Stacey F.D., Davis P.M. Physics of the Earth
Подождите немного. Документ загружается.

//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C19.3D
–
317
– [314–336] 13.3.2008 2:45PM
high temperature for kT > h, so that the energy
levels are effectively blurred into a continuum,
allowing the classical approximation (Eq. (19.3))
to be used. However, the mode theory is the
starting point for an understanding of the
Gr ¨uneisen parameter, as in the following
section.
The level to which a mode is excited ther-
mally is determined by the average thermal
energy, kT, of the other modes with which it
interacts. The probability of excitation to the
nth level is
pðnÞ¼expðnh=kTÞ=
X
1
n¼0
expðnh=kTÞ: (19:5)
This is the Boltzmann energy distribution, with a
denominator that is a normalizing factor to
make the sum of all p(n ) equal to unity. The
state n, when it occurs, contributes energy nh,
so that its contribution to the average energy of
the mode,
E,isnhp(n). Summing over all states,
n ¼0to1,
E ¼ h
X
1
n¼0
n expðnh=kTÞ=
X
1
n¼0
expðnh=kTÞ:
(19:6)
Writing x ¼exp(h/kT), the denominator of
Eq. (19.6) is recognized as a geometric progression,
X
1
n¼0
x
n
¼ 1=ð1 xÞ; (19:7)
and the numerator is
h
X
1
n¼0
nx
n
¼ h
X
1
n¼0
xdðx
n
Þ=dx
¼ hxd
X
1
n¼0
x
n
!
=dx ¼ hx =ð1 xÞ
2
:
(19:8)
Using Eqs. (19.7) and (19.8) in (19.6), with
substitution for x,
E ¼ hx=ð1 xÞ¼h=½expðh=kTÞ1: (19:9)
This is the Einstein model of specific heat,
applicable to harmonic oscillators all of the same
frequency, .Athightemperatures,kT h,it
reduces to the classical situation,
E kT,andat
low temperatures, kT h, there is negligible
excitation above the ground state,
1
/
2
h.Thecor-
responding contribution to specific heat is
obtained by differentiating Eq. (19.9) with respect
to T at constant V, with the assumption that is
independent of T if V is held constant,
C
V
¼ð@E=@TÞ
V
¼ kðh=kTÞ
2
expðh=kTÞ=½expðh=kTÞ1
2
:
(19:10)
This reduces to k for h/kT 1 (the classical
limit). The assumption that is constant at con-
stant V would be valid if a lattice mode were a
perfect harmonic oscillator, but, as in the classi-
cal theory referred to above, it must be seen as an
approximation when applied to an anharmonic
oscillator, with a potential function having a
form represented by Fig. 18.4.
