Stacey F.D., Davis P.M. Physics of the Earth
Подождите немного. Документ загружается.

//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C18.3D
–
297
– [294–313] 13.3.2008 11:43AM
checks on the theories discussed in this chapter
as well as the composition and mineral structure
of the Earth. The several techniques each have
advantages and limitations. The first to be used
was hydrostatic compression in fluid-filled pres-
sure vessels, as in early work by P. W. Bridgman.
This still has an important role, in spite of more
recent developments of methods of attaining
much higher pressures, because it allows precise
measurements of acoustic velocities as functions
of pressure and therefore yields equation of state
derivatives, K
S
¼(@P/@ln)
S
and (@K
S
/@P)
T
at mod-
est pressures. It also offers the surety that pres-
sure is hydrostatic, which is not always
straightforward with higher pressure methods.
The next derivative, d
2
K/dP
2
, either isothermal or
adiabatic, is sometimes reported, although pres-
sure calibration is not generally precise enough
to make this useful.
Bridgman also experimented with the com-
pression of small, solid samples between tapered
anvils of very hard materials (sometimes crossed
anvils that required no special care in align-
ment). The modern use of diamond anvils pur-
sues this idea with great success (Fig. 18.2),
thanks to the remarkable properties of diamond.
Pressures of a few hundred GPa (a few megabars)
have been achieved, although most experiments
have been restricted to a range below 100 GPa.
The transparency of diamond allows both sam-
ple observation and laser heating. Diamond is
reasonably transparent also to X-rays, permitting
in situ measurement of X-ray diffraction by the
very small samples and therefore determination
of both crystal structure and lattice spacing (and
hence density) at very high pressures. The
method has proved to be extremely versatile,
with care to ensure that pressure is reasonably
close to hydrostatic and that, in heating experi-
ments, temperature is uniform as nearly as
possible. As with other techniques, pressure cal-
ibration is problematic. But the small size and
relative simplicity of diamond anvil cells have
made them readily accessible and they are used
for a wide range of experiments in many
laboratories.
An early success in the application of dia-
mond anvils to mineral physics was a series of
experiments by Liu (1976), who heated a variety
of minerals by lasers to 1000 8C under strong
compression, showing that at a pressure corre-
sponding to the 660 km transition in the mantle
(23.5 GPa) these minerals were transformed pre-
dominantly to an orthorhombic perovskite
phase of (Mg,Fe)SiO
3
. Although such a transition
had been anticipated on the basis of similar tran-
sitions at lower pressures in chemical analogues,
the direct confirmation that it occurred in man-
tle silicates was a crucial step in our understand-
ing of the lower mantle. It also highlighted the
usefulness of diamond anvils in high pressure
mineralogy.
Some experiments require much larger speci-
men sizes than are possible with diamond anvils.
Static experiments on larger specimens use
multi-anvil presses with sample enclosures of
cubic or octahedral shape compressed by six or
eight rams driven by hydraulic pistons.
Specimens can be surrounded by relatively soft
material to ensure a close approximation to
hydrostatic compression and heated by internal
electric elements from which the massive
Ruby powder
50 µm
Stainless steel
gasket
Diamond
Diamond
Sample
FIG U R E 18.2 Geometry of diamond
anvil cells in which pressures up to
250 GPa have been reached. Powdered
ruby fluoresces at a wavelength that
varies with pressure and is used for
pressure calibration, although not
generally to the highest pressures.
18.2 HIGH-PRESSURE EXPERIMENTS 297

//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C18.3D
–
298
– [294–313] 13.3.2008 11:43AM
pistons are insulated, with temperature moni-
tored by thermocouple. A particular use of such
apparatus is the production of high pressure
minerals, notably the perovskite mentioned
above, which is believed to be the dominant
component of the lower mantle but survives in
a metastable state at laboratory pressure and
temperature. After crystal growth at high pres-
sure and temperature it is quenched (cooled to
laboratory temperature while still at high pres-
sure) before decompression. Some other miner-
als, including Ca-silicate perovskite, which is
almost certainly a minor lower mantle constitu-
ent, do not survive decompression and must be
observed in situ in the high pressure apparatus.
The highest pressures are achieved transi-
ently in shock wave experiments. This method
is a by-product of atomic weapons development,
and the original application to terrestrial materi-
als was intended, at least partly, as a check on the
technique because the densities of these materi-
als at very high pressures were believed to be
known. Early results (McQueen and Marsh,
1966; McQueen et al., 1967) confirmed that
phase transitions converted familiar minerals
to denser forms, consistent with the lower man-
tle, but that the core could not be explained by
further phase transitions and must be inter-
preted as an alloy dominated by iron, with
minor addition(s) of lighter element(s).
Observations are made by high speed photogra-
phy of the motions of samples subjected to vio-
lent impacts by projectiles fired at them
(Fig. 18.3). Interpretation recognizes that shock
compression causes heating greater than adia-
batic compression. It must also be noted that
the compression is so sudden as to invite doubt
about how nearly specimens come to hydrostatic
and thermodynamic equilibrium. Anderson
(1995, Chapter 12) discusses the problems and
Stacey and Davis (2004) draw attention to doubt
about pressure calibration. It appears that shock
compression is a compromise between hydro-
static compression, described by K, and linear
compression, represented by the higher modu-
lus, . A plausible explanation is that stronger
(faster) shocks give less time for adjustment
towards a hydrostatic state, giving the impres-
sion of bulk modulus increasing faster with
pressure than it really does and leading to over-
estimates of pressure. Ignoring these doubts,
there is a reasonably simple interpretation of
compression in terms of the speeds of the
advance of a shock front through a specimen
and of the following shocked material.
