Shani G. Radiation Dosimetry: Instrumentation and Methods
Подождите немного. Документ загружается.

180 Radiation Dosimetry: Instrumentation and Methods
The results show that the C
e
factors for converting the
in-phantom air-kerma-calibrated chamber reading, to the
absorbed dose to water, are nearly constant for incident
electron energy between 4 and 11 MeV, for prototype
chambers with 0.018 mm thick copper layers, and between
4 and 15 MeV for chambers with 0.035 mm thick copper
layers. The C
e
factors are proportional to the product of
water/air stopping-power ratio and perturbation factor.
When the designated chamber is irradiated by an electron
beam at a depth d in a water phantom the absorbed dose
to water is
(3.192)
The variation of , with energy for a particular elec-
tron chamber, depends on both the electron fluence–per-
turbation correction factor and the stopping power ratio
for the phantom material to air, . The conversion
factor can be written as
(3.193)
where is a correction factor for a secondary-standard
NE2561 chamber to account for the lack of air equivalence
of the chamber wall, electrode, and build-up cap, and
is a correction factor for the effect of absorption and
scattering in the chamber wall, electrode, and build-up
cap at the calibration of the chamber irradiated in air by
a
60
Co beam or a 2-MV x-ray beam. g is the fraction of
the energy of the charged particles lost to bremsstrahlung.
The perturbation factor, , corrects for the effect of
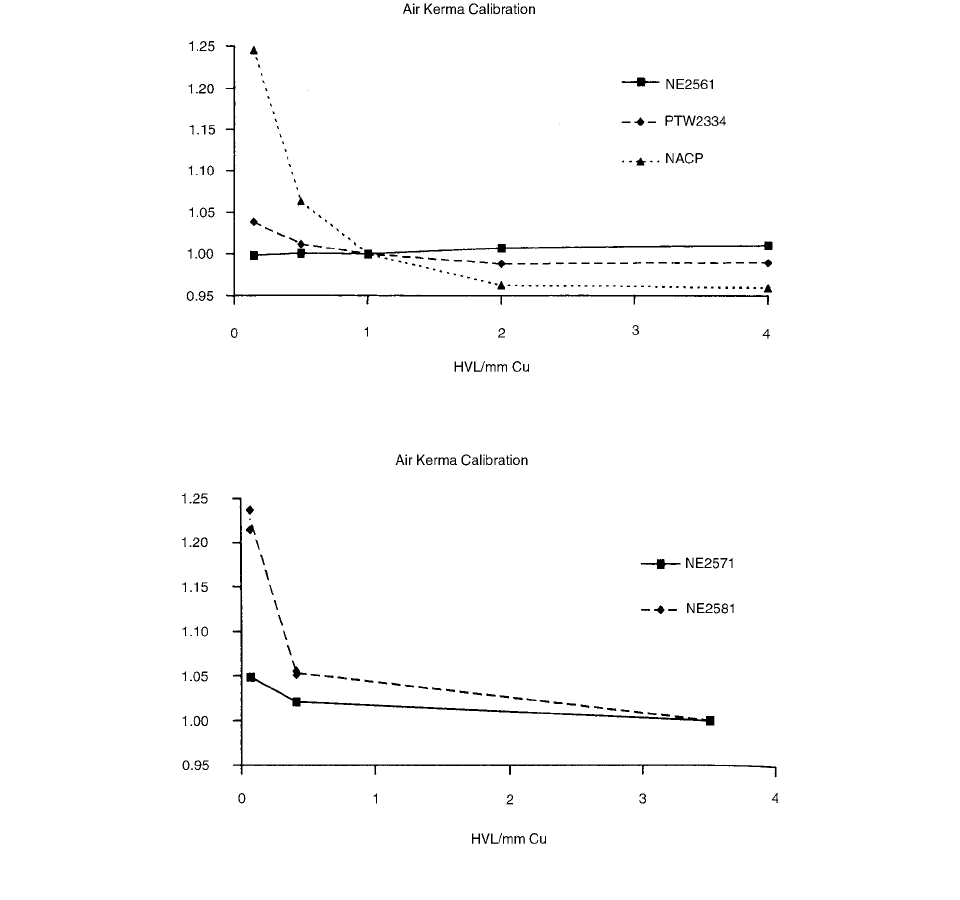
FIGURE 3.87 The variation of the chamber calibration factor with beam quality. (From Reference [92]. With permission.)
FIGURE 3.88 The variation of the chamber calibration factor with beam quality. (From Reference [92]. With permission.)
D
w
N
f
C
e
E
e
C
e
p
u
e
S
med,air
C
e
C
e
k
ch
s
k
att
s
1 g()k
s
{}P
cal
s
P
cal
e
()S
water,air
P
u
e
k
ch
s
k
att
s
p
cal
Ch-03.fm(part 2) Page 180 Friday, November 10, 2000 11:59 AM
Ionization Chamber Dosimetry 181
non-medium-equivalent wall material and central elec-
trode and for the deviation of the effective point of mea-
surement of the chamber from the chamber center during
the intercomparison of a secondary-standard NE2561
chamber and an electron chamber.
