Satas D., Tracton A.A. (ed.). Coatings Technology Handbook
Подождите немного. Документ загружается.

582
CALLAIS
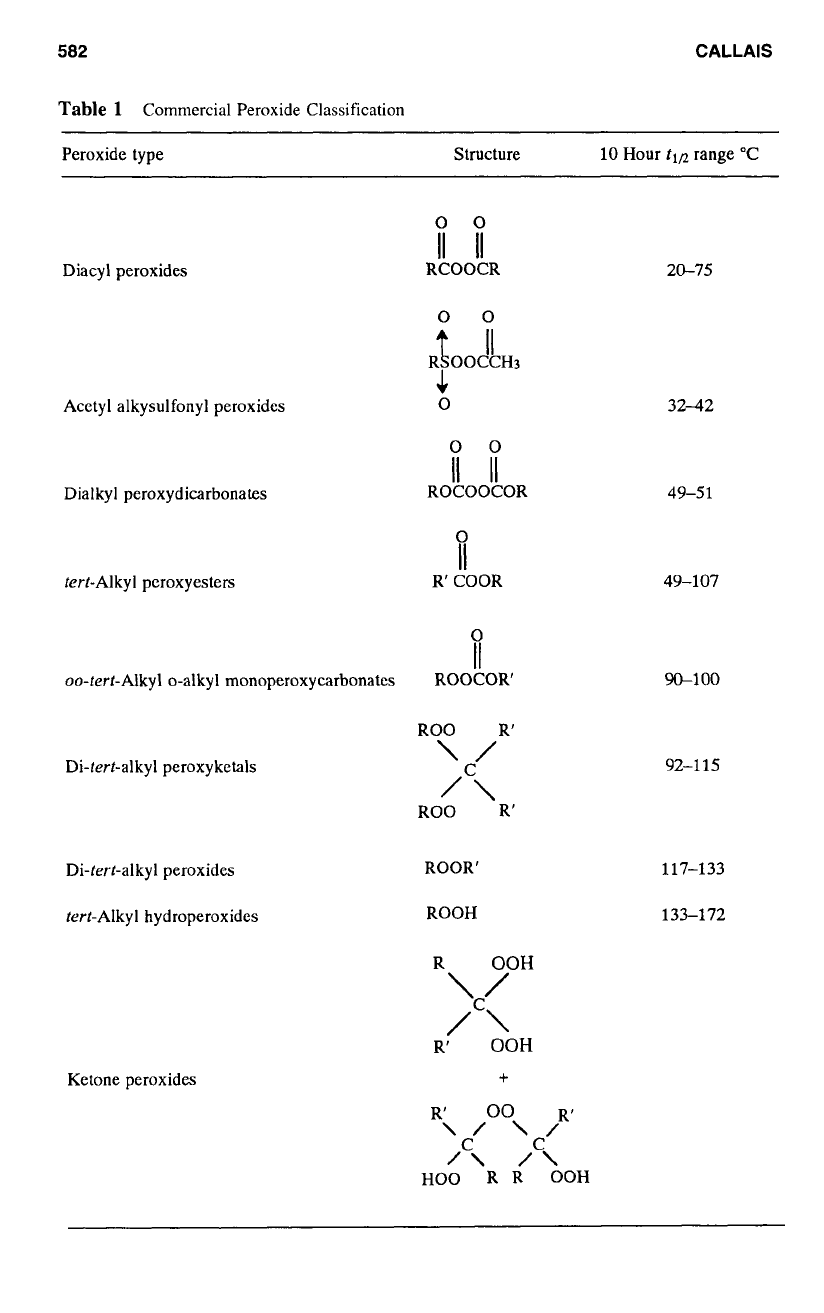
Table
1
Commercial Peroxide Classification
Peroxide type Structure 10
Hour
tl/~
range
"C
Diacyl peroxides
Acetyl alkysulfonyl peroxides
Dialkyl peroxydicarbonates
terf-Alkyl peroxyesters
00
II
II
RCOOCR
J.