Applying the principle that the mass of mate-
rial is conserved as the shock front advances
through it, at speed v relative to the uncom-
pressed material but speed (v u) relative to the
shock-compressed material, to which speed u is
imparted, we have
v
0
¼ðv uÞ: (18:5)
By observing both v and u by high speed photog-
raphy the compression is measured:
0
¼ 1
u
v
1
: (18:6)
The same observations give the pressure gener-
ated by the shock because this is equated to the
rate of change of momentum per unit area of
shock front,
P ¼
0
vu: (18:7)
That is, per unit time a mass (
0
v) per unit area is
given speed u. A series of observations with dif-
ferent shock intensities, produced by a range of
impactor speeds, gives a P() curve.
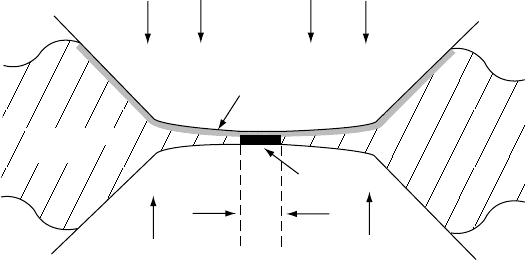
IMPACTOR
Shock front
Pressure, PP
= 0
u
v
ρ
ρ
0
FI G U R E 18.3 Geometry of shock wave propagation.
A high speed impact from the left initiates a shock wave
travelling at speed v through the initially stationary
sample. The shock-compressed material, density ,
moves at speed u < v. The unshocked material, density
0
, has not yet moved. The broken line indicates the
extent of the sample before impact.
298 FINITE STRAIN AND HIGH-PRESSURE EQUATIONS OF STATE
//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C18.3D
–
299
– [294–313] 13.3.2008 11:43AM
The compression is dynamic and is not, in
general, adiabatic. The heating can be calculated
by appealing to the conservation of energy
through a shock front, across which there is a
pressure difference P, causing acceleration of the
material to speed u. Since the pressure is exerted
by the compressed material, which advances at
speed u, the power per unit area of shock front is
Pu. This is equated to the rate at which energy is
imparted to the material, being the sum of
kinetic energy and increased internal energy,
Pu ¼
0
vðu
2
=2 þ U
U
0
Þ: (18:8)
U
and U
0
are the internal energies per unit mass
in the compressed and uncompressed states, the
asterisk being introduced to distinguish these
quantities from the total internal energy U of
an arbitrary mass, which is the convention adop-
ted in Appendix E. With substitutions from
Eqs. (18.6) and (18.7), we have
U
H
U
0
¼
1
2
P
H
1
0
1
: (18:9)
This is the Hugoniot equation, named after one of
its original authors. A shock compression curve
along which internal energy varies in the manner
of this equation is referred to as a Hugoniot and
the subscript H emphasizes that this relationship
applies only to such compressions.
Equation (18.9) may be used to estimate either
adiabatic or isothermal compressions from
Hugoniot data. The calculation of an adiabat is
simpler and an isotherm can be calculated from
it by standard thermodynamics. From Table E.2
(Appendix E), (@U/@V)
S
¼P, so that the variation
of internal energy with adiabatic compression is
dU
S
¼P
S
dV
(18:10)
with subscript S to indicate adiabatic variations
and continuation of the asterisk for unit mass
parameters. Integrating Eq. (18.10) and combin-
ing it with Eq. (18.9), we have
U
S
U
H
¼
ð
V
V
0
P
S
dV
1
2
P
H
ðV
0
V
Þ; (18:11)
which is the difference between internal ener-
gies along an adiabat and a Hugoniot at the same
density, 1/V
. For constant volume changes we
have, from Table E.2,
@U
@P
V
¼
m
¼
V
; (18:12)
so that, in the reasonable approximation that the
Gr ¨uneisen parameter, (Eq. (18.2)), is independ-
ent of temperature at constant volume,
U
S
U
H
¼ðV=ÞðP
S
P
H
Þ: (18:13)
Therefore the ‘correction’ of Hugoniot pressure
to an adiabat is given by
P
S
¼ P
H
1 =2ðÞ=
0
1ðÞ½
ð
V
V
0
P
S
dV
: (18:14)
The integral in Eq. (18.14) assumes knowledge
of P
S
(), which it is the purpose of the calcula-
tion to determine, so use of this equation is iter-
ative. Calculation of an isotherm from Hugoniot
data, either directly or via an adiabat, assumes
knowledge of the variation of with by
an appeal to one of the theories in Sections 18.3
to 18.5.