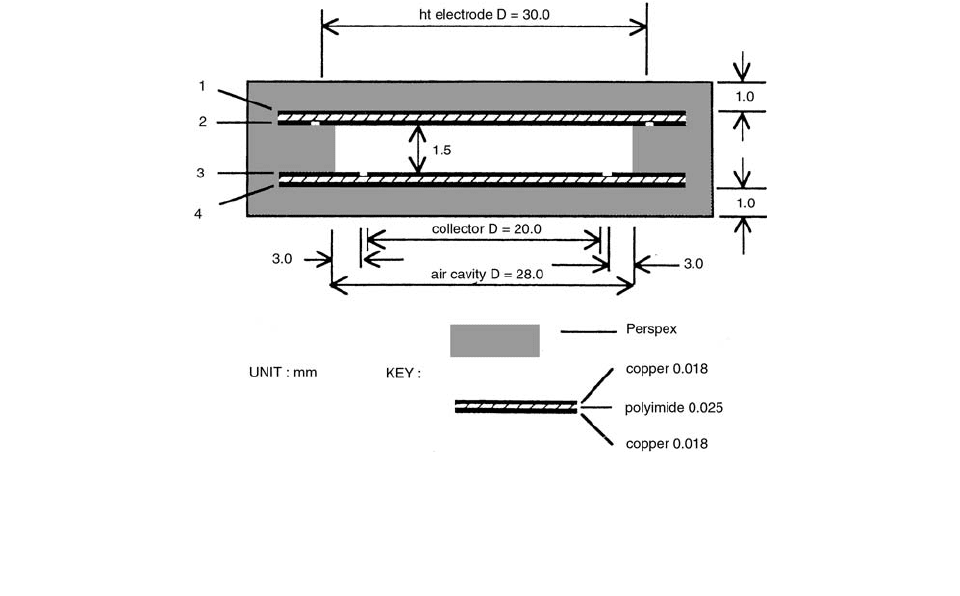
The geometrical details of the NPL chambers are shown
in Figure 3.89.
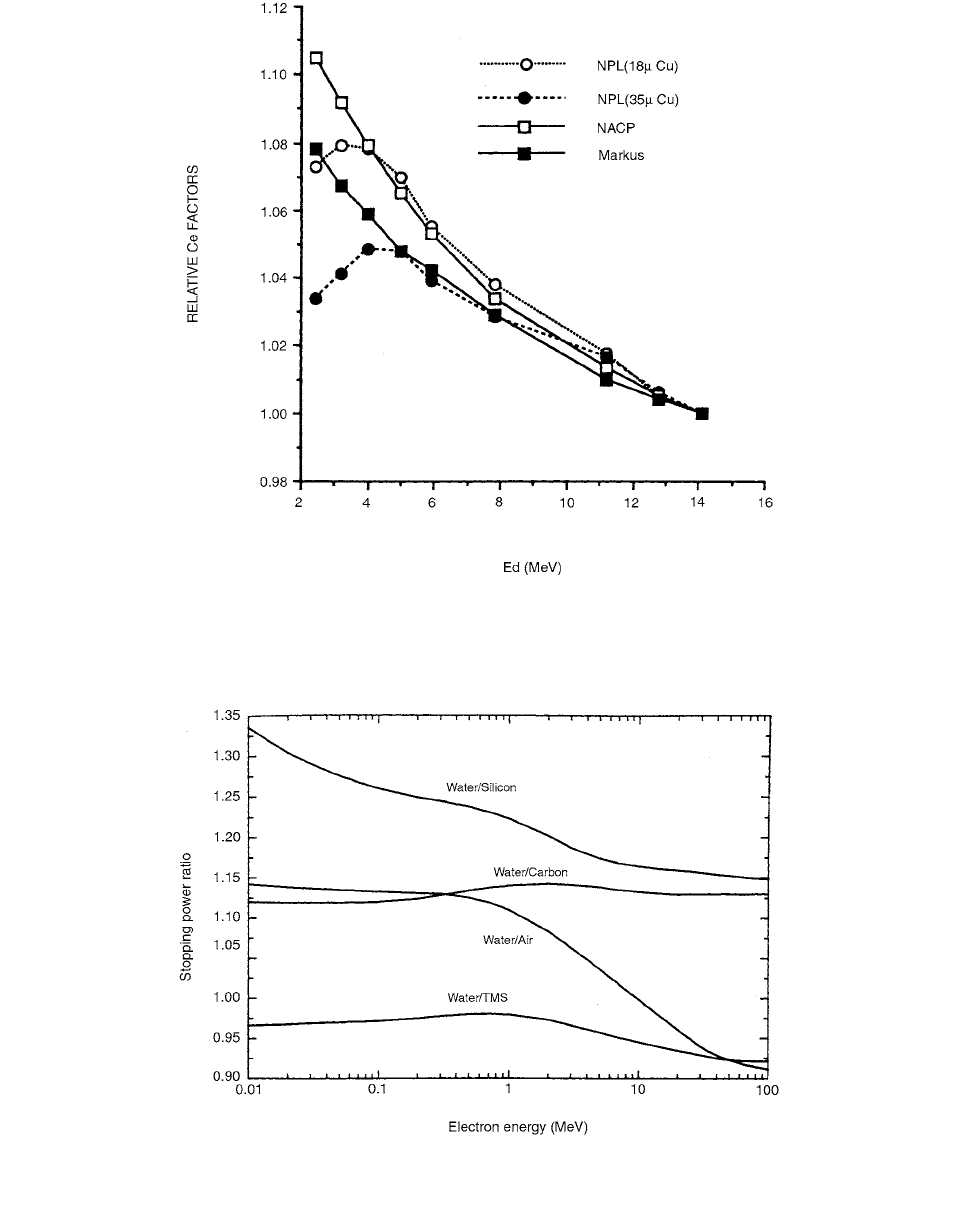
The conversion factor for a particular electron cham-
ber varies with both the electron fluence–perturbation correc-
tion factor and the stopping power ratio for water to air,
. Relative values of C
e
are shown in Figure 3.90.
X. LIQUID DIELECTRIC IONIZATION
CHAMBERS
Ionization chambers filled with a dielectric liquid instead
of a gas have many basic advantages. The sensitive volume
of a liquid-ionization chamber (LIC) can be made much
smaller than that of a gas-ionization chamber and still
provide the same ionization current. A high spatial reso-
lution, not possible with a gas chamber, can thereby be
achieved. The stopping power ratio for the dielectric liquid
to water varies by only a few percent in the range 1–50
MeV, compared to about 20 percent for air to water in the
same energy range (Figure 3.91).
