Satas D., Tracton A.A. (ed.). Coatings Technology Handbook
Подождите немного. Документ загружается.


592
MORELL
A closer view of the lamella (the foam bubble wall) would reveal the continuous
motions caused by concentration gradients along the surface. Drainage of the bulk liquid,
due to gravitational forces. results
in
surface pressure variations. This pressure differential
is alleviated by the movement
of
surfactant molecules from concentrated high pressure
areas
to
lower concentrated areas. Along with this movement is surface transport of water
molecules associated with the surfactant, which in turn drags along other bounding water
molecules. This counter drainage movement is known as the Plateau Maringoni Gibbs
effect and is responsible for restoring the bubble wall and giving it its surface elasticity
and stability.
A good example demonstrating surface transport occurs
in
cocktail glasses. A water/
alcohol mixture swirled around
in
a glass leads to wine term, drops that move up and
down the side of the glass. Alcohol evaporation
of
the liquid film
on
the glass increases
its surface tension and thus lowers its surface pressure. The surface is, therefore, continu-
ously pulled from the bulk liquid up the side of the glass. Liquid pumped up
in
this fashion
accumulates to form wine tears.
In an acetylenic diol solution. for example, drainage takes place. but the hydrophobic
nature of these molecules allows them to migrate
mpiclly
to
the interface and help alleviate
any pressure differential. At the final moment, these molecules slip away from each other.
due to low intermolecular attraction. and the wall collapses. This explains why an acetyle-
nic diol solution does not foam.
Acetylenic diols also help control foamy systems generated by other surfactants,
since they interfere with the close packing of these foam-stabilizing species at the interface
and thereby reduce surface monolayer rigidity.
It
is further theorized that due to the hydro-
phobic nature of defoamers they are likely to compete with the watedair interface by
capturing the hydrophobic ends
of
surfactant molecules and therefore rendering the lamella
unstable.
There appears to be some confusion with respect
to
foam control jargon. i.e.. non-
foaming, antifoaming. defoaming, and deairentrainment. Let us try to clarify these points
in order properly
to
discern the differences.
To illustrate
rlonfoarving,
a
0.1%
solution of an alkylphenol ethoxylate in water and
an equal surfactant concentration of the acetylenic diol is prepared. After both cylinders
are simply hand agitated. considerable foam
is
generated by the alkylphenol ethoxylate.
yet no foam appears with the acetylene-based solution. The lack of foam generated by
the acetylenic diol is what makes it
norzjomnir1g.
When
a
0.1%
solution containing
a
50/50
mixture of these two surfactants is pre-
pared, the acetylenic diol will demonstrate its
ar~r$wrnir~g
property. that is, the prevention
of foam normally generated by other substances.
Finally. acetylene based surfactants help to
defimr?
systems that already contain
foam. Adding some
to
an existing foam will demonstrate that property as well.
To summarize. what is observed is that acetylenic diols are not only nonfoaming
but also reduce the foam generated from substances such as alkylphenol ethoxylates.
Besides water solutions, acetylenic diols are
also
effective in binder systems. SBR
latex.
in
particular. is recognized for its foamy tendency
as
a result of the surfactants used
to
stabilize that emulsion, sodium laurel sulfate.
200
mils
of
an
SBR
latex is poured into
a Hobart and blended for five minutes. Remeasuring the volume
of
blended latex reveals
another class
of
foam that a coatings formulator must consider.
crir
er1frai17r??m.
The addition of most surfactants. like sulfosuccinates. will only aggravate the prob-
lem. However. when the acetylenic diol is formulated into this latex. its deairentrainnlent
properties can be easily seen. especially when compared
to
the other systems.

SURFACTANTS
FOR
WATERBORNE COATINGS APPLICATIONS
593
5.0
WETTING
One of the most important functions
of
a surfactant in a coating formulation is to facilitate
the wetting
of
various substances including the substrate to be coated or the pigment
particle to be dispersed.
A
coatings formulator should consider surfactants that are particu-
larly effective wetting agents under
dynanlic
conditions where new surfaces are continu-
ously being formed. The property of dynamic wetting is a measure of the ability of
a
surfactant
to
wet instantaneously any newly created surfaces. During coating production
and application new surfaces are continuously generated.
The traditional methods of measuring surface tension using the DuNouy ring or the
Wilhelmy plate presume that the surface is static or at equilibrium. Unfortunately, that
does not simulate real-world conditions or operations.