18.3 The appeal to atomic potentials
An atomic potential function is an expression for
the mutual potential energy, , of neighbouring
atoms as a function of their separation, r. Every
finite strain theory implies a potential function
with the general form of Fig. 18.4, the essential
feature of which is an asymmetry about the
potential minimum. It is easier to stretch atomic
bonds than to compress them. There are two
related consequences: bulk modulus increases
with pressure and materials normally expand
when heated. A finite strain theory is concerned
with the first of these effects, but it is closely
linked to theories of thermal properties, espe-
cially the Gr ¨uneisen parameter (Section 19.3).
There is a conceptual advantage in starting a
finite strain theory with a potential function; it
makes the underlying physical assumptions
clear and it leads naturally to the link with ther-
mal properties. Normal elasticity theory (infini-
tesimal strain theory) considers very small
18.3 THE APPEAL TO ATOMIC POTENTIALS 299
//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C18.3D
–
300
– [294–313] 13.3.2008 11:43AM
displacements from the minimum in Fig. 18.4. In
this range varies as the square of displacement,
giving forces proportional to displacement and
therefore elastic moduli that can be treated as
constants. There is no unique representation of
for extrapolation outside this range but only
numerous empirical potential functions that
have been used to represent the behaviour of
strongly compressed material. Computer models
(molecular dynamic calculations) can handle
analytically unmanageable potential functions,
but their validity still depends on assumed func-
tions There are two general forms, power law
potentials and functions incorporating exponen-
tials. They all have a minimum in ’ at r ¼a, the
equilibrium zero pressure spacing, at which
d/dr ¼0. Thus density variation is given by
=
0
¼ V
0
=V ¼ða=rÞ
3
; (18:15)
where subscript zero indicates zero pressure
values.
Consider a crystal with N atoms, each of
which has 6f neighbours and to each of which
it is bonded with energy . For a simple cubic
crystal f ¼1, for an atomic close-packed struc-
ture f ¼2 and for the diamond structure f ¼2/3.
Since each bond is shared by two atoms, the total
bond energy of the crystal is
E ¼ 3Nf : (18:16)
Let the volume of the crystal be
V ¼ Ngr
3
; (18:17)
where g is another dimensionless constant with
a value of unity for a simple cubic crystal. It is the
volume per atom, relative to a cube of side r;
it has a minimum value of 1=
ffiffiffi
2
p
for atomic
close-packed structures and a maximum value
of 8=3
ffiffiffi
3
p
for the diamond structure. Ignoring,
for the present, thermal effects, the pressure at
arbitrary atomic spacing, r,is
P ¼
dE
dV
¼
dE=dr
dV=dr
¼
f
g
1
r
2
d
dr
(18:18)
and bulk modulus is
K ¼V
dP
dV
¼V
dP=dr
dV=dr
¼
f
3g
1
r
d
2
dr
2
2
r
2
d
dr
!
:
(18:19)
We are also interested in
dK
dP
¼
dK=dr
dP=dr
¼
d
3
dr
3
3
r
d
2
dr
2
þ
4
r
2
d
dr
3
2
r
2
d
dr
1
r
d
2
dr
2
!
(18:20)
because we wish to find functions that match
the gradients of the K(P) curves for the deep
Earth. For some purposes we need higher deriv-
atives but it is generally more convenient to treat
each case specifically than to pursue the general
form. The algebra is simplified by noting that the
denominator of Eq. (18.20) has the form of K
(Eq. (18.19)), so that the next stage is obtained
by differentiating the product K(dK/dP).
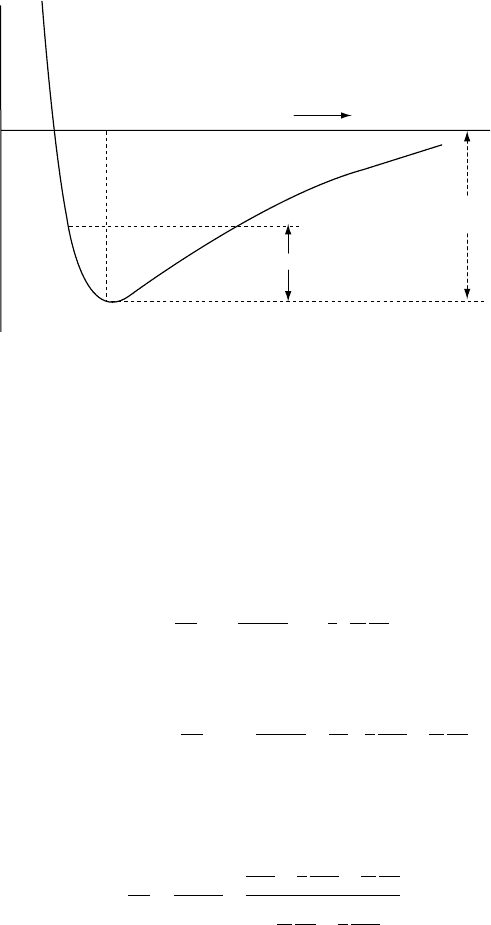
Binding
Thermal energy
a
φ
r
A
B
energy
FI G U R E 18.4 The form of an atomic
potential function, representing the
interaction energy of neighbouring atoms
as a function of their separation, r. The
equilibrium separation, a, is the result of a
balance between attractive and repulsive
forces. The increasing gradient with
decreasing r causes the bulk modulus
to increase with compression. Thermal
oscillation between A and B causes thermal
expansion by allowing greater bond
extensions than compressions.