Two dielectric liquids, isooctane and tetramethylsilane
(TMS), have been tested by Wickman and Nystrom [94]
as sensitive media in parallel-plate ionization chambers.
The ionization chambers use graphite electrodes and have
a high spatial resolution due to a coin-shaped ionization
volume of 2 mm
3
(diameter 3 mm, height 0.3 mm). The
detectors show a calibration stability within l% over
several years. In neither of the liquids did general recom-
bination exceed 2% in pulsed radiation fields and dose
rates up to 7 Gy min
1
.
The mobility of ions is reported to be extremely high.
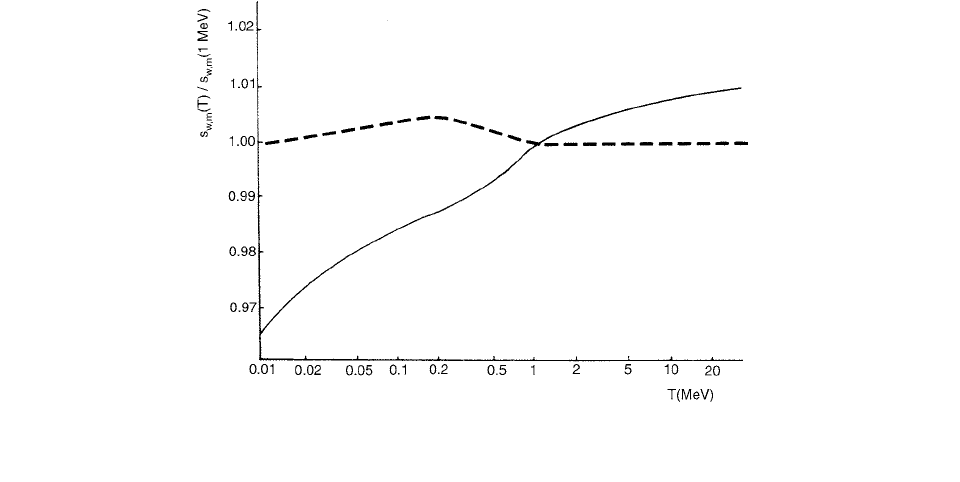
The collision stopping power ratio to water is found to be
almost constant (within about 0.2%) in the electron
energy range of 0.01–50 MeV, while isooctane has a mod-
erate variation of 2% (Figure 3.92).
The leakage current is a rough measure of the amount
of impurities in a dielectric liquid. Obviously, there are
some initial chemical exchanges due to liquid and chamber
wall interactions, as a new ionization chamber has to be
rinsed several times by emptying and filling it with fresh
liquid during the first weeks before a low and stable resid-
ual current is established. A typical residual current at room
temperature that can be achieved at a polarization potential
of 900 V (3.0 MV m
1
) is about 5 10
14
A in isooctane,
as well as in TMS. This current corresponds to a resistivity
of about 3
10
14
m. The resistivity reported for highly
purified hydrocarbon liquids is 3 to 30 times higher. The
residual current is temperature-dependent, as well as field
strength–dependent. The leakage current is slightly
increased directly after the polarization voltage has been
connected but stabilizes within a few minutes. When a
FIGURE 3.89 A cross-sectional view of the NPL plane-parallel chamber with 0.018-mm-thick copper layers. The thickness of each
copper layer (1,2,3, or 4) is 0.018 mm and the thickness of the polymide layer is 0.025 mm. The diameter of the collecting electrode
is 20 mm and the width of the guard ring is 3 mm. The diameter of the air cavity is 28 mm and the thickness is 1.5 mm. The thickness
of the front and back Perspex wall is 1 mm. For a 0.035-mm-thick copper-layered version, the geometry is exactly the same, except
that the copper layers are 0.035 mm thick. (From Reference [93]. With permission.)
C
e
p
u
e
S
water, air
Ch-03.fm(part 2) Page 181 Friday, November 10, 2000 11:59 AM
182 Radiation Dosimetry: Instrumentation and Methods
FIGURE 3.90 Relative factors for the plane-parallel chambers used by Ma et al. The values normalized to the values for
. (From Reference [93]. With permission.)
FIGURE 3.91 Stopping power ratio to water of the dielectric liquid TMS and other materials commonly used as sensitive media
for dosimeters. (From reference [99]. With permission.)
C
e
E
d
14.4 MeV
Ch-03.fm(part 2) Page 182 Friday, November 10, 2000 11:59 AM
Ionization Chamber Dosimetry 183
chamber has been rinsed and a low current has been estab-
lished, this will typically remain stable for years and
appears to be unaffected by normal use of the chamber.
A residual current of 5 A corresponds to a dose
rate from ionization radiation of 0.2 mGy min
1
in
isooctane and 0.13 mGy min
1
in TMS for the type of
chamber used in this investigation.