Crystals have vibrational modes with a wide
range of frequencies and can be represented as
collections of Einstein oscillators with different
values of . There is a corresponding wide range
of temperatures, below which the modes are
‘frozen out’, becoming thermally inactive. Thus
the transition from low to high temperature
regimes is more spread than in the Einstein
model with a single mode frequency. With
detailed knowledge of atomic bonding, and
resort to numerical methods, realistic vibra-
tional spectra can be calculated, even for quite
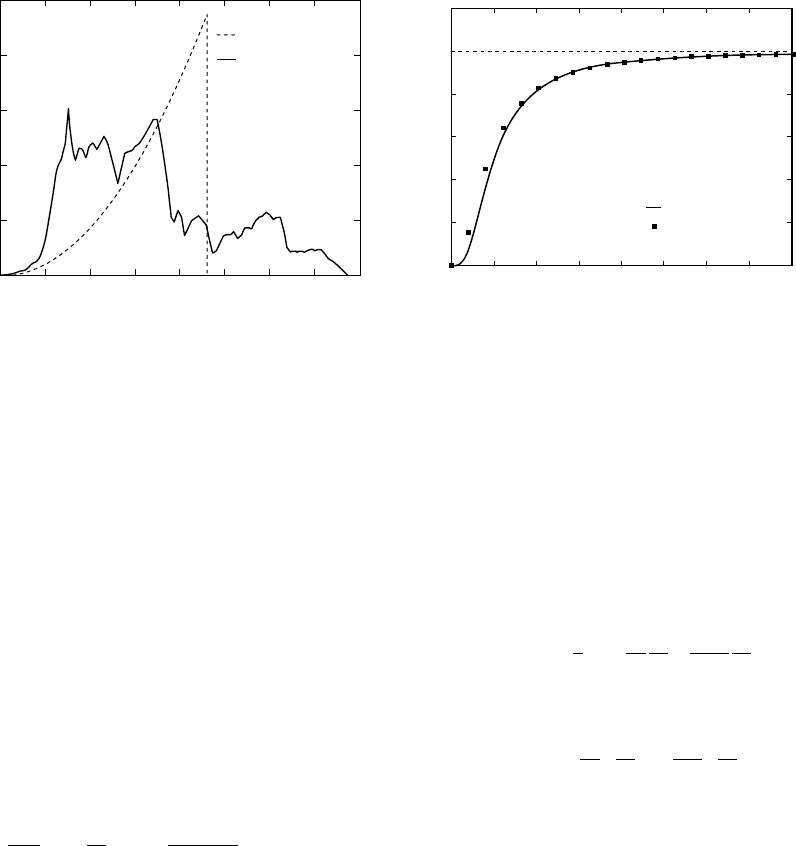
complicated crystal structures. Figure 19.1 gives
an example. It is compared with a mathemati-
cally simple, widely used reference model by
P. Debye, dating from the early twentieth century.
The Debye theory treats a material as an elas-
tic continuum, with standing waves of all orien-
tations and wavelengths that can be fitted into it
down to a limit that gives the required total
number of modes, three times the number of
atoms. The number of standing waves in the
frequency range to ( þd) that can be fitted
into a crystal is proportional to
2
d and this is
the spectrum of frequencies that the Debye
model assumes, with frequency related to wave-
length by an averaged acoustic velocity. Since
the total number of modes in an N atom crystal
is 3N, the spectrum is cut off at the frequency,
D
,
that gives this number. Thus the Debye model
19.2 SPECIFIC HEAT 317
//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C19.3D
–
318
– [314–336] 13.3.2008 2:45PM
has a characteristic temperature, the Debye
temperature,
D
, which is related to
D
by
k
D
¼ h
D
: (19:11)
At temperatures much higher than
D
, all of the
modes are fully excited and C
V
approaches the
classical, high temperature limit, C
V1
, given by
Eq. (19.3). At lower temperatures the high fre-
quency modes become inactive and C
V
has a
reduced value. Integration of Eq. (19.10),
weighted by the
2
d spectrum, gives the Debye
function, which is the sum of specific heats of a
collection of Einstein oscillators (Eq. (19.10))
with a Debye spectrum of frequencies
C
V
C
V1
¼ 3
T
D
3
ð
D
=T
0
x
4
e
x
ðe
x
1Þ
2
dx: (19:12)
Although the Debye theory cannot accurately
represent the fine details of thermal properties,
because the spectrum of mode frequencies is not
very realistic, in many situations it is a conven-
ient approximation, especially its identification
of a characteristic (Debye) temperature. The
spectra in Fig. 19.1 look very different, but
when both are used to calculate specific heat
curves, as in Fig. 19.2, the Debye theory is seen
to reproduce the essential features. The mineral
represented in this figure, MgSiO
3
, perovskite,
has a high Debye temperature, about 950 K, and
at laboratory temperature C
V
(290 K) 0.65 C
V1
.
For all higher temperatures the discrepancy
between the curves of Fig. 19.2 is quite small.
There is no analytical solution for the Debye
function (Eq. (19.12)) but for T >
D
thermal
energy and specific heat can be expanded as
polynomial functions of (
D
/T):
EðDebyeÞ¼C
V1
T
3
8
D
þ
1
20
2
D
T
1
1680
4
D
T
3
þ
;
(19:13)
C
V
ðDebyeÞ¼C
V
1
1
1
20
D
T
2
þ
1
560
D
T
4
þ
"#
:
(19:14)
In estimating the total stored energy in the Earth
(Chapter 21), the (3/8)
D
term in Eq. (19.13) is
important, especially as
D
increases systemati-
cally with pressure. This term is represented by
the area between the Debye curve and the clas-
sical limit extrapolated to T ¼0 in Fig. 19.2.
It is useful to keep in mind the accuracy of the
approximation that C
V
is constant at high tem-
peratures. It is not valid for metals, as discussed
below, but for insulators we can differentiate
Eq. (19.14) for an adequate indication:
ð@ ln C
V
=@ ln TÞ
V
¼ð1=10Þð
D
=TÞ
2
ð3=1400Þð
D
=TÞ
4
þ:
(19:15)
Debye model
Full calculation
Number of lattice modes per
unit frequency interval
0 9 18 27 36
Frequency (THz)
FI G U R E 19.1 The spectrum of lattice modes of
MgSiO
3
perovskite, compared with the Debye theory.
Redrawn from Oganov et al. (2000).
Debye model
Full calculation
0
500
1000
1500
2000
30
20
10
0
Temperature (K)
Classical limit
C
V
(J K
–1
mol
–1
)
FI G U R E 19.2 Specific heat of MgSiO
3
perovskite
calculated from the lattice mode spectrum in Fig. 19.1,
compared with Debye theory. Redrawn from Oganov
et al. (2000).
318 THERMAL PROPERTIES
//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C19.3D
–
319
– [314–336] 13.3.2008 2:45PM
In the deep Earth (T/
D
) 1.6, giving (@lnC
V
/
@lnT)
V
0.04. This is no more than various uncer-
tainties, including the mean atomic weight and
the effect of anharmonicity (Section 19.8). For
the Debye model (@lnC
V
/@lnV)
S
¼0, as demonstra-
ted following Eq. (19.29), so by Eq. (E.6) in
Appendix E (@lnC
V
/@lnV)
T
0.05.
Up to this point the discussion concerns only
the lattice heat capacity. This is the total for
insulators, including the crust and mantle, but
for metals there is an addition al c ontribution by
conduction electrons. In the core it accounts for
about 30% of the total specific heat. In solids the
electron energy levels are spread by interac-
tions in to bands with wide energy ranges.