0
00
II
II
ROCOOCOR
R' COOR
oo-tert-Alkyl o-alkyl monoperoxycarbonates
ROOCOR'
ROO R'
\/
Di-tert-alkyl peroxyketals
/"\
ROO R'
Di-tert-alkyl peroxides
tert-Alkyl hydroperoxides
Ketone peroxides
ROOR'
ROOH
R\
YH
/c\
R'
OOH
+
20-75
32-42
49-5
1
49-107
W100
92-1
15
117-133
133-172
ORGANIC
PEROXIDES
583
Peroxides
of
certain types, such as hydroperoxides and ketone peroxides, are primarily
used
in
combination with promoters and are employed at temperatures much lower than
their measured 10-hour
til?
temperature.
2.1
Peroxide Selection
To a large extent, the half-life range at which an organic peroxide decomposes determines
the application and controls overall process efficiency and product quality. In their product
bulletins, organic peroxide producers often provide half-life data over a wide range of
temperatures. For optimum efficiency, however, a peroxide is usually chosen
so
that the
half-life under the reaction conditions is in the range of
10-20
minutes. This ensures the
steady generation of radicals
at
such a rate that the heat of reaction can be safely contained
and a high conversion
of
monomer
to
polymer results.
It
is important
to
note, however, that peroxide half-life data are usually determined
in
select inert solvents and low peroxide concentration. Decomposition rates can be af-
fected by solvent polarity, radical-induced decomposition, and peroxide concentration.5
The temperature activity is not the sole consideration
in
selecting an organic peroxide
for a particular application. Other factors to be taken into account include cost, solubility,
safety, efficiency and type
of
radicals produced, necessity for refrigerated storage and
shipment, compatibility with production equipment, effect on the final product, and the
ability
to
be activated.
2.2
Radical Types