A
better approach makes use of
more sophisticated instrumentation such as the maximum bubble pressure tensiometer.
which can measure surface tension under both equilibrium and dynamic conditions.
This apparatus has two Teflon tubes immersed
in
the sample liquid. The tubes are
connected
to
a supply
of
gas and
to
a
pressure-measuring transducer. When the gas flows
through the tubes, measurements are recorded as bubbles begin to form in the liquid. The
gas flow rate can be regulated
to
change the rate of bubble formation. Measurements at
one bubble per second, for example, simulate equilibrium or static conditions, while six
bubbles per second are certainly more dynamic. Therefore the faster the bubble rate, the
faster the rate
of
surface formation.
Let us examine a practical benefit
of
dynamic surface tension reduction with respect
to coverage over difficult-to-wet substrates.
A
very common problem with waterborne coatings is that they are particularly
susceptible to surfaces that have not been properly cleaned or prepared. Surface contamina-
tion problems, which can result from oil, fingerprints. or dirt particles. can cause the final
coating to exhibit poor coverage, edge pool. craters, pinholes, and even reduced adhesion.
These problems can be effectively reduced with the addition
of
surfactants that allow
extremely low dynamic surface tensions.
To illustrate this point, we start with two identical panels. To sinlulate problems
that can occur in practice, we apply mineral oil on each panel. This is spread evenly Over
the face of the panels with
a
paper tissue.
In
a
recommended acrylic emulsion coil coating formulation, both the wetting agent
m~d
the defoamer were replaced with an acetylenic diol. When the acetylene-based formu-
lation is drawn down. good coverage was noted even under the adverse conditions of an
oil
contaminated surface. The control formulation encountered significant problems with
film retraction under the same conditions.
6.0
CONCLUSION
Additives play a very important role in the emerging technology of waterborne coatings.
They affect all aspects
of
the coating process-production, storage, application, and perfor-
mance. Hopefully, this paper communicated a better understanding of how specifically
wetting agents and defoamers work and the contributions they provide
to
the coatings
industry.
This Page Intentionally Left Blank

71
Surfactants, Dispersants, and
Defoamers for the Coatings, Inks, and
Adhesives Industries
Edward
W.
Orr and Gary
T.
Mallalieu
BYK-Clwmie
USA,
Wd1iqfi)rd
Comecticwt
1
.O
INTRODUCTION
Over the history of coatings, inks, and adhesives, many evolutionary changes have oc-
curred; such changes have affected not
only
the constituents of the formulations themselves
but also the application, curing. and performance parameters.
Of course, each trend poses challenges to both raw material suppliers and formulators
alike. Since additives are used to enhance system performance-the evolution of resins.
pigments, solvents, and application technologies poses special challenges to additive sup-
pliers.
Given the evolution of technologies toward more environmentally acceptable
sol-
vents, resins. and applicationkuring techniques, increased usage of interfacially active
substances is required. In bygone days. resin systems and solvent combinations oftentimes
had quite low surface tensions and therefore made fortnulation easier in comparison to
modern times.
Interfaces
play an important role
in
the production and application of these systems.
For the chemist, the following interfaces are of special interest: solidhir, solid/liquid.
liquidhir, liquid/liquid.
During the dispersion process. the solidair (pigmenuair) interface is replaced by
the solid/liquid (pigmenuresin solution) interface. During production and application, addi-
tional interfaces are important: solid/liquid (substrate/liquid, others). liquidliquid
(oil
con-
tamination. wet-on-wet application. others) as well as liquidhir (entrapped air, surface
leveling, others). The additive provides means of manipulation and control of these inter-
faces. This is precisely how it benefits the formulator and solves problems.
To cope with the challenges
of
advanced coating, ink, and adhesive systems, addi-
tives have evolved in both structure and performance. With this in mind, an overview of
595

596
ORR
AND MALLALIEU
additive technologies is presented. Special emphasis is placed upon three areas: surfactants,
dispersants, and defoamers. The chemical determinants of additive performance are out-
lined for
a
variety of systems. Not only will structure-performance correlations be dis-
cussed but also practical examples will be displayed.
Semantically speaking, the three phenomena introduced in the title of this chapter
are overlapping; accordingly, the objective
of
this chapter will be a clear delineation-as
much as possible within the editorial confines of a mere “overview chapter”-of the basic
physicochemical phenomena that lie at the base of surfactant, dispersant, and defoamer
technology.