These bands are occupied by electrons up to a
level, known as the Fermi energy, set by t he
number of electrons. The characteristic of met-
als is that they have electron energy bands that
overlap and are partly filled, so that electrons
with energies close to the Fermi level may
change states in response to an electric field
(giving electrical conduction) or to thermal agi-
tation. Some are thermally excited to energies
of order kT above the Fermi level, leaving vacant
states below that level. The number of electrons
so excited and their average excitation energy
are each proportional to T, so that t he electron
thermal energy is proportional to T
2
and elec-
tron specific heat is proportional to T.Thisis
represented by
C
e
¼ T: (19:16)
For laboratory iron, ¼4.98 mJ K
2
mol
1
¼
0.0892 J K
2
kg
1
. This means that a tem-
perature of 5000 K would make C
e
equal to the
lattice heat capacity by Eq. (19.3), but compres-
sion increases the spread of energy bands and
so decreases the density of energy states at
the Fermi level, reducing the value of .
Boness et al. (1986) reported a numerical cal-
culation of the band structure of iron with diffe-
rent crystal structures over the core pressure
range and summarized their results in a sim-
ple analytical approximation for as a function
of density, , relative to the zero pressure
value,
0
.
With substitution of the atomic weight of
pure iron, this is
¼ð6:113
0
= 1:144ÞmJ K
2
mol
1
¼ð0:1094
0
= 0:0205ÞJK
2
kg
1
:
(19:17)
The band structure and the value of hardly
depend on the crystal form and at core pressures
we assume that Eq. (19.17) is a good approxima-
tion also for liquid iron. Minor amounts of
lighter solute would have only a minor effect
on band structure. This equation is used to esti-
mate the electron contribution to core heat
capacity in the thermal model in Appendix G,
noting that Eqs. (19.16) and (19.17) give the elec-
tron contribution to C
V
and that Eq. (19.4) must
be used to obtain C
P
. It is important also to elec-
trical conductivity (Section 24.4) and conse-
quently also to the thermal conductivity of the
core (Section 19.6).
19.3 Thermal expansion and the
Gr¨uneisen parameter
A particular reason for geophysical interest in
thermal expansion is that the dilation of heated
material makes it buoyant relative to cooler,
surrounding material. In an extended medium
this causes convection, with an upward transfer
of heat by rising hot material. Thermal convec-
tion generates the mechanical energy driving
plate tectonics and the geological activity that
results from it. A uniform medium in which the
temperature gradient exceeds the adiabatic
one, that is the rate at which temperature
increases with depth due to compression, is
convectively unstable. Thus convection tends
to reduce any steeper gradient to the adiabatic
oneandthroughmuchoftheEarththetem-
perature gradient is believed to be close to
adiabatic. Calculations of this gradient
(Section 19.5) and of the mechanical energy of
convection (Chapter 22) rely on knowledge of
the volume coefficient of thermal expansion, ,
but there is no direct observation of for the
deep Earth. It is estimated indirectly from the
Gr ¨uneisen parameter, .
The physical reason why materials expand
when heated is seen in Fig. 18.4. A bond between
neighbouring atoms that oscillates in length
19.3 THERMAL EXPANSION AND THE GRU
¨
NEISEN PARAMETER 319
//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C19.3D
–
320
– [314–336] 13.3.2008 2:45PM
(between A and B) is extended more than it is
compressed and it spends more time in the
extended state because the restoring force (repre-
sented by the gradient of the curve) is then
weaker. The time-averaged extension is the
thermal expansion, which is a consequence of
the bond asymmetry. This has another effect. As
a material is compressed, the externally applied
pressure can be represented in Fig. 18.4 by an
added positive gradient, pushing the energy mini-
mum to smaller r and sharpening it. This means
increasing the elastic moduli. The increase in elas-
tic moduli with pressure is well observed by seis-
mology, which provides a direct measure of the
bond asymmetry. Thus thermal expansion and
the pressure dependences of elastic moduli have
a common cause and can be related by an appro-
priate theory. The Gr
¨
uneisen parameter, , makes
this theoretical connection, which is the reason
for the effort by geophysicists to understand it.
The parameter originally established in the
solid state physics literature by E. Gr ¨uneisen, and
bearing his name, was derived from the equa-
tions for an Einstein oscillator (Eqs. (19.9) and
(19.10)). For a vibrational mode of a crystal lat-
tice, identified as an oscillator of frequency
i
,
the Gr ¨uneisen definition is
i
¼ð@ ln
i
=@ ln VÞ
T
: (19:18)
i
is the mode Gr ¨uneisen parameter, or mode
gamma, and is not in general identical to the
i
of other modes. The interest in it arises from the
fact that, as shown below, an appropriate aver-
age of all of the
i
is equivalent to the dimension-
less combination of thermodynamic parameters
in Eq. (19.1). In common usage, mention of the
Gr ¨uneisen parameter means the definition in
Eq. (19.1), but if this needs to be distinguished
from Gr ¨uneisen’s definition it is referred to as
the thermodynamic gamma. Its usefulness in
thermodynamic relationships is particularly
obvious in two frequently used identities (see
Table E.2 in Appendix E):
ð@ ln T=@ ln Þ
S
¼ð@ ln T=@ ln VÞ
S
¼ K
S
ð@ ln T=@PÞ
S
¼ ; (19:19)
ð@P=@TÞ
V
¼ K
T
¼ C
V
: (19:20)
Equation (19.19) gives the adiabatic temperature
variation in terms of either density or pressure
and Eq. (19.20) is the differential form of the
Mie–Gr ¨uneisen equation, relating (thermal) pres-
sure to thermal energy of a material heated at
constant volume,
P
Thermal
¼
ð
T
o
C
V
dT E
Thermal
=V; (19:21)
where the approximate equality invokes the
assumption that is independent of T at con-
stant V. Over large depth ranges it varies rather
little and the assumption of constant may be a
useful approximation. Then Eq. (19.19) integra-
tes to give
T
1
=T
2
ðV
2
=V
1
Þ
: (19:22)
This equation is familiar in ideal gas physics, for
which ¼(C
P
/C
V
1), because T ¼1, simplifying
Eq. (19.4). It illustrates the point that by using
we introduce some of the simple insights of ideal
gas physics to solids.