Although organic peroxides initially cleave at the oxygen-oxygen bond, other bond cleav-
ages can and do occur. either simultaneously with or sequentially
to
the oxygen-oxygen
bond dissociation. The relative stability of the
R
radical determines whether a peroxide
undergoes single- or multiple-bond homolysis. The relative stability of the
R
radicals,
in
turn, can be correlated with the carbon-hydrogen or oxygen-hydrogen bond dissociation
energies
of
the parent compound, as shown
in
Table
2."
"'
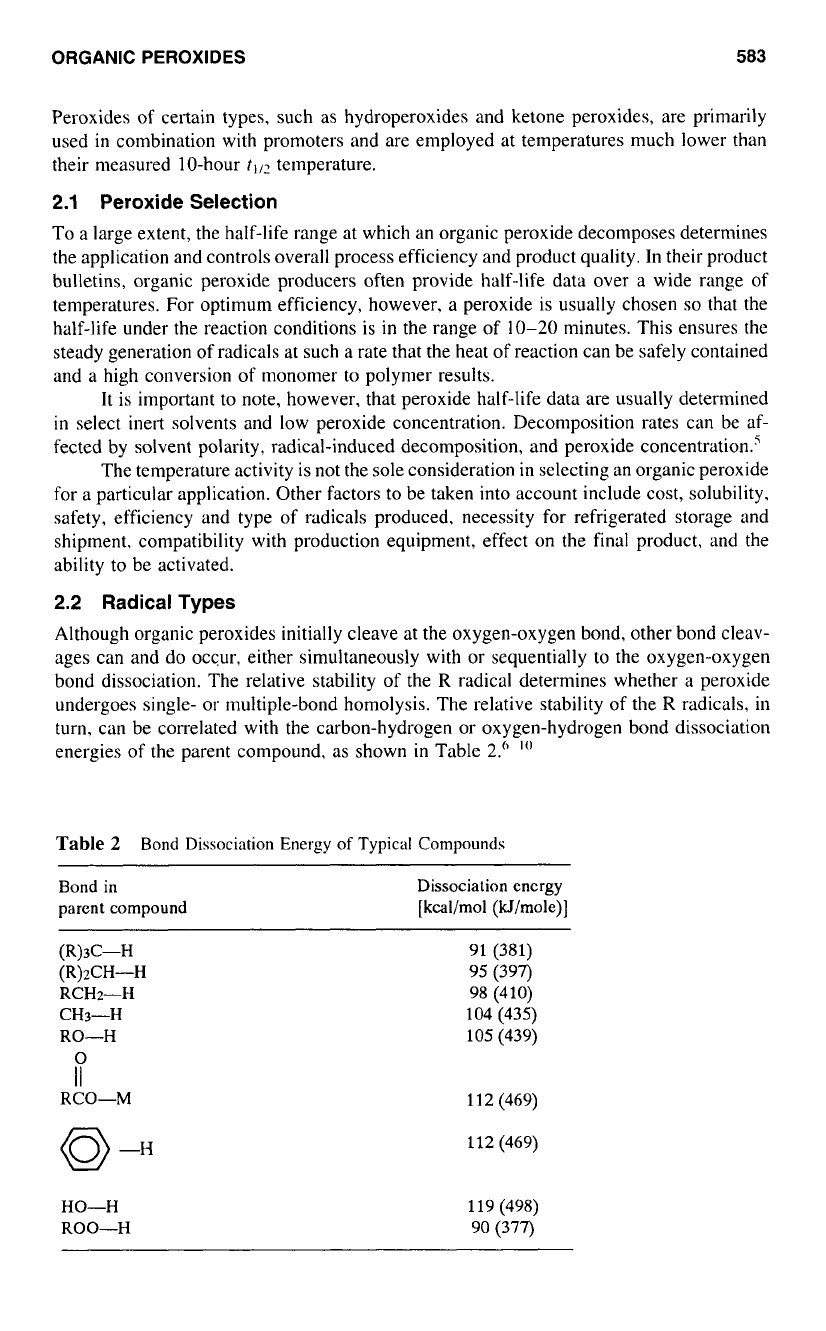
Table
2
Bond
Dissociation
Energy
of
Typical Compounds
Bond in
parent compound
Dissociation
encrgy
[kcal/mol
(kl/mole)J
(R)d-H
(R)zCH-H
RCH2-H
CH3-H
RO-H
i
RCO-M
91 (381)
98 (410)
104 (435)
95 (397)
105 (439)
11
2
(469)
112
(469)
HO-H
ROO-H
119 (498)
90 (377)
584 CALLAIS
The higher the bond dissociation energy, the less stable (more reactive) is the corre-
sponding radical that
is
formed by removing the hydrogen atom. Thus, the phenyl radical
is significantly more reactive than the alkyl radicals. The relative stability
of
alkyl radicals
is in the order
of
fer?-alkyl
>
sec-alkyl
>
n-alkyl
>
methyl. The methyl radical is about
as reactive as an alkoxy radical.
One reaction that follows the peroxide linkage dissociation is the p-scission reaction:
One of the
R
groups splits
off
to
form a new alkyl radical and a ketone. The group that
splits off forms the most stable radical. If the radical that splits
off
is more stable than
the alkoxy radical, the p-scission reaction will be fast and the predominant initiating
species will be the alkyl radical rather than the alkoxy radical. The rate of p-scission also
depends on temperature and pressure; that is, more scission occurs
if
the alkoxy radical
is generated at higher temperatures and pressures.