One could indeed devote several dozen pages to the many definitional idiosyncrasies
that occur (for instance, no fewer than nine different definitions
of
the word ”surfactant”
exist), but this would be rather counterproductive, especially in a brief introductory chapter.
Therefore, one must logically set the stage in this chapter by providing a simplified and
unified definitional approach.
As
a
result, the three aforementioned phenomena will be
both defined and refined within the logic
of
a three-part framework*:
1.
Wetting and dispersing additives
2.
Silicones and surface flow control agents
3.
Defoaming additives
2.0
WETTING AND DISPERSING ADDITIVES
Wetting and dispersing additives are designed to prevent defects such as flocculation,
gloss reduction, flooding and floating, formation of Btnard cells, settling of pigments,
and rheology problems.
2.1
The Wetting and Dispersing Process
While using the aforementioned products, the pigment grinding process takes place as
follows: During the first phase of pigment wetting, the air and humidity at the pigment
surface are replaced by binder solution. This means that the solidgaseous interface
(pigmentlair) must be transformed into a solidliquid interface (pigmenthinder).
In order successfully to complete the wetting phase, the difference in interfacial
tension between the pigment and the binder solution must be bridged over,
so
to
speak.
This bridging process can be supported by the usage of appropriate wetting agents.
Homogeneous particle distribution enhances the transformation process and provides
the key
to
improved performance; interfacially active substances, which bridge the bounda-
ries between solid and liquid media, are therefore essential. From
a
practical standpoint,
proper distribution is required not only during production and application but also upon
storage.
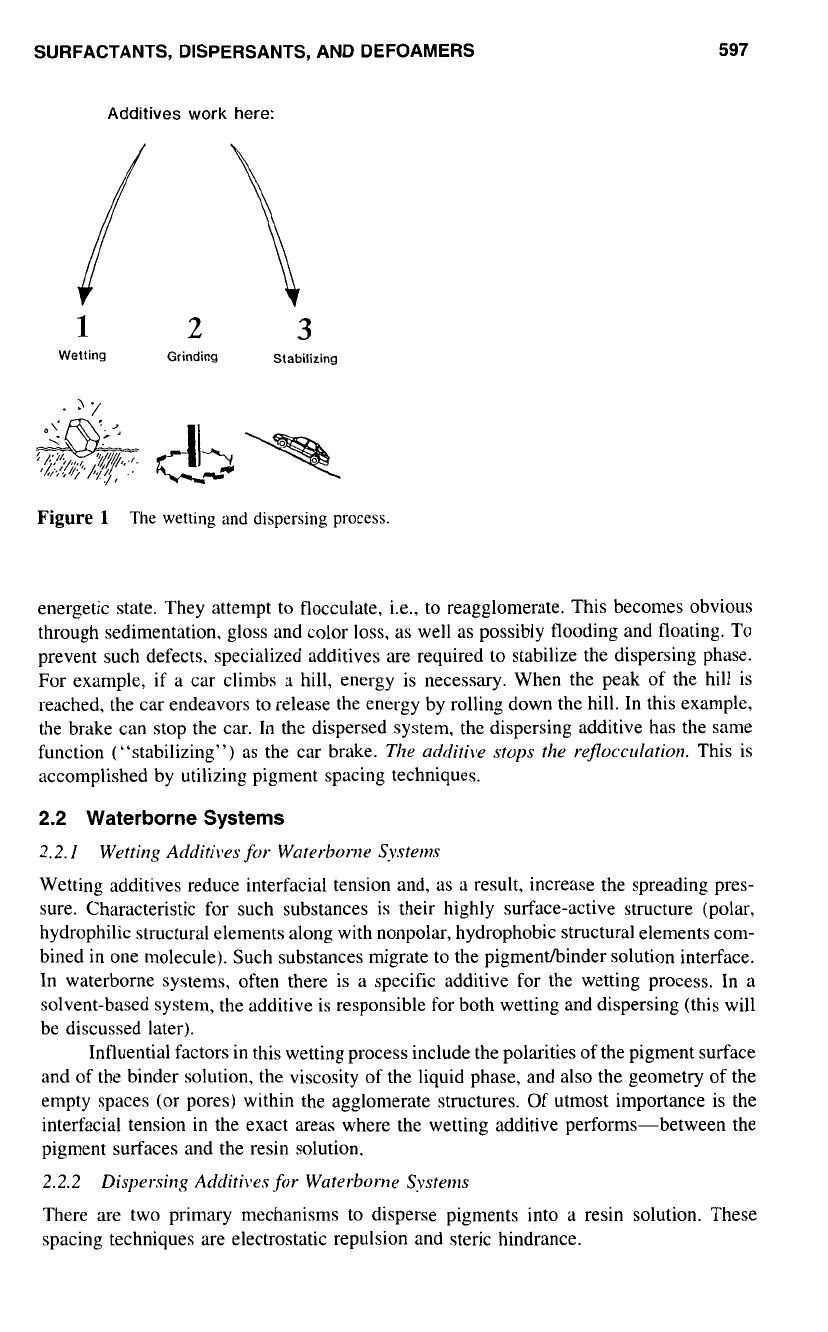
The transformation process is displayed in Fig.