To relate by Eq. (19.1) to Gr ¨uneisen’s defini-
tion (Eq. (19.18)), we differentiate Eq. (19.9) with
respect to V at constant T and substitute for the
resulting two terms by Eqs. (19.9) and (19.10),
with introduction of subscript i to indicate that
we are considering a single mode. This gives the
volume dependence of mean mode energy,
@E
i
=@VðÞ
T
¼
@ ln
i
@ ln V
T
1
V
h
i
exp h
i
=kTðÞ1
kT
V
h
i
=kTðÞ
2
exp h
i
=kTðÞ
exp h
i
=kTðÞ1½
2
)
¼
@ ln
i
@ ln V
T
E
i
V
T
V
@E
i
@T
V
¼
@ ln
i
@ ln V
T
T
2
V
@
@T
E
i
T
V
: ð19:23Þ
Now E
i
is the internal energy attributable to
mode i and we make use of a general identity
for internal energy, U (see entries for @U
T
and @P
V
in Table E.2 of Appendix E),
ð@U=@VÞ
T
¼ð@P=@TÞ
V
T P ¼ T
2
½@ðP=TÞ=@T
V
: (19:24)
320 THERMAL PROPERTIES
//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C19.3D
–
321
– [314–336] 13.3.2008 2:45PM
Identifying this expression with (@E
i
/@T)
V
in
Eq. (19.23) and dividing both sides by T
2
,
½@ðP
i
=TÞ=@T
V
¼ð@ ln
i
=@ ln VÞ
T
ð1=VÞ½@ðE
i
=TÞ=@T
V
:
(19:25)
By making the assumption that
i
and therefore
i
¼(@ln
i
/@lnV)
T
are independent of T at con-
stant V, as in the Einstein theory (the starting
point of the Gr ¨uneisen theory), we can integrate
Eq. (19.25) with respect to T at constant V to give
the relationship between thermal pressure due
to mode i and its thermal energy:
P
i
¼ð@ ln
i
=@ ln VÞ
T
ðE
i
=VÞ: (19:26)
This has the form of the Mie–Gr ¨uneisen equation
(Eq. (19.21)), requiring Gr ¨uneisen’s definition of
i
by Eq. (19.18). Note that the Einstein assump-
tion, that
i
is independent of T at constant V,
neglects anharmonicity (Section 19.8).
The value of for materials having modes
with different
i
is an average over all modes,
weighted according to the heat capacities of the
modes. Then, noting that is the coefficient
relating thermal pressure to thermal energy, as
in Eq. (19.12),
C
V
¼
X
C
i
i
; (19:27)
where the C
i
are the various mode C
V
by Eq. (19.10).
If we restrict interest to high temperatures then
C
i
k (Boltzmann’s constant) for each mode and
is then a simple average of the
i
. A temperature-
dependence arises at low temperatures (addi-
tional to the small anharmonic effect of vibra-
tion amplitude on the
i
) when modes of
different
i
contribute in different proportions
because of their reduced C
i
by Eq. (19.10).
However, even in this situation the effect is com-
monly small. This is evident from a thermody-
namic identity (see Tables E.3 and E.1 in
Appendix E),
ð@=@ ln TÞ
V
¼ð@ ln C
V
=@ ln VÞ
S
; (19:28)
which vanishes identically in the Debye approx-
imation. This situation is represented by a
Debye version of Gr ¨uneisen’s definition of
(Eq. (19.18)),
D
¼ð@ ln
D
=@ ln VÞ
T
¼ð@ ln
D
=@ ln VÞ
T
;
(19:29)
because
D
and
D
are related by Eq. (19.11). With
the assumption that, as for all
i
in Gruneisen’s
theory,
D
is independent of T at constant V, the
isothermal derivative in Eq. (19.29) is the same as
an adiabatic derivative, so that in comparing
Eqs. (19.19) and (19.29) we see that (T/
D
) and
therefore C
V
are constant on an adiabat. Of
course Debye theory is only an approximation,
but it is useful in understanding why C
V
is
approximately constant on an adiabat.