300 FINITE STRAIN AND HIGH-PRESSURE EQUATIONS OF STATE
//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C18.3D
–
301
– [294–313] 13.3.2008 11:43AM
The simplest potential functions are those
that represent as a function of 1/r:
¼a
1
ða=rÞ
m
þ a
2
ða=rÞ
n
þ a
3
ða=rÞ
p
þ a
4
ða=rÞ
q
þ;
(18:21)
where a is the equilibrium spacing, a
1
, a
2
,...are
coefficients with the dimensions of energy and n,
m,... are dimensionless exponents, commonly
but not necessarily integers. There are numerous
variants. With just two terms and arbitrary m,
n (n > m so that a
1
is negative) we have the
potential proposed by G. Mie before the intro-
duction of quantum mechanics suggested differ-
ent forms. If m ¼1 to represent Coulomb
attraction it is known as the Born–Mie potential.
If m ¼6, n ¼12 we have the Lennard-Jones poten-
tial for dipole–dipole interactions. J. Bardeen
proposed a three-term potential with exponents
1, 2, 3 and the theory of Birch (1952) can be
represented by Eq. (18.21) with either 2, 3 or 4
terms having exponents 2, 4, 6, 8. With all four
terms it is referred to as the fourth-order theory,
although there cannot in principle be fewer than
two terms, so, with this nomenclature, there is
no first-order theory. With exponents that are all
multiples of 2, it is evident that Eq. (18.21) is a
polynomial in (/
0
)
2/3
so that repeated differen-
tiation is straightforward. Writing (/
0
) ¼x, dif-
ferentiating Eq. (18.21) with respect to x as far as
K
00
¼d
2
K/dP
2
and eliminating coefficients by sub-
stitution of zero pressure (x ¼1) values of K, K
0
and K
00
, the fourth-order Birch theory gives
P ¼dE=dV ¼ðx
2
=V
0
ÞdE=dx
¼ð9=16ÞK
0
ðAx
5=3
þ Bx
7=3
Cx
3
þ Dx
11=3
Þ;
(18:22)
where
A ¼K
0
K
00
0
þðK
0
0
4ÞðK
0
0
5Þþ59=9;
B ¼3K
0
K
00
0
þðK
0
0
4Þð3K
0
0
13Þþ129=9;
C ¼3K
0
K
00
0
þðK
0
0
4Þð3K
0
0
11Þþ105=9;
D ¼K
0
K
00
0
þðK
0
0
4ÞðK
0
0
3Þþ35=9:
(18:23)
For the third-order theory, a
4
and D are assumed
to be zero so that
K
0
K
00
0
¼ðK
0
0
4ÞðK
0
0
3Þ35=9;
simplifying A, B, C:
A ¼2ðK
0
0
4Þþ8=3;
B ¼4ðK
0
0
4Þþ8=3;
C ¼2ðK
0
0
4Þ
¼ B A:
(18:24)
For the second-order theory, with a
3
and C also
zero,
K
0
0
¼ 4; K
0
K
00
0
¼35=9; A ¼ B ¼ 8=3: (18:25)
Advantages and limitations of the Birch theory
and reasons for its prominence in geophysics are
discussed in Section 18.4.
Doubts about the fundamental validity of
equations with the power law form of Eq. (18.21)
arose as soon as quantum mechanics suggested
that at least the repulsive term of a potential
function should have an exponential form. Early
theoretical attempts to develop equations of this
form were based on studies of vibrational spectra
of diatomic molecules, first by P. N. Morse in 1929
and then by R. Rydberg in 1932. The Rydberg
potential, which now receives more attention, is
¼ A½1 f ð1 r=aÞexp½ð1 r=aÞ: (18:26)
Differentiation, as for the Birch theory above,
gives the corresponding finite strain equation,
P ¼ 3K
0
x
2=3
ð1 x
1=3
Þexp½ð1 x
1=3
Þ; (18:27)
where zero pressure conditions fix A and ¼(3/2)
(K
0
0
1). This equation was given strong support
by Vinet et al. (1987) and is sometimes referred
to as the Vinet equation, although it antedated
his work by many years. However, for appli-
cation to the pressures in the deep Earth the
Morse and Rydberg potentials share a crippling
shortcoming. In Section 18.6 we refer to the
thermodynamic requirement that a finite
strain theory must give a value of K
0
that exceeds
5/3 as P !1. This is the quantity K
0
1
which is
equal to the highest exponent of x in equations
such as 18.22 or 18.27. Equation (18.27) gives
K
0
1
¼2/3 and so fails the thermodynamic crite-
rion by a wide margin, but Stacey (2005) pointed
out that a very simple modification overcomes
this problem and gives sensible fits to terrestrial
18.3 THE APPEAL TO ATOMIC POTENTIALS 301
//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C18.3D
–
302
– [294–313] 13.3.2008 11:43AM
data. He suggested a generalized Rydberg equa-
tion with arbitrary K
0
1
,
P ¼ 3K
0
x
K
0
1
ð1 x
1=3
Þexp½ð1 x
1=3
Þ; (18:28)
where now
¼ð3=2ÞK
0
0
3K
0
1
þ 1=2
¼3K
0
K
00
0
ð3=4ÞK
02
0
þ 1=12: (18: 29)
The approach to finite strain via potential
functions is fundamental and leads naturally
to the relationship to thermal properties
(Section 19.3). However, while it is useful to
remember that every finite strain theory implies
a potential function, the form of the required
function is not known precisely enough to
account adequately for properties that depend
on high derivatives of it. One such property is
K
0
¼d K/dP and especially its infinite pressure
asymptote, for which a thermodynamic argu-
ment in Section 18.6 leads to a lower bound,
5/3 (Eq. (18.56)). Most theories give fixed values
for this parameter, with a wide range, mostly
below this limit (Stacey, 2005, Table 1). A
quite different approach to finite strain,
designed to avoid this difficulty, is presented in
Section 18.5.