1.
As
shown, the pigment agglomer-
ates are dispersed by means of mechanical energy (grinding)
so
that their particle size is
therefore reduced. The technology of the equipment (in combination with properly adjusted
millbase formulations and grinding media) is very important for the effectiveness
of
the
grinding process. The mechanically dispersed pigment particles strive to adopt a weaker
*
Because of the overview nature of thls brief chapter, please note that
all
references and cross-references are
grouped
and organized according
tu
the three-part framework.
SURFACTANTS, DISPERSANTS, AND DEFOAMERS
597
Additives work here:
l
2
3
Wetting
Grinding
Stabilizing
Figure
1
The wetting
and
dispersing
process.
energetic state. They attempt
to
flocculate, i.e., to reagglomerate. This becomes obvious
through sedimentation, gloss and color
loss,
as
well as possibly flooding and floating. TO
prevent such defects. specialized additives are required to stabilize the dispersing phase.
For example, if a car climbs a hill, energy is necessary. When the peak of the hill is
reached, the car endeavors to release the energy by rolling down the hill. In this example,
the brake can stop the car. In the dispersed system, the dispersing additive has the same
function (“stabilizing”)
as
the car brake.
The
acirliti\le
stops
the
rejlocculation.
This is
accomplished by utilizing pigment spacing techniques.
2.2
Waterborne Systems
2.2.
I
Wetting
Additilves
for
Waterborne Svstetns
Wetting additives reduce interfacial tension and, as a result, increase the spreading pres-
sure. Characteristic for such substances is their highly surface-active structure (polar,
hydrophilic structural elements along with nonpolar, hydrophobic structural elements com-
bined in one molecule). Such substances migrate to the pigmenthinder solution interface.
In waterborne systems, often there is a specific additive for the wetting process. In
a
solvent-based system, the additive is responsible for both wetting and dispersing (this will
be discussed later).
Influential factors in this wetting process include the polarities of the pigment surface
and of the binder solution, the viscosity of the liquid phase, and also the geometry of the
empty spaces (or pores) within the agglomerate structures. Of utmost importance is the
interfacial tension
in
the exact areas where the wetting additive performs-between the
pigment surfaces and the resin solution.
2.2.2
Dispersing
Arlcliriws
for Waterborne
Systerm
There are two primary mechanisms to disperse pigments into a resin solution. These
spacing techniques are electrostatic repulsion and steric hindrance.

598
ORR
AND MALLALIEU
Concerning electrostatic repulsion, the pigment particles in the liquid carry electrical
charges on their surfaces. Through the usage of special additives, it is possible
to
strengthen
the charges and furthermore
to
make all pigment particles equivalently charged. Counteri-
ons concentrate around the pigment particles
so
that an electrical double-layer is formed;
stabilization increases along with layer thickness. This particular electrostatic repulsion
stabilization mechanism is especially useful in waterborne latex dispersion systems. Chem-
ically speaking, the additives utilized
for
dispersion in such systems are polyelectro-
lytes-tailored higher molecular weight products with electrical charges in the side chains.
In
addition to polyphosphates. many polycarboxylic acid derivatives are employed as
polyelectrolytes
in
the coatings industry. These adsorb onto the pigment surface and conse-
quently transfer their charge to the pigment particle. Through electrostatic repulsion be-
tween equally charged pigments, the detlocculated state is stabilized. This is the primary
mechanism in many waterborne systems.
Concerning steric hindrance, dispersing additives that function by steric hindrance
display two special structural features. First, such products contain one or more so-called
pigment-affinic groups-anchor groups or adhesive groups-all
of
which provide strong
adsorption upon the pigment surface. Second, such products contain resin-compatible
chains (hydrocarbon entities), which, after additive adsorption upon the pigment. protrude
as far as possible from the pigment surface into the surrounding resin solution. This layer
of adsorbed additive molecules with the protruding chains produces steric hindrance or
“entropic stabilization.”