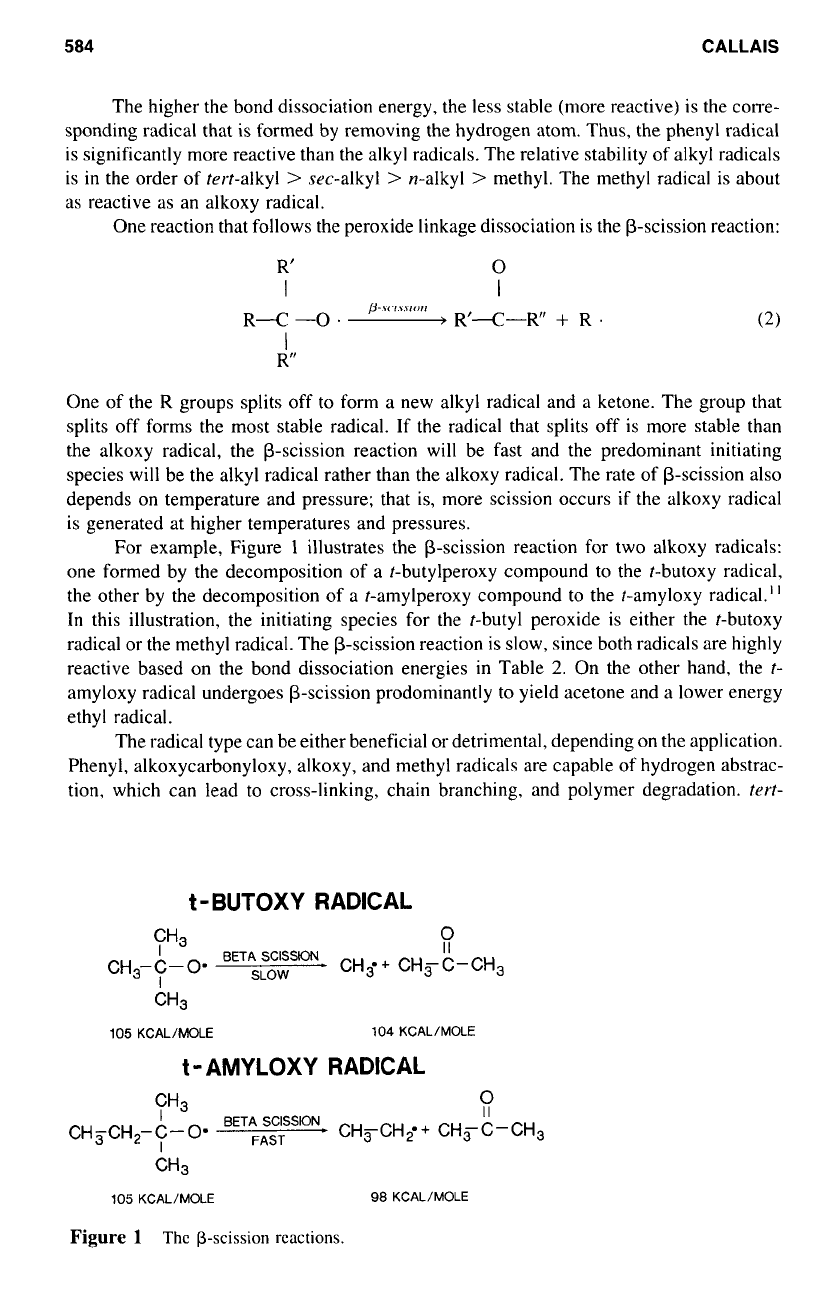
For example, Figure
1
illustrates the (3-scission reaction for two alkoxy radicals:
one formed by the decomposition of a t-butylperoxy compound to the t-butoxy radical,
the other by the decomposition of a t-amylperoxy compound to the t-amyloxy radical.”
In
this illustration, the initiating species for the t-butyl peroxide is either the t-butoxy
radical or the methyl radical. The p-scission reaction is slow, since both radicals are highly
reactive based on the bond dissociation energies in Table
2.
On the other hand, the
t-
amyloxy radical undergoes p-scission prodominantly
to
yield acetone and a lower energy
ethyl radical.