18.4 Finite strain approaches
To most geophysicists ‘finite strain’ means the
theory of Birch (1952) that leads to Eq. (18.22),
but from a quite different starting point. It origi-
nated from an attempt by Love (1927) to extend
conventional elasticity theory. This defines
strain as a fractional change in a dimension,
D l/l
0
, which is assumed to be infinitesimal. In
his extension of the theory to finite deformation,
Love imposed the mathematical requirement
that, when treated in three dimensions, strain
should appear invariant with rotations or inter-
changes of coordinate axes. His method of
achieving this was to define strain in terms of
the squares of the separations of material points.
Elastic shear strains are very small in all situa-
tions in the Earth, so we are interested in finite
strain only for the case of hydrostatic compres-
sion, with extension (or compression) the same
in all directions. Then, if the separation of two
material points is S
0
in the unstrained state and
becomes S in the strained state, the Love-defined
strain, "
L
, is given by
ðS=S
0
Þ
2
¼ 1 þ 2"
L
(18:30)
and the ratio of volumes in the strained and
unstrained states is
V=V
0
¼ðS=S
0
Þ
3
¼ð1 þ 2"
L
Þ
3=2
; (18:31)
so that
"
L
¼½ðV=V
0
Þ
2=3
1=2: (18:32)
When strain energy is written as a polyno-
mial in "
L
, starting with "
L
2
, it is not convergent.
Prompted by a comment by F. D. Murnaghan,
Birch (1952) redefined strain relative to the
strained state, that is D l/l instead of D l/l
0
, and
found that this greatly improved the conver-
gence. For convenience in dealing with compres-
sion, Birch also reversed the sign of strain to
make it positive for compression. With these
changes the Birch-defined strain is
"
B
¼½ðV
0
=VÞ
2=3
1=2 ¼½ð=
0
Þ
2=3
1=2: (18:33)
"
L
is referred to as Lagrangian strain and "
B
as
Eulerian strain. In the limit of very small strains
both converge to the conventional definition in
elasticity theory (but with a sign difference). In
the Birch theory strain energy is written as a
polynomial in "
B
,
E
S
¼ c
2
"
B
2
þ c
3
"
B
3
þ c
4
"
B
4
þ (18:34)
with the implication that this is an infinite
series, but not usable beyond the "
B
4
term.
Substituting for "
B
by Eq. (18.33) and multiply-
ing out the terms, we see that Eq. (18.34) is a
polynomial in (/
0
)
2/3
and so is completely
equivalent to the 2, 4, 6, 8 power law potential
that gives Eq. (18.22). Although the Birch theory
is generally presented in terms of "
B
, this is
algebraically much less convenient than
Eq. (18.22).
The claim for convergence that made
Eq. (18.34) interesting to geophysicists is based
on the observation that for the second-order
theory (c
3
¼0, c
4
¼0) K
0
0
¼4 (Eq. 18.25) and this
is not far from the values for many minerals.
302 FINITE STRAIN AND HIGH-PRESSURE EQUATIONS OF STATE
//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C18.3D
–
303
– [294–313] 13.3.2008 11:43AM
Thus, for these minerals c
3
c
2
. However, when
lower mantle data are fitted to the Birch theory
they give c
4
c
2
. Equation (18.34) is not intrinsi-
cally convergent but relies on "
B
1. Several
authors have drawn attention to difficulties
with the Birch theory. For example, if
Eq. (18.22) is fitted to lower mantle or core data
it gives negative D or, if the third order theory
(D ¼0) is used, it gives positive C, and in either
case this means negative pressure at strong com-
pressions. But the Birch theory survives because
the geophysical community has not been con-
vinced that there is anything better. So, we
draw attention to Eq. (18.28) and the theories in
Section 18.5.