Equation (19.18) is the starting point for a
derivation of the most widely used formula for
estimating for the Earth’s interior. This is the
‘acoustic gamma’,
A
, which is based on the
assumption that there are only two kinds of lat-
tice mode, corresponding to compressional and
shear waves, with two shear modes for each
compressional mode. This is better than the
Debye method of averaging wave speeds. It
assumes an isotropic medium but makes the
best possible use of the seismologically observed
P- and S-wave speeds, being a weighted sum of
gammas for compressional (P) and shear (S)
modes:
A
¼ð1=3Þ
P
þð2=3Þ
S
: (19:30)
Taking mode i, controlled by elastic modulus X
i
,
to be a standing wave of speed V
i
¼(X
i
/)
1/2
, with a
wavelength l
i
that is a fixed number of lattice
spacings and therefore proportional to V
1/3
, the
mode frequency is
i
¼ V
i
=l
i
/ X
1=2
i
V
1=6
; (19:31)
and so, by differentiation,
i
¼
@ ln
i
@ ln V
T
¼
1
2
V
X
i
@X
i
@V
T
1
6
¼
1
2
K
T
X
i
@X
i
@P
T
1
6
;
(19:32)
where K
T
¼V(@P/@V)
T
is the isothermal bulk
modulus. For compressional waves X
i
¼(K
S
þ(4/
3)) and for shear waves X
i
¼, so that by
Eq. (19.30)
19.3 THERMAL EXPANSION AND THE GRU
¨
NEISEN PARAMETER 321

//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C19.3D
–
322
– [314–336] 13.3.2008 2:45PM
A
¼
1
6
K
T
K
S
þ
4
3
@K
S
@P
T
þ
4
3
@
@P
T
þ
1
3
K
T
@
@P
T
1
6
: ð19:33Þ
The temperature variation through most of
the Earth is believed to be close to adiabatic, so
that, to the extent that seismological models give
reliable gradients of the elastic moduli, they give
(@K
S
/@P)
S
and (@/@P)
S
. To convert these to the
isothermal derivatives required by Eq. (19.33)
we need the temperature derivatives, which are
written in dimensionless forms, as defined in
Appendix E,
S
¼ð1=K
S
Þð@K
S
=@TÞ
P
; (19:34)
" ¼ð1=Þð@=@TÞ
P
: (19:35)
By writing a derivative identity for any para-
meter X,
ð@X=@PÞ
T
¼ð@X=@P Þ
S
ð@X=@TÞ
P
ð@T=@PÞ
S
; (19:36)
with (@T/@P)
S
¼T/K
S
from entries in Table E.2 of
Appendix E, taking X to be either K
S
or , and
using Eqs. (19.34) and (19.35) to substitute for the
temperature derivatives, we obtain the required
relationships:
ð@K
S
=@PÞ
T
¼ð@K
S
=@PÞ
S
þ T
S
; (19:37)
ð@=@PÞ
T
¼ð@=@PÞ
S
þ T"=K
S
: (19:38)
These expressions may be obtained from entries
in Table E.3 of Appendix E.
The calculation of
S
and " (Eqs. (19.34) and
(19.35)) is a subject of Section 19.7, which is con-
cerned with the interpretation of deep seismic
velocity anomalies. For the lower mantle, for
which there are sufficient data for detailed calcu-
lations, numerical values are given in Appendix G.
The temperature terms in Eqs. (19.37) and (19.38)
add 0.1 to 0.3 to the estimate of
A
at deep Earth
temperatures and pressures, although they have
often been neglected.
A critical assessment of the assumptions
and approximations implicit in the derivation
of Eq. (19.33) is given by Stacey and Davis
(2004), who conclude that it survives the doubts
very well, whereas alternative formulae face
unresolved difficulties. However, care is
required in applying seismological estimates
of dK/dP and d/dP for the deep Earth. Models
such as PREM (Appendix F) represent seismic
velocities and density as polynomials in radius
over each of several radius ranges. This is a
mathematically convenient but unphysical rep-
resentation of material properties, so that differ-
entiation to obtain dK/dP and d/dP yields
satisfactory averages but implausible depth var-
iations. The difficulty is overcome by fitting the
PREM model to a finite strain theory (Chapter 18)
for which derivative properties have been prop-
erly constrained by thermodynamic principles.
The Gr ¨uneisen theory and derivation of
the acoustic formula (Eq. (19.33)) are specific to
solid insulators, but the thermodynamic defini-
tion of (Eq. (19.1)) is applicable to every material.
We need to consider its application to metals and
to liquids because is as important to the core as
it is to the mantle. There are other approaches
that we now consider, but they require calibra-
tion by the acoustic calculation.
The ‘free’ conduction electrons in metals
respond to heat as well as electric fields. Their
contribution to specific heat is discussed in
Section 19.2 and thermal conductivity in
Section 19.6. In the core the electron heat
capacity is approximately 30% of the total and
there is a corresponding electron component of
thermal pressure. This is related to thermal
energy by an electronic Gr ¨uneisen parameter,
e
. The total for a metal is the sum of lattice
and electron contributions, weighted according
to the contributions to specific heat in the same
way as the lattice contributions are added in
Eq. (19.27). In principle it is possible to calculate
e
with a sufficiently accurate model of electron
band structure, but then we do not have an inde-
pendent estimate of the lattice because the
conduction electrons contribute to elasticity. In
any case most of the core is liquid, precluding
use of Eq. (19.33), in which is prominent, so a
different approach is required.