The radical type can be either beneficial or detrimental, depending
on
the application.
Phenyl, alkoxycarbonyloxy, alkoxy, and methyl radicals are capable
of
hydrogen abstrac-
tion, which can lead to cross-linking, chain branching, and polymer degradation. tert-
t
-
BUTOXY RADICAL
CH3
105
KCALIMOLE
104
KCAL/MOLE
t
-
AMYLOXY RADICAL
p
0
BETA SCISSION
II
CHzCH,-C”Og
FAST
-
CHgCHf+ CHeyC-CH,
I
CH3
105
KCAL/MOLE
98
KCAL/MOLE
Figure
1
Thc
p-scission
reactions.
ORGANIC
PEROXIDES
585
Alkyl, .Tec-alkyl, n-alkyl, and rrrr-cumyl radicals are less energetic, more Selective radicals.
These radical types contribute to greater chain linearity and a narrower molecular weight
distribution.
3.0
APPLICATIONS IN COATINGS
Organic peroxides are commonly used in the preparation of solution acrylic thermoplastic
and thermosetting resins and the graft modification
of
alkyd resins. Some organic peroxides
that are suitable
as
initiators for acrylic polymers and copolymers are listed in Table
3."-Is
Efforts in recent years have been directed toward the production of hydroxy-func-
tional acrylic oligomers for use in high solids coatings applications.'""9 To achieve high
solids coatings, acrylic polyol resins
of
low molecular weight and
a
narrow molecular
weight distribution must be used to ensure an acceptable solution viscosity
in
the coatings
formulation. In the synthesis
of
these resins, azonitrile and peroxide initiators, alone or
in combination with high concentration of a chain transfer agent and high temperatures,
are However, certain peroxide initiators that abstract hydrogens cause
chain branching, a broadening
of
the molecular weight distribution, and a high solution
viscosity.
In
addition, the use
of
mercaptan chain transfer agents is known to produce
objectionable odors,
as
well as color and light instability in the coating."
In
a
recent report, the use of r-amyl peroxides has been shown
to
produce acrylic
oligomers with low molecular weight, narrow molecular weight distribution, low color,
and low solution viscosity without added chain transfer The t-amyl peroxide
initiated resins
also
gave better gloss and
gloss
retention in accelerated weathering.
In
the graft modification of alkyd resins, the proportion
of
the graft copolymer
formed has been shown to be dependent on the nature of the radical initiator u~ed.'~~'~)
t-
Butyl peroxides have been shown to be preferable to azonitriles because of the formation
of a more reactive radical favoring graft copolymer formation.
Table
3
Organic
Peroxide
Initiators
for
the
Polymerization
of
Acrylics
Half-life
temperature
("C)
Peroxide
10
h
Ih
Ref.
Dibenzoyl
peroxide
r-Butylperoxy-2-ethylhexanoate
t-Butylperoxybenzoate
Dicumyl
peroxide
Di-t-butyl
peroxide
t-Amylperoxy-2-ethylhexanoate
r-Amylperoxybenzoate
t-Amylperoxyacetate
I,
1
-Di-(t-amy1peroxy)cyclohexane
2,2-Di-(t-amylperoxy)propane
Ethyl
3,3-di-(r-amylperoxy)butyrate
73
77
102
116
126
75
100
100
93
108
112
91
95
1
20
136
I49
92
121
120
112
128
125
12
13
13
14
14
15
15
15
15
15
15

586
CALLAIS
4.0
SAFETY FACTORS AND PRODUCERS
Organic peroxides are useful initiators because
of
their thermal instability. Since peroxide
initiators encompass a wide temperature activity range, a safe temperature for one peroxide
may be unsafe for another. Manufacturers’ recommendations should be carefully followed
for handling, storage, and disposal. In addition, compounds such as transition metals,
amines. strong acids and bases, and reducing agents can accelerate peroxide decomposition.