Another strain-based theory was presented
by Poirier and Tarantola (1998), who pointed
out that there is no logical reason to define strain
relative to either strained or unstrained states. If
the concept of strain is used it should be defined
as a small increment in deformation relative to
the ambient state and integrated over the total
range. This gives the total strain as a logarithm of
the ratio of final and initial states, which, for
volume compression, is
"
H
¼ð1=3ÞlnðV=V
0
Þ: (18:35)
The factor 1/3 arises because strain is defined as a
change in a linear dimension. The subscript H
acknowledges that Poirier and Tarantola
referred to "
H
as Hencky strain, adopting the
definition from structural geology, in which it
represents large inelastic deformations. Writing
strain energy as a polynomial in "
H
, as for "
B
in
Eq. (18.34), they obtained their logarithmic finite
strain equation,
P ¼ xK
0
½ln x þð1=2ÞðK
0
0
2Þðln xÞ
2
þ: (18:36)
This is the third-order equation, terminating at
"
H
3
. The fourth-order term is given by Stacey and
Davis (2004), but is doubtfully useful, justifying
the claim that Eq. (18.36) is more strongly con-
vergent than Eq. (18.22). Although, for most pur-
poses, the logarithmic equation is better than
the Birch equation, in the extreme pressure
limit it gives K
0
1
¼1 for all orders, falling short
of the thermodynamic lower bound, 5/3, dis-
cussed in Section 18.6.
18.5 Derivative equations
The equations discussed in Sections 18.3 and
18.4 have trouble with derivative properties, in
particular K
0
and especially its infinite pressure
asymptote, K
0
1
. In this section we consider equa-
tions that are derived by starting with physical
arguments about the behaviour of K
0
and inte-
grating to obtain P() relationships, instead of
working the other way. Most finite strain theo-
ries consider isothermal pressure derivatives,
but in geophysics adiabatic derivatives are
often more directly useful. Equations in this sec-
tion apply equally well to either and subscripts T
and S are dropped.
We can regard Murnaghan’s equation,
K
0
¼constant, as a special case and the precursor
to a class of equations that we refer to as K-
primed equations or derivative equations. With
K
0
d K/dP ¼K
0
0
, K ¼K
0
þK
0
0
P, integration gives
=
0
¼ðK=K
0
Þ
1=K
0
¼ð1 þ K
0
0
P=KÞ
1=K
0
: (18:37)
As Fig. 18.1 shows, this is a sufficiently good
approximation to be useful over moderate pres-
sure ranges and it is easy to apply. However,
closer inspection of the figure shows that the
gradients of the lower mantle and core graphs
decrease with pressure, that is K
00
is negative and
not zero as assumed by the Murnaghan equation.
Since the equations of the previous two sections
give negative K
00
, Murnaghan’s equation itself is
not a step forward, but is merely a pointer to a
new direction.
The first real insight on the behaviour of K
0
appears in a paper by Keane (1954). Keane recog-
nized that K
0
decreases from its zero pressure
value, K
0
0
, towards a finite limit, K
0
1
,asP !1.
Although he had no direct evidence of values of
K
0
1
, he argued that it must be bounded by limits
ðK
0
0
1Þ > K
0
1
> K
0
0
=2; (18:38)
which we refer to as Keane’s rule. Evidence that
we now have strongly supports this rule
(Section 18.9). Derivation of a tighter limit is
one of the current challenges in finite strain
theory because if K
0
1
is known, or is related to
K
0
0
, then fitting of equations such as (18.28) or
18.5 DERIVATIVE EQUATIONS 303
//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C18.3D
–
304
– [294–313] 13.3.2008 11:43AM
those in this section requires one fewer fitting
constant and is correspondingly more secure. By
making the assumption that pressure is a quad-
ratic function of Birch strain (Eq. (18.33)), with
related coefficients, Keane derived the relation-
ship that we refer to as Keane’s equation:
K
0
¼ K
0
0
þðK
0
0
K
0
1
ÞK
0
=K; (18:39)
which integrates to give
K=K
0
¼ 1 þðK
0
0
=K
0
1
Þðx
K
0
1
1Þ; (18:40)
P=K
0
¼ðK
0
0
=K
0 2
1
Þðx
K
0
1
1ÞðK
0
0
=K
0
1
1Þln x
(18:41)
with x ¼/
0
. In manipulating these equations it
is convenient to note the simple forms for higher
derivatives,
KK
00
¼K
0
ðK
0
K
0
1
Þ; (18:42)
K
2
K
000
¼ K
0
ðK
0
K
0
1
Þð3K
0
K
0
1
Þ: (18: 43)
Although Keane’s equation has been used very
occasionally its merit has not been widely recog-
nized. It is one of the equations to be taken
seriously.
The next step in development of K-primed
equations was recognition that
K
0
1
¼ðP=KÞ
1
1
; (18:44)
which is a universal algebraic feature of all equa-
tions for which K
0
1
is positive, as proved by
Stacey and Davis (2004). Although this is a stand-
ard condition of all potentially useful equations,
serious use of it is possible only if a finite strain
equation is written as a relationship between K
0
and (P/K), giving it a fixed end point at P !1.