Newly designed and very specialized chemistries use the above stabilization mecha-
nism. Furthermore, this mechanism is accentuated by the interaction of the polymeric
segments of the additive with the resin polymers in such a way that the envelope,
SO
to speak, around the pigment particles is enlarged. Through specific structural elements
composed of pigment-affinic groups (polar) and resin-compatible chains (nonpolar). these
additives exhibit definitive surface-active properties. In other words. they not only stabilize
the pigment dispersion but also function as wetting additives.
2.3
Solvent-Based Systems
“Wetting and dispersing” as a term should not be separated when discussing nonaqueous
systems. The process first involves wetting, which is usually the removal or displacement
of air or moisture from the pigment surfaces. and then subsequently. dispersing, which is
the utilization
of
appropriate mechanical forces to produce primary particles. Finally,
stabilization of these particles is needed
to
keep them from reflocculating. If the defloccu-
lated system is not stabilized (as discussed previously), then the system reverts
to
a
state
of lower energy.
The primary mechanism
of
stabilization for solvent-based systems is through steric
hindrance.
as
discussed
in
the previous section (“Dispersing Additives for Waterborne
Systems”).
Additionally, electrostatic repulsion plays an important role, especially when more
than one pigment is present in a mixture. It has been proven that certain high molecular
weight species
in
the proper formulation are very beneficial in depositing a positive charge
on
various pigments (independent of initial pigment charge or type; even difficult-to-
stabilize organic pigments can be controlled). Further details are discussed in later sections
of
this chapter.

SURFACTANTS, DISPERSANTS, AND DEFOAMERS
599
ideal controlled uncontrolled
agglomerates dispersion flocculation fiocculation
hiding power prevents: reduction
of
gloss
gloss
flooding-
viscosity
viscosity
settling
aging
floating
mill base increase on
color
strength
reduced
color strength
reduced opacity
sagging
Figure
2
State
of
dispersion.
2.4
Classification
Wetting and dispersing additives for solvent-based systems can be classified by their
chemistry and mode of action. Chemically, they may be classified as ”anionic,” “ca-
tionic,” or “electroneutral.” Experience shows. however, that this classification does not
necessarily allow conclusions concerning effectiveness
of
any type.
A
more meaningful classification is
to
differentiate between detlocculating and con-
trolled flocculating types, shown in Fig.
2.
(Please keep
in
mind that an unabridged descrip-
tion of the complexity of surfactant behavior would require several thousand pages; accord-
ingly, only
a
cursory examination
of
surfactant behavior is included in this overview
chapter.)
Dejloccukrtior7
means that pigments are to the greatest possible extent stabilized as
single particles and sterically separated from each other into an ideal dispersion. This
means a change in rheology toward Newtonian flow. Thus one achieves excellent flow
properties, high gloss. optimal hiding power. reduction of nlillbase viscosity. high transpar-
ency, and development
of
the exact color features.
C~~r~trolleclflocc~rlatir~~
means that pigments and extenders are stabilized as defined
and selectively interactive units of several particles. Rheology is modified
in
a slightly
thixotropic manner. The resulting effects are antisettling, antisagging and improved
antifloodingltloating.
2.4.
l
Dqflocculrrting
Adiitit9e.s
Depending upon the actual ingredients of the system, wetting and dispersing behavior can
be tailored on a case-by-case basis. One of the more important variables is the pigment
surface polarity. Highly polar surfaces generally require the usage of low molecular weight
polymeric additives. whereas nonpolar surfaces require the higher molecular weight spe-
cies.
Deflocculating products have at least one pigment affinic group. Higher molecular
weight additives generally have several pigment affinic groups. They are arranged in such
a
way that all groups
of
an additive molecule adsorb onto a pigment particle.

600
ORR
AND MALLALIEU
Apart from pigment affinic groups. the binder-compatible molecular chains achieve
a form of "interchanging" reaction of the additives between pigment and binder. This
causes various beneficial stabilization effects. For instance, the pigment affinic groups
cause adsorption
of
the additives upon the pigment surface. In conjunction with the binder,
the binder-compatible chains are responsible for steric hindrance. thus enveloping the
pigment particles with polymeric substance and preventing direct pigment-pigment con-
tact.
Deflocculation generally leads to an improved, more efficient pigment utilization,
which (especially
in
the case of the sometimes rather expensive organic pigments) is not
economically unimportant. The degree of deflocculation or flocculation exerts a dramatic
influence upon the developed shade or tint of a pigment.