Consequently, peroxides should be kept free of contamination. Only peroxides and perox-
ide formulations that can be shipped and utilized with
a
reasonable degree of safety are
produced commercially.
5.0
FUTURE TRENDS
The current trend
in
organic peroxide research is to commercialize new multifunctional
initiators, that is, peroxides with functionality other than simply being free radical sources.
For example, organic peroxides that contain
a
hydroxyl group have recently been intro-
duced
Polymeric peroxides that contain the peroxide linkage
in
the main chain of the
polymer, on the end of a polymer, and pendent to the polymer chain have been investi-
gated.3”37 Organic peroxides containing a ultraviolet light absorbing3x or a hindered amine
light group have been used to synthesize polymers with the stabilizer group
chemically bound to the polymer.
REFERENCES
1.
2.
3.
4.
S.
6.
7.
8.
9.
IO.
11.
12.
13.
14.
15.
16.
D.
Swern,
Orgtrnic
Prro.xitles,
Vol.
I.
New York, Wiley-Interscience, 1970.
D. Swern,
Orpu~ic
fermides,
Vol.
2,
New York, Wiley-Interscicnce, 1971.
C.
S.
Sheppard and
0.
L. Magcli,
in
E~~cycloywditr
of
Cllertliccrl
Trchnolop,
Vol.
17, 3rd ed.,
H.
F.
Mark,
D.
F.
Othmer, C. G. Overbergcr, and G. T. SCaborg, Eds.. New
York.
Wiley-
Interscience, 1982,
p.
27.
R.
L.
Pastorino
and
R.
N.
Lewis,
In
Motlml
Plrsrics
Eucyclopedicr,
Vol.
64,
IOA,
R.
Juran,
Ed., New
York.
McGraw-Hill, 1988,
p.
165.
C.
S.
Sheppard,
in
E~~cyclopedi~
of
Polyrner
Scierlw
crrd
Techology,
Vol.
1
I,
2nd
ed.,
H.
F.
Mark.
N.
H.
Bikalcs, C. G. Overberger, and
G.
Menged,
Eds.,
New York, Wiley-Intcrscience,
1988,
p.
I.
D.
M.
Golden
and
S.
W. Benson.
Clrertl.
RC\,.,
69,
125 (1969).
P.
Gray,
A.
A.
Herod, and
A.
Jones,
cl ten^.
Re.v..
71.
289
(
197
I
).
A.
A.
Zavitsas,
J.
Anl.
Cl~rt~.
Soc..
94,
2779, 2788 (1972).
R.
T. Sanderson,
J.
Am.
Chertr.
Soc.,
97,
1367
(
1975).
R.
S.
Nangla
and
S.
W. Bcnson,
J.
Phys.
Cl~cw.,
83,
I
138
(
1979).
R.
J. Kirchgcssner,
V.
R.
Kamath, C.
S.
Sheppard.
and
S.
E. Stromberg,
Mod.
Plnst..
61(
1
I),
66
(
1984).
Ditrcyl
Peroxides
(product bullctin), Lucidol Division, Pennwalt Corporation, Buffalo,
NY,
1986.
Peroxyosters
(product bulletin). Lucidol Division, Pennwalt Corporation, Buffalo,
NY,
1986.
Ditrlkyl
Peroxides
(product bulletin), Lucidol Division, Pennwalt Corporation, Buffalo,
NY,
1985.
t-Atrlyl
Pero.rides
(product bullctin), Lucidol Division, Pennwalt Corporatlon, Buffalo,
NY,
1985.
M. Takahashi.
Polyn.
PImt.
Tecltrlol.
EILX..
IS.
I
(1980).

ORGANIC
PEROXIDES
587
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
31.
32.
33.
34.
.
35.
36.
37.
38.
39.
40.
L. W. Hill and
Z.
W. Wicks,
Jr.,
Pros.
Org.
Cocrtirlgs,
10.
55
(1982).