Several such equations have been tried. The most
successful is the ‘reciprocal K-primed equation’
1=K
0
¼ 1=K
0
0
þð1 K
0
1
=K
0
0
ÞP=K: (18:45)
By writing it in this form Eq. (18.44) is automati-
cally incorporated. Eq. (18.45) is best represented
as a graph of 1/K
0
vs P/K, as in Fig. 18.5. On this
graph Eq. (18.45) is a straight line from 1/K
0
0
at P/K ¼0 to its intersection with Eq. (18.44),
which is marked ‘Infinite pressure limit’, a
straight line of gradient 1 through the origin,
along which the end points of all equations
must lie. Stacey and Davis (2004) presented a
method of integrating Eq. (18.45). Its integral
and derivative forms are
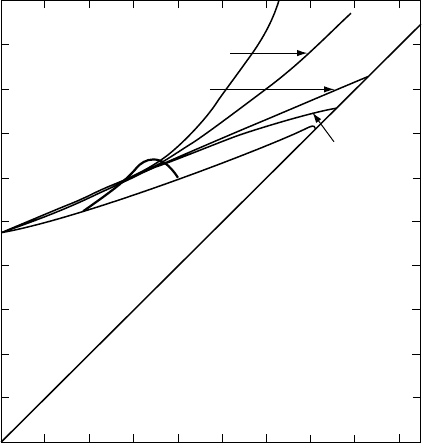
0.2
0.1
0.3
0.4
0.5
0
0.10 0.2 0.3 0.4
P
/K
PREM
Birch 3
Infinite pressure limit
Birch 4
Rydberg
Keane
Reciprocal K
′
1/K
′
FI G U R E 18.5 Plots of 1/K
0
(¼dP/dK)vsP/K for
five equations fitted to the PREM model of
the lower mantle (for the radius range 3630 km
to 5600 km). Birch 4 (Eq. (18.23)); Birch 3
(Eq. (18.23) with three terms); Rydberg
(Eq. (18.27)); Keane (Eq. (18.39)); reciprocal
K-primed (Eq. (18.45)).
304 FINITE STRAIN AND HIGH-PRESSURE EQUATIONS OF STATE
//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C18.3D
–
305
– [294–313] 13.3.2008 11:43AM
K=K
0
¼ð1 K
0
1
P=KÞ
K
0
0
=K
0
1
;
(18:46)
lnð=
0
Þ¼ðK
0
0
=K
02
1
Þlnð1 K
0
1
P=KÞ
ðK
0
0
=K
0
1
1ÞP=K; (18:47)
KK
00
¼ðK
02
=K
0
0
ÞðK
0
K
0
1
Þ; (18:48)
K
2
K
000
¼ðK
03
=K
02
0
ÞðK
0
K
0
1
Þð3K
0
2K
0
1
þ K
0
0
Þ:
(18:49)
Although Eqs. (18.39) and (18.45) appear very
different, in fact they are sufficiently similar that
we can choose between them only on the basis of
convenience of use. This is seen by comparing
the higher derivative relationships and noting
that Eqs. (18.42) and (18.48) become identical at
P ¼0, so that the relationship between K
0
0
, K
0
K
00
0
and K
0
1
is the same for both equations. For appli-
cations to laboratory compression data on mate-
rials that exist at P ¼0, and for which
0
and K
0
are known, use of Eq. (18.41) is generally more
convenient. On the other hand, for an Earth
model such as PREM, with K tabulated but
0
and K
0
to be estimated by extrapolation,
Eq. (18.45) has the advantage that P/K can be
treated as an observed quantity and Eq. (18.47)
can be fitted without involving K
0
, requiring
one fewer fitting constant and giving corre-
spondingly greater certainty. K
0
is then obtained
from Eq. (18.46), with K
0
0
and K
0
1
already fixed by
Eq. (18.47). Alternatively, K
0
can be fitted by
Eq. (18.46) without involving . Equation (18.45)
and its integral forms were used for the data fits
in Appendix F, referred to in Section 18.9.
The use of P/K as the pressure parameter in
the Eqs. (18.45) to (18.47) has other important
advantages. We refer to it as normalized pres-
sure. Unlike P itself, P/K ‘saturates’ at a finite
value with indefinitely strong compression, as
in Eq. (18.44). Properties such as the thermody-
namic Gr ¨uneisen parameter, that approach
finite limits at extreme pressure, are much bet-
ter related to P/K than to P or even . At the
bottom of the lower mantle the value of P/K is
half of its infinite pressure limit and throughout
the core it is much nearer to the infinite pressure
limit than to P/K ¼0. This means that a theoret-
ical restriction on the infinite pressure limit, as
considered in Section 18.6, is an important con-
straint on the very high pressure behaviour of an
equation of state. The divergence of alternative
equations is seen in Fig. 18.5; thermal properties
calculated from higher derivatives of these equa-
tions (Section 19.3) are very different and are
useful only if equations with satisfactorily con-
strained derivatives are applied.
We need to emphasize that all finite strain
theories are empirical, whether dressed up in
apparently sophisticated theoretical arguments
or not. There is no unique agreed theory, but a
choice must be made on the basis of plausibility
arguments and convenience of use in particular
applications. However, for the deep Earth the
choice is limited. Only the equations for which
K
0
1
is an adjustable parameter can give plausible
derivative properties. New theoretical con-
straints are urgently needed and Section 18.6
gives an indication of what may be possible.