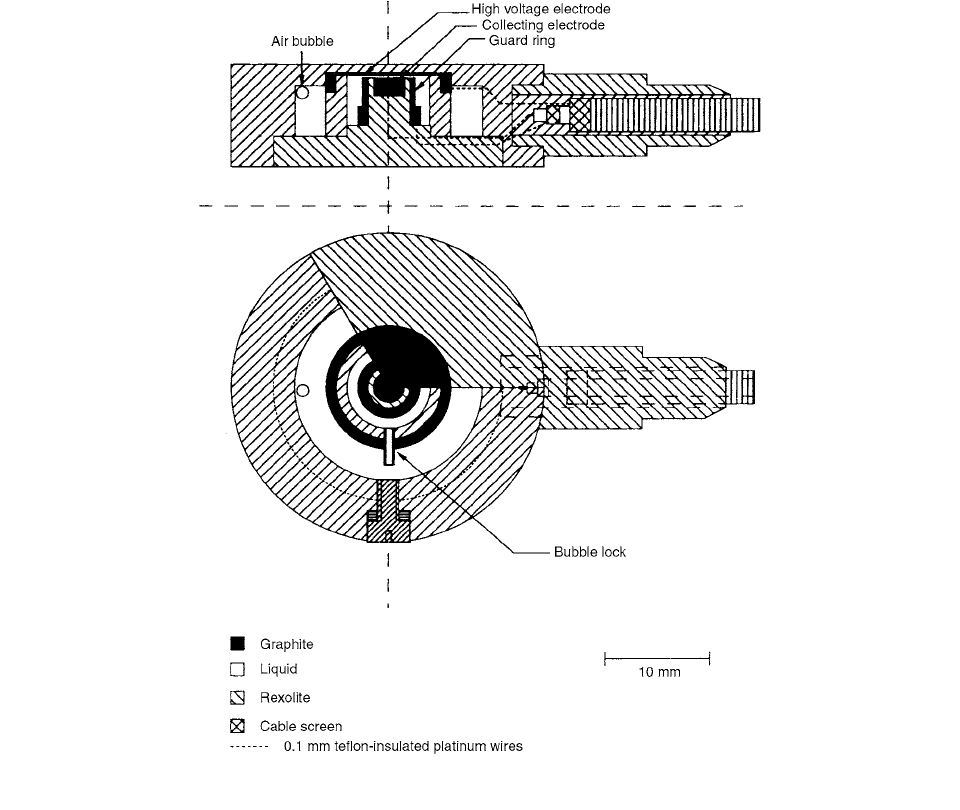
The basic chamber design is illustrated in Figure 3.93.
Strong emphasis during the development of the chamber
has been placed on the creation of a small, robust, versa-
tile, and easy-to-handle detector with a stable response
that can be used as simply as any gas-ionization chamber
for routine calibration and measurements in radiation ther-
apy fields. The chamber is watertight and can be used with
the same liquid for years.
The electrodes are made of thin discs of pure graphite.
In the earlier chamber designs, thin layers of evaporated
beryllium were used. The reason for the exchange was that
beryllium reacts with the liquid in a way not yet understood,
causing a slow and constant drop in the detector sensitivity.
No significant change in calibration stability has been found
when graphite discs are used as electrodes.
An air bubble has been introduced in order to com-
pensate for the approximately four times greater volume
change with temperature in the liquid compared to the
chamber body materials. The liquid container has been
divided into two compartments; the air bubble is placed
in the outer part, and a “bubble lock” between the two
compartments is introduced in order to prevent air from
coming between the electrodes.
The distances between the ions formed in the track
of an ionizing particle are much shorter than in the gas.
The ion recombination in the liquid is much more pro-
nounced and must often be corrected for. In the track
of a highly energetic particle, liquid ions are formed in
clusters containing one or a few ion pairs, and there is
a significant initial recombination of the ions in their
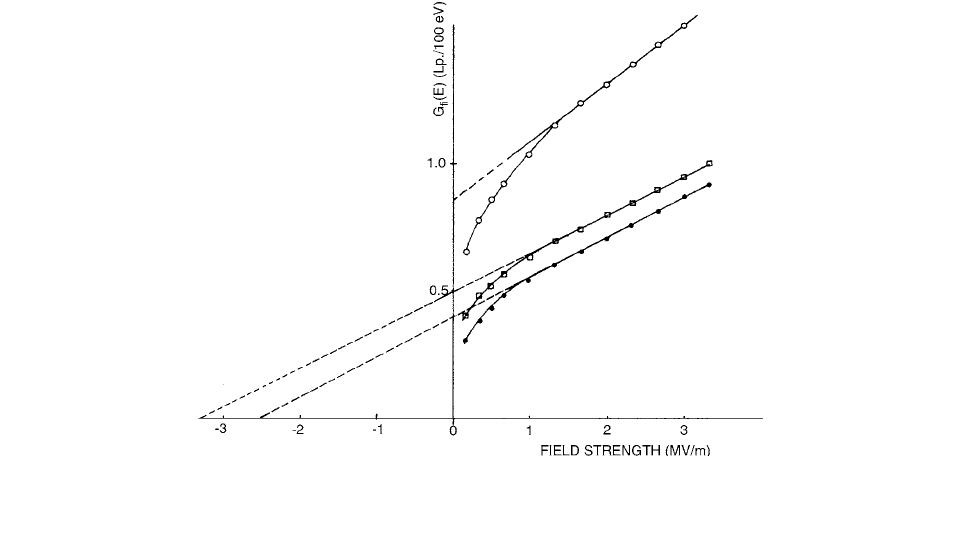
original clusters. The amount of ions liberated per 100
eV of absorbed energy at zero external electric field is
commonly defined as . The intra-cluster recombina-
tion is affected by the temperature and by the external
electric field. The value at room temperature varies
from approximately 0.1 to 1 for the various dielectric
liquids. For “impurity-free” 2, 2, 4-trimethylpentane
(isooctane) and TMS, is 0.33 and 0.74, respectively.
[94]
The free-ion yield, , is a measure of the probability
that the electrons formed by the ionization radiation have
sufficient energy to escape from the electric field of the
parent positive ions.