An alternative class of formulae, equally appli-
cable to solids and liquids, has the general form
¼½K
0
=2 1=6 ðf =3Þð1 P=3KÞ=½1 ð2 f =3ÞP=K;
(19:39)
322 THERMAL PROPERTIES
//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C19.3D
–
323
– [314–336] 13.3.2008 2:45PM
with different values of f according to what is
assumed about the thermal motions of atoms
(or electrons). We refer to these formulae as the
free volume type because Eq. (19.39) with f ¼2
was derived by Vashchenko and Zubarev (1963)
from free volume theory. A linear model by
Dugdale and MacDonald (1953), giving f ¼1, is
still sometimes used. A special case, Slater’s
gamma, for which f ¼0 (Eq. (18.54)), is used in
Section 18.6 for the infinite pressure extrapola-
tion. Slater’s formula is derived directly from
Gr ¨uneisen’s definition (Eq. (19.18)) with the
assumption that all
i
are equal because all
mode frequencies have the same volume
dependence. As in Eq. (19.32), this means that
all moduli have the same volume dependence,
that is Poisson’s ratio, (not to be confused with
mode frequency), is independent of pressure.
Although is observed to increase with compres-
sion, for reasons discussed in Section 18.8, caus-
ing Slater’s formula to overestimate , the final
paragraph of Section 18.8 points out that in the
infinite pressure extrapolation Slater’s assump-
tion becomes valid, making Eq. (18.55) rigorous.
It provides an important constraint on finite
strain theories, as outlined in Section 18.6. A
difficulty with Eq. (19.39) is that we have no
satisfactory theoretical value of f. An empirical
solution to this problem, adopted by Stacey and
Davis (2004), is to match Eq. (19.39) at P ¼0 to the
zero pressure extrapolation of the acoustic for-
mula (Eq. (19.33)). Fitted to lower mantle data, it
gives f ¼1.44. A justification for this is that it
gives values of indistinguishable from those
obtained by a third theory, based on thermo-
dynamic requirements on derivatives of ,as
discussed by Stacey and Davis (2004).
It is interesting to note that appears to be
little affected by melting. This can be understood
by recognizing thermal motion as vibrations of
lattice modes that are dominated by very high
frequencies (10
12
to 10
13
Hz). As we discuss in the
following section, the fluidity of a liquid is due to
the mobility of the crystal dislocations with
which it is saturated, but dislocations cannot
respond to stress cycles with these frequencies.
So, in the consideration of lattice vibrations, the
elasticities of liquid and solid are not very
different.
To use Eq. (19.39) for the core we need to
justify its application to metals with strong con-
tributions to by conduction electrons. The
point is that this equation is calculated by apply-
ing the Mie–Gr ¨uneisen equation (Eq. (19.21)),
which is a model-independent identity if is
independent of T. It simply depends on a calcu-
lation of thermal pressure and its relationship to
the volume dependence of bulk modulus, and in
this respect electron pressure is no different
from lattice pressure. While the electron and
lattice gammas may be very different, they con-
tribute to the total in the manner of Eq. (19.27).
The essential feature of Eq. (19.39) is that it
makes no appeal to but only to K and its pres-
sure dependence.
19.4 Melting
The standard thermodynamic identity for the
variation of melting point, T
M
, with pressure, P,
is the Clausius–Clapeyron equation,
dT
M
=dP ¼ V=S; (19:40)
where DV and DS are the volume and entropy
increments of the melting process. The deriva-
tion of this equation relies on the fact that for a
substance in thermodynamic equilibrium at
fixed pressure the Gibbs free energy, G (defined
in Table E.1 of Appendix E), is a minimum. (At
constant V the Helmholtz free energy, F,isa
minimum and at constant S it is the enthalpy,
H, that is a minimum.) The coexistence of solid
and liquid at the melting point means that G has
the same value for both states at all points on the
melting curve. By considering an increment dG
in G due to simultaneous increments in P and T
and substituting for derivatives by entries in
Table E.2 (Appendix E), we have
dG ¼ð@G =@PÞ
T
dP þð@G=@TÞ
P
dT
¼ VdP SdT:
(19:41)
Identifying T with T
M
, so that the increments
follow the melting curve, we require that dG be
the same for solid (subscript S) and liquid (sub-
script L) and therefore
19 . 4 M E LT I N G 323
//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C19.3D
–
324
– [314–336] 13.3.2008 2:45PM
V
L
dP S
L
dT ¼ V
S
dP S
S
dT; (19:42)
which rearranges to the form of Eq. (19.40). This
is an identity, applicable to all phase transitions,
and is referred to also in Section 22.3, in connec-
tion with convection through solid–solid phase
transitions in the mantle.
In the case of melting, DS ¼S
L
S
S
is always
positive because latent heat L ¼DST
M
must be
applied to cause melting. In what we refer to
here as ‘normal’ or ‘simple’ melting DV is also
positive, that is the liquid is less dense than the
solid, so that T
M
increases with P. The best
known exception is water for which DV is neg-
ative (at low pressure), and it is useful to keep
this case in mind in considering ‘normal’ or
‘simple’ melting and why there are exceptions.
A successful general theory of melting is that it is
a free proliferation of crystal dislocations (see
Fig. 14.4) and that it occurs when the free ener-
gies of the undislocated crystal and one satu-
rated with dislocations (identified with the
liquid) are equal. Accepting this as a theory of
‘simple’ melting we can see why water does not
fit in. The theory assumes that there is no major
change in atomic coordination between the solid
and liquid states, because it is not changed much
by the introduction of dislocations, but common
ice is structurally quite different from liquid
water. As in water, strongly oriented polar
bonds occur in silicates, which also do not fit
well with the concept of ‘simple’ melting, but
most metals (bismuth excepted) do so. When
very high pressure is applied both solid and
liquid structures become more close-packed
and, regardless of low pressure behaviour,
assume greater structural similarity, allowing
simple melting theory to be applied with increas-
ing confidence. The principal geophysical appli-
cation is to the melting point of iron and
solidification of the inner core.