18.6 Thermodynamic constraints
Many of the thermodynamic identities in
Appendix E connect elastic and thermal proper-
ties. They are often used in mineral physics to
assess the validity of approximations and
assumptions about material properties at high
pressures and temperatures. A particular exam-
ple that is central to the subject is the relation-
ship between the pressure dependence of
thermal expansion coefficient, , and the tem-
perature dependence of bulk modulus, K
T
,
ð@=@PÞ
T
¼ð1=K
T
2
Þð@K
T
=@TÞ
P
¼
T
=K
T
;
(18:50)
where
T
is one of the second derivative param-
eters defined in Table E.1. This is typical of the
conventional presentation of thermodynamic
relationships in that it considers isothermal
compression, both in (@/@P)
T
and in the use of
K
T
¼V(@P/@V)
T
. For application to geophysics
we are usually more interested in the variations
in properties on an adiabat, and one purpose of
the tables in Appendix E is to make adiabatic
properties as readily accessible as the more usu-
ally quoted isothermal ones.
18.6 THERMODYNAMIC CONSTRAINTS 305

//FS2/CUP/3-PAGINATION/SDE/2-PROOFS/3B2/9780521873628C18.3D
–
306
– [294–313] 13.3.2008 11:43AM
In the limit of extreme pressure many of
these relationships simplify and the simplified
forms impose constraints that equations of state
must satisfy. This means that we are considering
the extrapolation of equations to P !1 and
need to be careful about the implications of the
extrapolation. The extrapolated values of mate-
rial properties at infinite pressure are simply
equation of state parameters. They are not
directly observable, even in principle, but are
coefficients of equations fitted to the observed
pressure range. If any familiar material were to
approach infinite compression it would undergo
phase transitions to exotic forms with quite dif-
ferent equations of state. Theoretical properties
and equation of state parameters of materials at
extreme pressure have no relevance to the pro-
perties of lower pressure phases. The extrapo-
lated properties must nevertheless satisfy
physical laws, in particular thermodynamic rela-
tionships, even though they refer to conditions
under which the material cannot exist. The laws
do not break down at the (P, T) conditions at
which another phase of lower free energy
appears. An obvious analogy is extrapolated
zero pressure properties,
0
, K
0
, K
0
0
, of materials
that do not survive decompression to P ¼0. They
are often quoted in high pressure mineral
physics and follow the conventional relation-
ships, but they are not the same as the properties
of the zero pressure phases of the same materi-
als. Infinite pressure extrapolation is no differ-
ent. We labour this point to allay any doubt
about the legitimacy of the important conclu-
sions that are derived from the extrapolation.
We are imposing constraints on parameters of
equations of state, in particular K
0
1
, which
appears in Eqs. (18.39) to (18.49). The practical
significance of this constraint is emphasized by
pointing out that in the core and the lower half
of the lower mantle K
0
is nearer to K
0
1
than it is to
K
0
0
. This applies quite generally to derivative
properties, including the Gr ¨uneisen parameter,
, (Eq. (18.2)). Deep in the Earth they are all much
closer to their infinite pressure extrapolations
than to zero pressure values.
Consider the product (T). This product
appears in many of the identities in
Appendix E, including Eqs. (E.1) to (E.3), which
relate adiabatic and isothermal bulk moduli
and their pressure derivatives. For solids and
liquids it is a small quantity, even at tempera-
tures of the Earth’s interior, and it decreases
with pressure. As we demonstrate, it vanishes
identically at P !1, simplifying these identities.
Equation (E.15) combines adiabatic derivatives
of g, and T, from Table E.3, with substitutions
by other identities in Table E.4 and, like all of
the equations from which it is derived it is an
identity, without approximation. Noting the
proof by Stacey and Davis (2004) that if K
0
remains positive and finite then P !1 means
V !0, we can integrate Eq. (E.15) from P ¼0
(V ¼V
0
)toP ¼1(V ¼0),
ð
ðTÞ
1
ðTÞ
0
dðTÞ
Tð1 þ TÞ
¼ ln 1 þ
1
ðTÞ
0
ln 1 þ
1
ðTÞ
1
¼
ð
0
V
0
ð
S
þ qÞ
dV
V
: ð18:51Þ
(
S
þq ) must remain finite, so the volume inte-
gral in Eq. (18.51) is 1. The only way this can
be achieved is by (T)
1
¼0 identically. (Stacey
and Davis showed that the other apparent alter-
native, (d
S
þq )
1
¼0, implies the ideal gas equa-
tion, which is not relevant to solids.) Vanishing
(T) means not only K
S1
¼K
T1
but also
K
0
S1
¼K
0
T1
. Moreover, these equalities apply at
any temperature because the proof does not
depend on which adiabat is considered, so all
these quantities become independent of tem-
perature. Thus K
0
1
is an unambiguous equation
of state parameter, with the same value for all
adiabats and all isotherms of any particular
material. But it is a material constant, not a
universal one. We note that Eq. (E.14) is an
adiabatic derivative, so the integration that
gives Eq. (18.51) follows an adiabat. As we dis-
cuss below, remains finite at V !0, and from
Table E.2, ¼(@lnT/@lnV)
S
,soT !1 on an
adiabat as V !0. In spite of this (T) !0. Thus
decreases more strongly than T increases, mak-
ing the product T vanish at V !0.
Now consider the definition of q:
q ¼ð@ ln =@ ln VÞ
T
: (18:52)
306 FINITE STRAIN AND HIGH-PRESSURE EQUATIONS OF STATE