In the measurements of the collection efficiency, the
absorbed dose in water was simultaneously monitored by
a small plane-parallel air-ionization chamber with a well-
known and small general recombination that was cor-
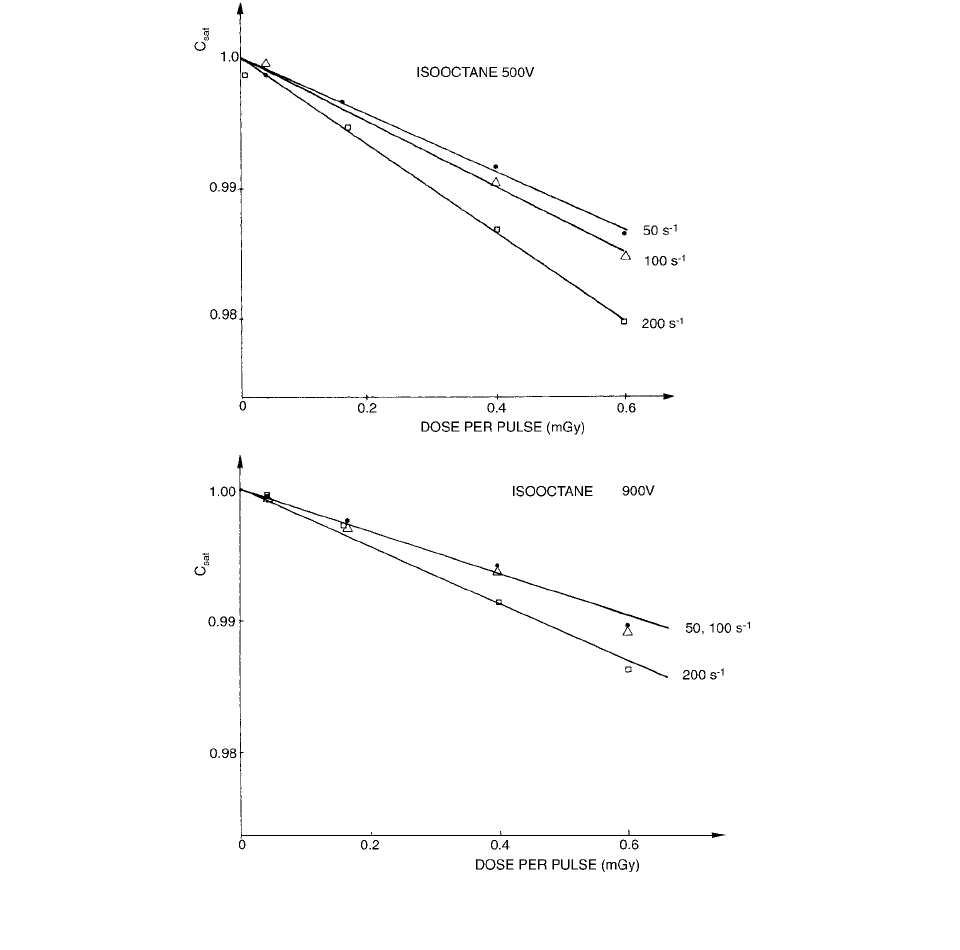
rected for. The results are presented in Figures 3.94. It is
seen from the figures that the general recombination in
isooctane is noticeably dependent on the pulse repetition
rate when the polarizing voltage is 500 V. This effect
decreases when the voltage is increased to 900 V and
is, at that voltage, almost negligible for pulse rates under
100 pulses per second. This pulse rate effect depends on
the fact that the transport time of some of the ions is longer
than the pulse interval; this means that new ions are
injected by the following pulse before these slow-motion
FIGURE 3.92 Mass collision stopping power ratios, water to isooctane (—) and TMS (- -), normalized to 1.00 at the electron energy
T 1 MeV. (From Reference [94]. With permission.)
10
14
G
fi
0
G
fi
0
G
fi
0
G
fi
Ch-03.fm(part 2) Page 183 Friday, November 10, 2000 11:59 AM
184 Radiation Dosimetry: Instrumentation and Methods
ions have been cleared from the liquid. This then causes
an increased recombination when the pulse repetition rate
increases. The ion transport in the chamber has been stud-
ied by the use of a DC-amplifier with high sensitivity and
a fairly good response time.
Chemical decomposition of the liquid by radiation-
induced bond fissions and an uncontrolled recombination
of the fragments to form other molecules than the original
compound can affect the calibration.
One kg of 224-trimethylpentane contains 5.3
molecules, and the absorbed energy of 1 J creates 3.2
affected molecules. From these figures, the relative
number of affected molecules per Gy of absorbed dose in
the liquid is (3.2 ) (5.3 ), or 6 %.
Most hydrocarbons have their G-values within a factor of
three of that of isooctane. The dependence of G
fi
on the
field strength is shown in Figure 3.95.
The absorbed dose to liquid, , at a field strength
E is given by
(3.194)
The absorbed dose to water can then be calculated from
(3.195)
where is the absorbed dose to water, is the
absorbed dose to liquid, is the free-ion yield per
absorbed energy at field strength E, is the correction
factor for general recombination, is the mass of
active liquid volume, and , is the collected charge in
liquid. The factor f accounts for different cavity-theory
effects in the conversion from dose in the liquid layer of
the chamber to dose in water. In electron beams,
, where is the Spencer-Attix stopping
power ratio of water to liquid with a cut-off energy cor-
responding to the energy of electrons with a range cor-
responding to the average distance across the liquid layer,
i.e., 0.02 g cm
2
. corrects for any electron fluence
change due to the Rexolite-graphite wall and should be
close to 1, as the materials are thin and are very nearly
“water equivalent.”
FIGURE 3.93 Cross-sectional views of the chambers used. (From Reference [94]. With permission.)
10
24
10
16
10
16
10
24
10
6
D
liq
D
liq
Q
liq
C
sat
m
liq
G
fi
E()[]
D
w
D
liq
f
D
w
D
liq
G
fi
E()
C
sat
m
liq
Q
liq
fS
Wliq,
SA
p
u
S
Wliq,
SA
P
u
Ch-03.fm(part 2) Page 184 Friday, November 10, 2000 11:59 AM
Ionization Chamber Dosimetry 185
In photon beams the factor f is dependent on the
energy and on the physical dimensions of the cavity and
wall. At very low energies, the major contribution to the
energy deposition in the liquid is from electrons released
by photon interactions in the liquid. In this case the factor
f can be approximated by
(3.196)
At high energies, on the other hand, where the liquid
layer and walls are thin compared with the secondary elec-
tron ranges, only a small fraction of the electrons in the
liquid is generated in the liquid or in the wall. In this case
the sensitive volume behaves like a Bragg-Gray cavity:
(3.197)
In the megavoltage range, however, none of these sit-
uations is approximated very accurately. Burlin and Chan
(94a) suggested a weighting factor, d, related to cavity
size for the intermediate situation in which the cavity
behaves neither as a Bragg-Gray cavity nor as a large
cavity:
(3.198)
FIGURE 3.94 Collection efficiency with pulse dose at pulse radiation (pulse length ~3
s) and different pulse repetition
frequency and polarization voltages. (From Reference [94]. With permission.)