Equation (19.40) is basic to the theory of melt-
ing, but it can be extrapolated to high pressures
only with assumptions about both DV and DS and
this is not directly useful, so that several alter-
native theories of melting have arisen. The one
with the strongest influence on modern ideas
originated with F. A. Lindemann, who deduced
that melting point varied with volume and
Debye temperature as V
2/3
D
2
. Using Eq. (19.29)
and assuming ¼
D
(Eq. (19.29)), this differenti-
ates to give (Problem 19.6)
ð1=T
M
ÞdT
M
=dP ¼ 2ð 1=3Þ=K
T
: (19:43)
Equation (19.43) is often attributed to Gilvarry
(1956), although comparison with his equations
(38) and (31) shows that his theory gives an addi-
tional factor [1 þ2( 1/3)T
M
]
1
on the right-
hand side. The basis of its derivation was an
assumption that melting occurs when the ampli-
tude of atomic vibration reaches a critical frac-
tion of the atomic spacing. There are now
derivations of the same or very similar formulae
with stronger fundamental foundations, one of
which has a thermodynamic basis and is pre-
sented here. But there is an underlying, implicit
assumption that the melting process is at least
very similar to a proliferation of dislocations,
and a simple model by Stacey and Irvine (1977)
shows why. Atoms in a dislocation are displaced
from their equilibrium positions so that some
bonds are stretched and others compressed, but
the forces are balanced. The Stacey and Irvine
calculation simulated this situation with two
lines of atoms locked together at their ends but
having unequal numbers of atoms, so that one
line was compressed and the other stretched.
Bond asymmetry, as in Fig. 18.4, causes an ave-
rage extension, which is identified with DV in
Eq. (19.40), and the energy increment, relative
to the undislocated lines, is identified with latent
heat, T
M
DS. This allows the equation to be writ-
ten in terms of derivatives of the atomic poten-
tial function and when K, P and K
0
are substituted
by Eqs. (18.18) to (18.20), the equation has the
form of Eq. (19.43) with the one-dimensional
(Dugdale–MacDonald) formula for (Eq. (19.39)
with f ¼1). This general conclusion is fundamen-
tal and important because it shows that an equa-
tion with the form of Eq. (19.43) is independent
of assumptions about dislocation structure.
We present here a more rigorous, thermo-
dynamic derivation of the Gilvarry-type melting
law. Adopting an argument by Stacey et al.
(1989), if we consider a mass m of solid to be
heated at constant volume (without melting)
then the applied heat mC
V
DT is (neglecting
324 THERMAL PROPERTIES
//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C19.3D
–
325
– [314–336] 13.3.2008 2:45PM
anharmonicity) equipartitioned between increases
in atomic kinetic energy DE
K
and bond potential
energy DE
P
,sothat
E
K
¼ð1=2ÞmC
V
T: (19:44)
When the same temperature increment is
applied at constant pressure, heat mC
P
DT is
required, but since the temperature increment
is the same, DE
K
is still given by Eq. (19.44) and
therefore
E
P
¼ mðC
P
C
V
=2ÞT: (19:45)
With the relationship between C
P
and C
V
(Eq. (19.4)) this becomes
E
P
¼ð1=2ÞmC
V
Tð1 þ 2TÞ: (19:46)
This is the increment in average bond potential
energy caused by heating at constant P. At the
same time there is thermal expansion,
V ¼ VT; (19:47)
and the ratio is
V=E
P
¼ 2=ðm=VÞC
V
ð1 þ 2TÞ
¼ 2=½C
V
ð1 þ 2TÞ; (19:48)
which, with the definition of (Eq. (19.1)), is
V=E
P
¼ 2=½K
T
ð1 þ 2TÞ: (19:49)
Now melting (at constant pressure) is an appli-
cation of heat that causes no temperature
rise so that the latent heat is entirely bond
potential energy and Eq. (19.49) can be identi-
fied with Eq. (19.40) by writing DE
P
¼T
M
DS,so
that
ð1=T
M
ÞdT
M
=dP ¼ 2=½K
T
ð1 þ 2T
M
Þ: (19:50)
This is similar to Eq. (19.43), which, as mentioned
in the discussion of this equation, is often attrib-
uted to Gilvarry (1956) but does not correctly quote
him. His theory leads to an equation with the form
of Eq. (19.50) but with ( 1/3) replacing .
Equation (19.50) can be rewritten to repre-
sent the variation of T
M
with density at melting
by applying a melting curve modulus, derived by
Stacey et al. (1989),
K
M
¼ ð@P=@Þ
T¼T
M
¼ K
T
ð1 þ K
M
dT
M
=dPÞ: (19:51)
Combined with Eq. (19.50) it yields a simpler
result:
dlnT
M
=dln ¼ 2: (19:52)
Both Eqs. (19.50) and (19.52) slightly overesti-
mate the pressure dependence of T
M
. They rely
on the assumption that bonds are stretched and
compressed but not broken. In the melting of
atomic close-packed structures roughly 2% of
atomic bonds are broken and this is a measure
of the errors in these equations.