R.
H.
Kuhn, N. Roman. and
J.
D.
Whitman,
Mod.
Pcrirlt
Corrtirrgs.
71(5),
50
(1981).
R.
F.
Storey. in
Sur-rcc,
Corrtirlgs.
A.
L. Wilson,
J.
W. Nicholson. and H. J. Prosscr. Eds..
London, Elsevier Applied Science,
1987,
p.
69.
C.
J. Bouboulis,
U.S.
Patent
4,739,006 (1988).
D.
Rhum and P.
F.
Aluotto,
U.S.
Patent
4,075,242 (1978).
Y. Eguchi and
A.
Yamada.
U.S.
Patent
4,687,882
(
1987).
W. R. Berghoff,
U.S.
Patent
4,716,200
(
1987).
R.
A.
Gray,
J.
Techno/.,
57(728). 83
(
1985).
R. Buter.
J.
Techrlol..
59(749), 37 (1987).
D.
Rhurn and P.
F.
Aluotto,
J.
Techrlol..
53703). 75 (1983).
V.
R. Karnath and
J.
D.
Sargent, Jr..
J.
Cotrt.
Techrwl..
59(746).
S
1
(1987).
V.
R.
Karnath,
U.S.
Patent pending.
F.
M. Merrett,
Trtrr1.s.
Frrrtrdtry
Soc..
50,
759 (1954).
D.
H.
Solomon.
J.
Oil
Colour..
Clleru.
Assoc..
45
88
(1962).
J.
Sanchez,
U.S.
Patent
4,525,308 (1985).
A.
J.
D'A~~gelo
and
0.
L. Mageli,
U.S.
Patcnts
4,304,882 (1981), 3,952,041 (1976), 3,991.109
(1976), 3,706,818 (1972),
and
3,839,390 (1974).
A.
J.
D'Angelo,
U.S.
Patent
3,671,651 (1972).
R.
A.
Bafford,
U.S.
Patent
3,800,007 (1974).
R.
A.
Bafford, E. R. Kamens, and
0.
L.
Mageli,
U.S.
Patent
3,763.1
12
(1973).
0.
L.
Magcli, R.
E.
Light, Jr., and R. B. Gallagher,
U.S.
Patent
3,536,676 (1970).
H. Ohmura and M. Nakayarna,
U.S.
Patent
4,659,769 (1987).
C. S.
Sheppard and R. E. MacLeay,
U.S.
Patents
4,042,773 (1977)
and
4,045,427 (1977).
T.
N. Myers, European Patent Appl.,
233,476 (1987).
P.
A.
Callais.
V.
R. Kamath, and J.
D.
Sarpent,
Proc.
Wtrter-Borne
Higher.
Solids
Cntltiqs
Syrrlp..
15,
104 (1988).
This Page Intentionally Left Blank
70
Surfactants for Waterborne Coatings
Applications
1
.O
INTRODUCTION
As
governmental regulations become increasingly restrictive, waterborne coatings appear
to be the logical choice for many paint manufacturers. However, the technological switch
from solvent to waterborne systems requires an understanding of the challenges that lay
ahead with respect
to
wetting. foam control, and coverage over difficult-to-wet substrates.
This paper will help explain the important contribution of wetting agents and defoam-
ers
to
the emerging technology of waterborne coatings. Topics will include the chemistry
of several surfactants along with a thorough analysis and understanding of surface tension.
Surface tension reduction and mechanisms relating to foam stabilization will be reviewed.
2.0
CHEMISTRY
All
surfactants fall
into
two classifications, nonionic and ionic. Within the ionic category,
surfactants can be further subdivided into anionic, cationic, or amphoteric types. For coat-
ings. most surfactants utilized are either nonionic
or
anionic. For wetting agents. the
products we will compare include alkylphenol ethoxylates. sodium dioctyl sulfosuccinates.
sodium laurel sulfates, block copolymers of ethylene and propylene oxides, alkyl benzene
sulfonates, and. finally. a specialty class called acetylenic glycols. We start with this.