C
sat
1
f
---
en
()
W
liq
1
f
---
s
W
liq
1
f
---
ds
W
liq
1( d )
en
()
W
liq
Ch-03.fm(part 2) Page 185 Friday, November 10, 2000 11:59 AM
186 Radiation Dosimetry: Instrumentation and Methods
Here d is the fraction of the electrons in the liquid gener-
ated by photon interactions outside the liquid.
The first term of Equation (3.198) then becomes
(3.199)
where
is the fraction of the outside-cavity electrons
generated in the wall. The fact that the liquid-ionization
chamber in the present work has a front wall consisting
of two materials, graphite and Rexolite, demands a further
split of Expression (3.199):
(3.200)
Here
is the fraction of the wall-generated electrons
originating from the graphite and (1
) is the fraction
of those generated in the Rexolite. An approximate expres-
sion for the f-factor would thus be [94]
(3.201)
The general collection efficiency has been measured
by Johansson and Wickman [95] in liquid isooctane
(C
8
H
18
) and tetramethylsilane (Si(CH
3
)
4
) as the sensitive
media in a parallel-plate ionization chamber, with an elec-
trode distance of 1 mm, intended for photon and electron
dosimetry applications. The measurements were made at
potential differences of 50, 100, 200, and 500 V. Measure-
ments were performed for each liquid and electric field
strength, with the decay rate of
99m
Tc used as the dose-
rate reference. The maximum dose rate was about 150
mGy min
1
in each experiment.
If the general collection efficiency, defined as the ratio
of the ionization current to the saturation current, is
denoted by f, the ionization current–polarizing voltage
relation derived by Mie [96] can be expressed as
(3.202)
where and are the mobilities of the ions;
is the
recombination rate constant; e is the electronic charge; d
is the distance between the collecting electrodes; q is the
amount of charge liberated by the radiation and escaping
initial recombination per unit volume and time in the
medium; U is the polarizing voltage of the ionization cham-
ber;
r
is the permittivity of the medium in the chamber;
and
0
is the permittivity of free space. The recombination
FIGURE 3.95 The amount of free-ion pairs per 100 eV of absorbed energy at different field strengths in TMS () and isooctane
(
) irradiated with
60
Co photons. Isooctane irradiated with 190-kV x-rays (). (From Reference [94]. With permission.)
G
fi
d
en
()
w
wall
s
wall
liq
1
()S
w
liq
[]
d{
en
()
w
gr
s
gr
liq
1
()
en
()
w
rex
s
rex
liq
[]
1 a()s
w
liq
}
1
f
---
d{
en
()
w
gr
s
gr
liq
1
()
en
()
w
rex
s
rex
liq
[]
1
()s
w
liq
}
f
1
1
2
14
()10[]1 f(){}
2
------------------------------------------------------------------------------------------
2
1
6
---
m
2
d
4
q
U
2
--------
m
ek
1
k
2
-------------
12/
ek
1
k
2
()
r
0
-------------------------
k
1
k
2
Ch-03.fm(part 2) Page 186 Friday, November 10, 2000 11:59 AM
Ionization Chamber Dosimetry 187
rate constant and the ion mobilities are specific constants
of the sensitive medium.
If the ionization chamber contains air at normal pres-
sure and temperature with a
value of 3.56, the factor
in the square brackets in Equation (3.202) can be ignored
for . The equation for the general collection
efficiency is then reduced to
(3.203)
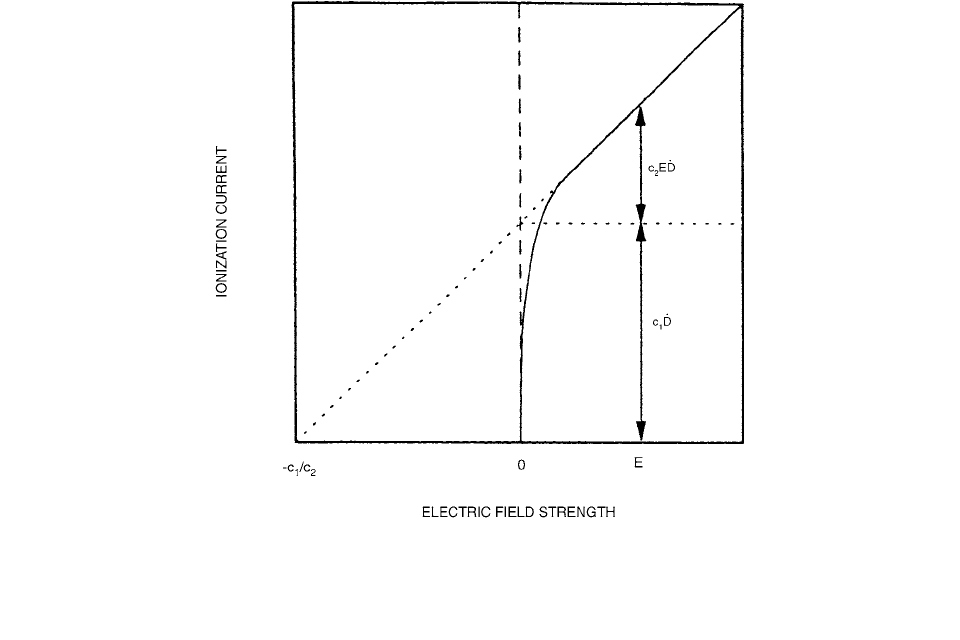
When using an ionization chamber with a dielectric liquid
instead of a gas as the sensitive medium, the ion-produc-
tion density is increased by a factor of about 300. The
ionization current never achieves saturation with respect
to the initial recombination. The characteristic relation
between the ionization current from an irradiated dielec-
tric liquid and the electric field strength is shown in Figure
3.96. The ionization current can be described as the sum
of two components. The first term consists of the product
, and the second term is the product , where
and are constants characteristic of the liquid, E is the
electric field strength, and is the dose rate.