At first sight comparison of Eqs. (19.19) and
(19.52) suggests a factor of two for the ratio of
melting point and adiabatic gradients, but it is
somewhat less than this because the variations
of density with pressure for the two equations
are represented by different moduli. This point is
elaborated in the following section.
Equation (19.50) or (19.52) can be applied to
iron under core conditions but are not satisfac-
tory for extrapolation from zero pressure. The
assumptions in the derivation break down in the
vicinity of a triple point and there are two (at
least) on the iron melting curve. The low pres-
sure (body centred cubic) structure converts
first to (face centred cubic) and then to " (hexa-
gonal close packed) or to something very similar.
As understood in the dislocation theory of melt-
ing, the structure of a melt in equilibrium with
solid crystals is a dislocated version of the crystal
structure. Under conditions remote from triple
points this is unambiguous, but in the vicinity of
a triple point there is a progressive transition in
liquid structure from a dislocated low pressure
form to a dislocated high pressure form. It is not
a sharp transition, as in the corresponding sol-
id–solid phase transition, but smeared over a
range in pressure and varying also with temper-
ature, as demonstrated by Sanloup et al. (2000)
for liquid iron in the vicinity of its ––liquid
triple point.
The melting point of iron at inner core boun-
dary (ICB) pressure is experimentally inaccessi-
ble. It is beyond the range of diamond anvils
and shock waves cause melting before that pres-
sure is reached. However, observations of the
melting point of iron in its " phase can be
extrapolated to ICB pressure because no further
phase transitions are involved. There is still a
19 . 4 M E LT I N G 325
//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C19.3D
–
326
– [314–336] 13.3.2008 2:45PM
problem that diamond anvil measurements (e.g.
Boehler, 1993) have given consistently lower
estimates of T
M
than shock waves, but in view
of difficulties with both temperature measure-
ment and pressure calibration in shock wave
experiments, we extrapolate the diamond
anvil results to ICB pressure by Eq. (19.52),
obtaining T
M
¼5750 K for iron. Depression of
the melting point by solutes is also uncertain,
but we allow 750 K, giving 5000 K as the ICB
temperature. This is a conveniently rounded
number that acknowledges the uncertainty,
which is about 500 K.
The density increment by freezing is also of
geophysical interest. The conventional approach
to ‘simple’ melting, as discussed by Poirier
(2000), gives an entropy increment for n moles:
S ¼ nR ln 2 þ K
T
V; (19:53)
which can be used with Eqs. (19.40) and (19.50) to
give
V ¼ð2T
M
=K
T
ÞnR ln 2: (19:54)
Applied to the core at ICB conditions this gives
a density increment of 200 kg m
3
(1.6%). The
difference between this and the observed
820 kg m
3
density contrast (Masters and
Gubbins, 2003) is a measure of the compositional
difference and the consequent gravitational
energy release by progressive freezing of the
inner core (Section 22.6).
19.5 Adiabatic and melting point
gradients
Global geological processes at all levels are con-
trolled or caused by convection, either thermal
convection, as in the mantle, or with a strong
compositional effect, as in the core. In the case of
thermal convection, heat sources must maintain
a temperature gradient steeper than the adiabat,
even if only slightly so, and mechanical stirring,
as by compositional convection, also establishes
or maintains an adiabatic gradient. The exis-
tence of adiabatic gradients is an essential fea-
ture of an active planet.
To see why thermal convection can occur
only in a medium with a temperature gradient
exceeding the adiabatic value, consider a homo-
geneous medium with an arbitrary temperature
gradient in which a small volume of material is
displaced vertically upwards without allowing
any heat transfer to or from it. Its temperature
falls by adiabatic decompression as it rises. If it is
then at the same temperature as its new sur-
roundings the medium has an adiabatic gra-
dient. If the gradient in the medium is less than
this then the element is cooler and therefore
denser than its new surroundings and tends to
sink back to its original level. The medium is
stable, with no tendency to convect. On the
other hand, if the gradient in the medium is
steeper than the adiabat, then the displaced vol-
ume element is hotter and less dense than its
new surroundings and so tends to rise further.
The medium is then unstable and may convect
spontaneously. The steeper is the temperature
gradient, the stronger is the convection. Since
hot, rising material is displaced by cooler falling
material there is a net upward transfer of heat,
and if the heat sources are not maintained the
temperature gradient falls and convection ceases
when it reaches the adiabatic value. In the core
the temperature gradient is believed to be very
close indeed to adiabatic and the PREM model of
the lower mantle indicates that it is only slightly
steeper there (Section 17.5).
The adiabatic gradient can be written in
terms of Eq. (19.19),
ð@T@zÞ
Adiabatic
¼ð@T@PÞ
S
dP=dz ¼ðT=K
S
Þg;
(19:55)
where g is gravity. With substitution for by
Eq. (19.1) we have an alternative form in terms of ,
ð@T@zÞ
Adiabatic
¼ Tg=C
P
; (19:56)
but, for the purpose of obtaining temperature
differences, integration by Eq. (19.55) or (19.19)
is more convenient. This is particularly obvious
if constant is a sufficient approximation, so
that Eq. (19.22) can be used. As pointed out in
Chapter 22, it is the adiabatic temperature ratios
between heat sources and sinks in a convecting
medium, calculated by Eq. (19.55) or (19.56), that
326 THERMAL PROPERTIES
