVE DECAY
LAW
Radioactive decay is spontaneous change of
a
nucleus. The change may result in
a new nuclide or simply change the energy of the nucleus. If there is a certain
amount of
a
radioisotope at hand, there is no certainty that in the next second
"so many nuclei will decay" or "none will decay." One can talk of the probabil-
ity that a nucleus will decay in a certain period of time.
The probability that a given nucleus will decay per unit time is called the
decay constant and is indicated by the letter
A.
For a certain species,
A
is
1.
The same for all the nuclei
2. Constant, independent of the number of nuclei present
3.
Independent of the age of the nucleus
Consider a certain mass m of a certain radioisotope with decay constant
A.
The number of atoms (or nuclei) in the mass m is equal to
where
N,
=
6.022
x
loz3
=
Avogadro's number
A
=
atomic weight of the isotope
:
v7
=
0.51
Figure
3.11
Electron-positron anni-
Ey
=
0.51
1
MeV
,+
hilation.
104
MEASUREMENT
AND
DETECTION
OF
RADIATION
Figure
3.12
A
complex decay scheme. For complete explanation of all the symbols and numbers see
Ref.
4.
Half-life is given for each element's ground state, and energy of each level is given at
intermediate states.
Q,
is the neutron separation energy. Transition probabilities are indicated as
percentages (from Ref.
4).
This number of atoms decreases with time, due to the decay according to
Decrease per unit time
=
decay per unit time
or mathematically,
The solution of this equation is
where
N(0)
=
number of atoms at t
=
0.
The probability that a nucleus
will
not
decay
in time t-i.e., it will survive
time t- is given by the ratio of
atoms not decaying in time t
N(0)e-At
-
- -
-
e-~t
(3.70)
atoms at
t
=
0
NO)
REVIEW
OF
ATOMIC
AND
NUCLEAR
PHYSICS
105
The probability that the nucleus will decay between t and t
+
dt is
p(t) dt
=
(probability to survive to time t)(probablility to decay in dt)
=
ePA'
A
dt
The average lifetime
i
of the nucleus is given by
One concept used extensively with radioisotopes in the half-life T, defined
as the time it takes for half of a certain number of nuclei to decay. Thus, using
Eq. 3.69,
N(T)
1
-A,
--
-
-
NO) 2
=
e
which then gives the relationship between
A
and T:
For a sample of N(t) nuclei at time
t,
each having decay constant A, the
expected number of nuclei decaying per unit time is
where A(t)
=
activity of the sample at time t.
The units of activity are the Becquerel (Bq), equal to
1
decay/s, or the
Curie (Ci) equal to 3.7
x
10'' Bq. The Becquerel is the SI unit defined in 1977.
The term specific activity (SA) is used frequently. It may have one of the two
following meanings:
1.
For solids,
activity
SA
=
-
mass
(Bq/kg or Ci/g)
2. For gases or liquids,
activity
SA
=
-
(Bq/m3 or Ci/cm3)
volume
Example
3.8
What is the SA of 60~o?
Answer
The SA is
A
AN In2
N,
SA=-=-=-m-=
(In 2)(6.022
x
loz3)
m m Tm
A
(5.2 y)(3.16
X
10' s/y)(0.060 kg)

106
MEASUREMENT
AND DETECIlON
OF
RADIATION
Example
3.9
What is the
SA
of a liquid sample of m3 containing
kg of
32~?
I
Answer
The
SA
is
A
AN In2
NA
SA=-
=
-
=-m-=
(In 2)(1OP6 kgN6.022
x
loz3)
V
V
VT
A
m3)(14.3 d)(864OO s/d)(O.OX kg)
There are isotopes that decay by more than one mode. Consider such an
isotope decaying by the modes 1,2,3,.
. .
,
i
(e.g., alpha, beta, gamma, etc.,
decay), and let
A,
=
probability per unit time that the nucleus will decay by the ith mode
The total probability of decay (total decay constant) is
A=
A,
+A2
+
-..
+Ai
+
0.-
If the sample contains N(t) atoms at time t, the number of decays per unit time
by the ith mode is
The term partial
haZf-lz$e
is sometimes used to indicate a different decay mode.
If
T,
is the partial half-life for the ith decay mode, using Eqs. 3.72 and 3.74, one
obtains
It should be pointed out that it is the total decay constant that is used by Eqs.
3.69 and 3.73a, and not the partial decay constants.
Example
3.10
The isotope 252~f decays by alpha decay and by spontaneous
fission. The total half-life is 2.646 years and the half-life for alpha decay is 2.731
years. What is the number of
spontaneous fissions per secondeper 10-' kg (1 g)
of
252
~f?
Answer
The spontaneous fission activity is
The spontaneous fission half-life is, using Eq. 3.75,
REVIEW
OF
ATOMIC
AND
NUCLEAR
PHYSICS
107
Therefore,
Sometimes the daughter of a radioactive nucleus may also be radioactive
and decay to a third radioactive nucleus. Thus, a radioactive chain
N,
-,
N2
-,
N,
-,
etc.
is generated.
An
example of a well-known series is that of 235~, which through
combined
a
and
P-
decays ends up as an isotope of lead. The general equation
giving the number of atoms of the ith isotope, at time
t
in terms of the decay
constants of all the other isotopes in the chain was developed by
at ern an.^
If
&(O) is the number of atoms of the ith isotope of the series at time
t
=
0 and
then the Bateman equation takes the form
Example
3.11
Apply the Bateman equation for the second and third isotope
in a series.
Answer
3.9
NUCLEAR REACTIONS
3.9.1
General Remarks
A
nuclear reaction
is an interaction between two particles, a fast bombarding
particle, called the
projectile,
and a slower or stationary
target.
The products of
the reaction may be two or more particles. For the energies considered here
(<
20
MeV), the products are also two particles (with the exception of fission,
which is discussed in the next section).