The ions can escape initial recombination by diffusion
or by the combined effect of diffusion and the influence
of the external electric field. Under constant irradiation
conditions and with negligible general recombination, the
ionization current will increase linearly with electric field
strength. In Figure 3.96, the constants and represent
the fractions of the ionization current that escape initial
recombination due to diffusion and due to the external
electric field, respectively.
The body of the parallel-plate liquid-ionization cham-
ber used by Johansson and Wickman for the experiments
was made of Rexolite
®
, a styrene copolymer with a density
of 1.05 g cm
1
, and the electrodes were made of pure
graphite. The chamber had cylindrical symmetry without
any guard. It was irradiated towards the plane frontal side
during the experiments. The thickness of the chamber wall
in the irradiation direction was 1 mm, consisting of
0.7 mm Rexolite
®
and 0.3 mm graphite. The sensitive
volume had a diameter of 3 mm and thickness of 1 mm,
and was filled through a hole with diameter of 0.3 mm in
the center of the second electrode. The total sensitive
volume of the chamber was 7.07 10
9
m
3
. The dielectric
liquids used were isooctane (C
8
H
18
; Merck, isooctane
analysis grade 99.5%) and tetramethylsilane (TMS;
Si(CH
3
)
4
; Merck, NMR calibration grade 99.7%). The
liquids were used without any further purification.
The relative dose-rate distribution in the liquid layer
at the irradiation geometry was calculated by the function
(3.204)
FIGURE 3.96 The characteristic relation of the ionization current from a dielectric liquid and the electric field strength in a liquid-
ionization chamber. The ionization current is the sum of the two components , where is the fraction of ions that
escape initial recombination due to diffusion, is the fraction of ions that escape initial recombination due to the external electric
field strength,
E is the electric field strength, and is the dose rate. (Fom Reference [95]. With permission.)
c
1
D
˙
c
2
ED
˙
c
1
c
2
D
˙
0.7 f 1
f
1
1
2
----------------
c
1
D
˙
c
2
ED
˙
c
1
c
2
D
˙
c
1
c
2
t
e
y
y
2
-------
y
t
s
h()
e
y
y
2
-------
yd
t
s
h
d
t
Ch-03.fm(part 2) Page 187 Friday, November 10, 2000 11:59 AM

188 Radiation Dosimetry: Instrumentation and Methods
where is the relative photon fluence,
the linear atten-
uation coefficient of the different materials, t is the thick-
ness of the different materials, is the linear attenuation
coefficient, and h is the thickness of the
99m
Tc solution.
For the theoretical general collection efficiency, a
mean q value for the sensitive volume was used. This mean
value was calculated as
(3.205)
where is the mean value of the amount of charge liber-
ated per unit volume and time in the liquid by the radiation
and escaping initial recombination, is an experimentally
determined correction factor, is the theoretical ioniza-
tion current in the absence of general recombination, and
is the liquid volume in the ionization chamber. The
general collection efficiency derived by Mie, Equation
(3.202), also contains the parameter
. For two ions of
opposite sign at a separation distance R and approaching
each other with a velocity, the recombination rate constant
is given by
(3.206)
where , the Onsager escape distance [56], is the sepa-
ration distance at which the Coulombic potential energy
is equal to the diffusion kinetic energy of the ions and D
is the sum of the diffusion coefficients of the ions. For
dielectric media with a low permittivity, the ratio is
large and the recombination rate constant can be approx-
imated by the equation (Debye 1942)
(3.207)
This approximation of the recombination rate constant
leads to a
value of 1 for dielectric liquids. [95]
m (in Equation 3.202) is a constant purely dependent
on the recombination rate constant and the mobilities of
the ions in the medium. From the experimental results,
the specific m values for isooctane and tetramethylsilane
were empirically determined by an iterative method, using
the values for the electrode distance and the polarizing
voltages and by assuming . Measured values of
m, k, k
2
, and
are given in Table 3.32.
From the definition of the m value (Equation (3.202))
and the approximation of the recombination rate constant
(Equation (3.206)), a relation between the mobilities of
the ions of different signs can be derived as
(3.208)
The experimentally determined general collection effi-
ciencies are well described by the theoretical general collec-
tion efficiency equation derived by Mie (Figures 3.97 and
3.98). However, probably as a result of the approximate
space charge correction, the theoretical expression shows a
lower general collection efficiency for all of the experiments.
The maximum deviation is less than 1% of the saturation
current at a general collection efficiency of 60%. For general
collection efficiencies down to 90%, the simplified equation
proposed by Greening [95a] can also be used, showing a
deviation from the experimental general collection efficiency
of less than 1% of the saturation current. [95]
Two new liquid-ionization chamber designs, consist-
ing of cylindrical and plane-parallel configurations, were
presented by Wickman et al. [97] They are designed to be
suitable for high-precision measurements of absorbed
dose to water at dose rates and photon energies typical for
LDR intermediate photon energy brachytherapy sources.
The chambers have a sensitive liquid–layer thickness of
1 mm and sensitive volumes of 7 mm
3
(plane-parallel) and
20 mm
3
(cylindrical). The liquids used as sensitive media
in the chambers are either isooctane (C
8
H
18
), tetramethyl-
silane (Si(CH
3
)
4
) or mixtures of these two liquids in the
approximate proportions 2 to 1.