If.
for example, a system tends
to
settle upon storage. then color shift can result. In situations where this is especially
critical (such
as
in the base component of a mixing system). the only acceptable method
for producing coatings with a constant and defined shade is the complete deflocculation
method described below:
A new group
of
additives has been developed over the past few years-high molecu-
lar weight polymeric wetting and dispersing additives. Such additives provide
complete
cleflocculrtion
mcl
consequently differentiate thrnlsel\~esf,-~~r~~ the con~~er~tioncrl
Ion,
wolec-
ulur
nlright
crn~logs
through molecular weights sufficiently high to allow the attainment
of
a
rosin-like chrrrcrctor.
In addition, the newer additives contain a considerably larger
number of pigment-affinic groups. Because of these structural features, such additives can
form durable adsorption layers upon many organic pigments. Stabilization arises,
in
part,
from steric hindrance (exactly as with the conventional products)
in
which well-solvated
polymer chains are utilized; however. optimal stabilization is possible only when such
polymer chains are properly unfurled and therefore quite compatible with the surrounding
resin solution. If this compatibility is obstructed. then the polymer chains collapse. Conse-
quently. all chances for steric hindrance and the resultant stabilization are lost.
2.4.2
Controllecl Flocculatirlg Adliti\w
If the pigment affinic groups are not merely confined
to
a small region of the additive
molecule, but are distributed in a special fashion over the entire molecule. then such an
additive can simultaneously contact two or more pigment molecules in a bridge-like fash-
ion. Controlled flocculation is the result. At this point. it is important
to
clarify the differ-
ence between the above controlled flocculation state and the normal flocculation state.
Without additives, the pigment particles make direct contact with one another
in
uncon-
trolled flocculates.
In
contrast,
no
direct pigment-pigment contact occurs
in
controlled
tlocculates; additive molecules are always present between the pigment particles.
An ordinary flocculation without additive (exhibiting direct pigment-pigment con-
tact) is not controllable. The formulation properties are oftentimes different from one
production process to another and may also change during storage. This causes variances
in
shades or perhaps even uncontrolled sedimentation behavior. Due to the light shear
forces that may occur during coating. rolling, or spraying, the flocculates and additive
structures are destroyed. However. they build up again immediately after shear stress.
Suitable processing properties with proper flow may result despite the rather high viscosity
of the formulation in the container.
2.5
Summary
The manipulation
of
both the solidgas and the solidAiquid interfaces created when a
.
pigment is incorporated into
a
system is the prime function of wetting and dispersing

SURFACTANTS, DISPERSANTS, AND DEFOAMERS
601
additives. Various chemistries and mechanisms are employed, but it is the selective control
of
the interfacial tension that optimizes the final properties.’
‘’
3.0
SILICONES AND SURFACE FLOW CONTROL AGENTS
3.1
Background
The proper use of silicone (polysiloxane) additives can help avoid a variety
of
surface
defects-orange peel, crawling, craters. foam. fisheyes, and floating. These six phenomena
may, at first glance, appear totally unrelated; however, a clear understanding of surface
science and interfacial tension uncovers many commonalities.
One method of tailoring the properties of a dimethylpolysiloxane is by varying the
molecular weight. According to the exact performance desired. molecules can be custom-
designed
to
function as flow control agents. slip aids, defoamers, or even hammer-finish
additives. As molecular weight increases, incompatibility increases. The key is to achieve
a proper balance of properties. This balance is more optimally achieved by modifying the
polysiloxane with organic chains in order to manipulate compatibility. A typical structure
is shown in Fig.
3.
3.2
Chemical Structure
of
“Silicones”
To
further elaborate upon the example structure indicated above, several variations can
be discussed. The variables of this molecule are
“X,”
“Y,”
the polyether chain. and the
organic modification. An essential point at the synthesis of such siloxanes is the ratio
of
X
to
Y.
The polyether chain orients itself toward the organophilic phase (in the resin),
whereas the dimethyl group orients toward the hydrophobic phase. The dimethyl group
can also be oleophobic at the gadair interface.
Proper molecular design allows control of such features as surface tension, interfacial
tension, binder compatibility, recoatability, flow properties, flood/float. slip, and even
defoaming. Recent chemical advances have therefore greatly expanded the role of silicone
additives in coatings systems.
la
1,
Figure
3
Polyether modified dimethylpolysiloxane (hydrolytically stable).