TABLE 3.32
Experimentally Determined m Values and the Corresponding Mobilities of
the Ions (k
1
, k
2
) and the Recombination Rate Constants (
) for Isooctane
and Tetramethylsilane
m (s
I/2
C
–1/2
V m
–1/2
) k
1
(m
2
s
–1
V
–1
) k
2
(m
2
s
–1
V
–1
)
(m
3
s
–1
)
Isooctane 2.0 10
9
2.9 10
–8
2.9 10
–8
5.4 10
–16
Tetramethylsilane 1.4 10
9
5.3 10
–8
9.0 10
–8
1.4 10
–15
Source: From Reference [95]. With permission.
s
qq
f
i
theor
V
liquid
-------------
q
q
f
i
theor
V
liquid
ek
1
k
2
()
0
r
-------------------------
1
r
c
D
R
2
---------
r
c
R
----
r
c
R
----
expexp
1
r
c
r
c
R
ek
1
k
2
()
0
r
-------------------------
q
f
1
k
2
1
m
2
r
0
1k
1
()
----------------------------------------
Ch-03.fm(part 2) Page 188 Friday, November 10, 2000 11:59 AM
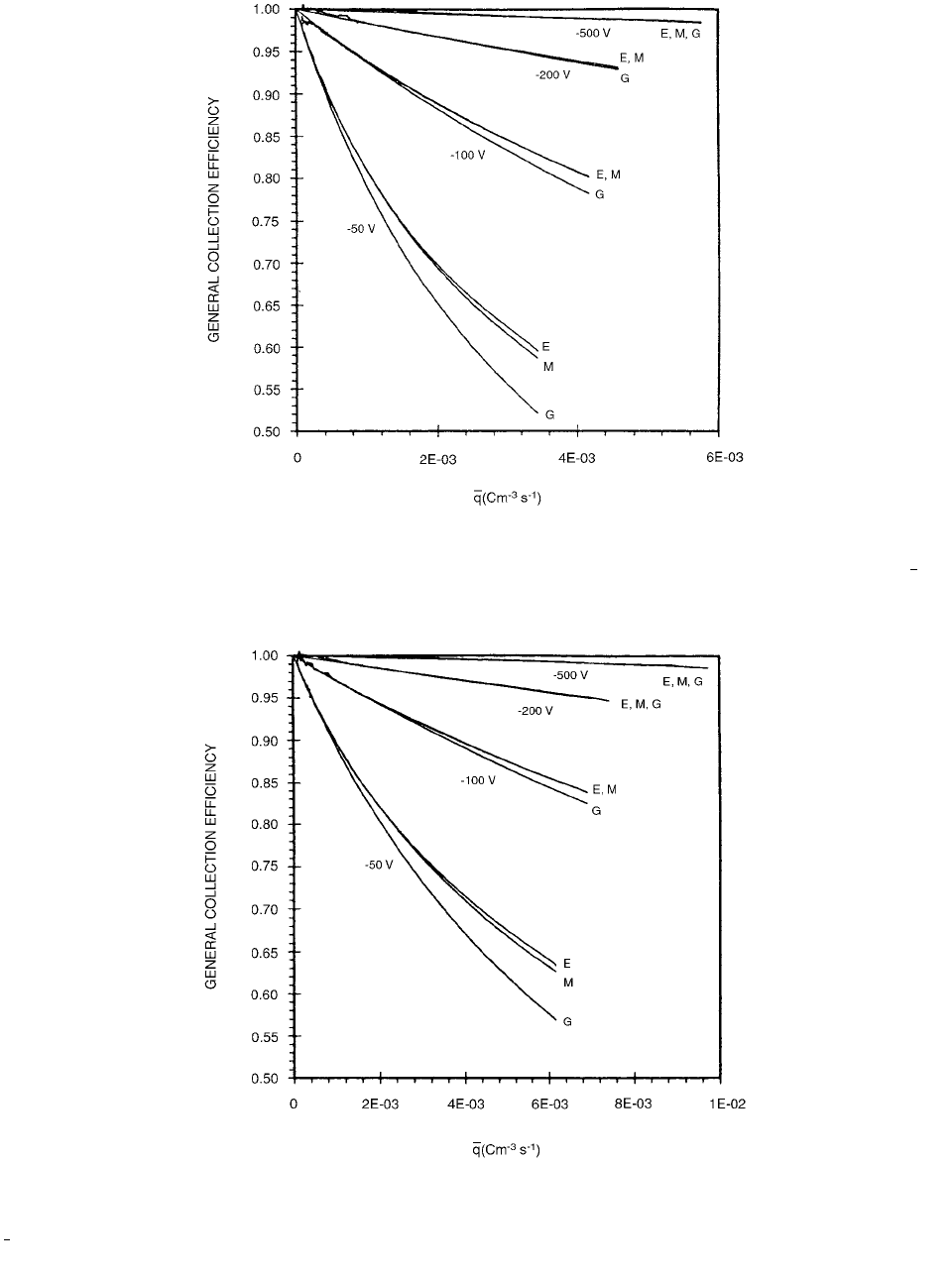
Ionization Chamber Dosimetry 189
FIGURE 3.97 Experimental (E) and theoretical (Mie (M); Greening (G)) general collection efficiency in the parallel-plate liquid-
ionization chamber, filled with isooctane and operated at the polarizing voltages 50, 100, 200, and 500 V, as a function of , the
mean value of the amount of charge liberated by irradiation and escaping initial recombination. (From Reference [95]. With
permission.)
FIGURE 3.98 Experimental (E) and theoretical (Mie (M); Greening (G)) general collection efficiency in the parallel-plate liquid-
ionization chamber, filled with tetramethylsilane and operated at the polarizing voltages 50, 100, 200, and 500 V, as a function of
the mean value of the amount of charge liberated by irradiation and escaping initial recombination (From Reference [95]. With
permission.
q
q
Ch-03.fm(part 2) Page 189 Friday, November 10, 2000 11:59 AM