Acetylenic glycols are a chemically unique group
of
nonionic surface active agents
that have been especially designed to provide multifunctional benefits to a wide array
of
waterborne coating products. Two key benefits include an unusual combination
of
wetting
and foam control properties.
Characterized as
an
acetylenic
diol,
we have a ten-carbon backbone molecule with
a triple bond, two adjacent hydroxyl groups. and four symmetrical methyl groups. Based
on
acetylene chemistry. this product
is
unlike any other surfactant molecule. The combina-
589

590
MORELL
tion of the triple bond and the two hydroxyl groups creates
a
domain of high electron
density, making this portion of the molecule polar and thus hydrophilic. The highly
branched methyl groups, along with the backbone, supply the hydrophobic property creat-
ing an excellent surface tension reducer. Additionally. this particular product was designed
to be nonfoaming by careful engineering of the hydrophobehydrophile ratio.
Alkylphenol ethoxylates are commonly used
in
many applications. The hydrophile
is the ethoxylated portion, which can be regulated by the amount of ethylene oxide
(EO)
employed. The hydrophobe is generally based on either octylphenol or nonylphenol.
Block copolymers are another class of nonionic surfactants. The geonletrical configu-
ration is similar to the acetylenic glycol except that
it
is reversed. The ”outside” hydro-
philic portions are
EO
links, and the central hydrophobe is based on propylene oxide
(PO)
links. Both of these can be manipulated to affect its overall molecular weight and
HLB
classification.
Sodium laurel sulfate. or
SLS,
is
a
popular anionic surfactant utilized
in
the emulsion
polymerization of many vehicle binders. The sodium sulfate component is the hydrophilic
part, while the lauryl portion is the hydrophobe.
Finally, sulfosuccinates are a class
of
interesting anionic surface active agents. The
central hydrophile is the sodium salt
of
the sulfosuccinate, while the hydrophobe is gener-
ally dioctyl. This ionic surfactant is sometimes referred to as
DOS
(sodium dioctyl sulfo-
succinate).
3.0
THEORY
Now, before we discuss the practical formulating benefits of surfactants in greater detail,
it
may be helpful
to
review the theoretical differences between some of these products.
This will particularly aid our understanding of how some can actually contribute to foam
control properties besides wetting. But first, let us review some fundamental concepts.
A good place to start is with a definition. What is a surfactant? First. the name itself
is an acronym formed by combining the words surface active agent.
A
surfactant can be
defined as any substance that will significantly reduce the surface tension of a liquid at
a
very low concentration. Even within the context of this definition, there may be terms
that are not fully understood. For example, what do we mean when we say “surface
tension”? To define this concept,
it
is
best first to review chemical bonds, both interatomic
and intermolecular. These include covalent, ionic, and intermolecular.
When we think
of
covalent bonds, generally the first thing that comes to mind is a
sharing of electrons. However, some atoms share more than others do. An equal distribution
of
shared electrons occurs with hydrogen gas. Each atom
of
this molecule has one electron
in its valence shell and is seeking another with a similar condition (i.e., another hydrogen
atom)
to
help complete its shell with two electrons. Since there is no need for the electrons
to
orbit more often over one compared to the other, this equitable distribution results
in
a nonpolar covalent association.
The water molecule is different. The oxygen atom has six electrons
in
its outer
valence shell and desperately needs two more to complete the required eight. This “hun-
ger” for electrons makes this particular atom more electronegative than hydrogen. There-
fore. when
it
attracts two hydrogen atoms,
it
surrounds itself more often than not with a
complete set. This disproportionate sharing results
in
a slight negative charge
to
the oxygen
atom while at the same time
it
renders a slight positive charge to the other two hydrogens.

SURFACTANTS FOR WATERBORNE COATINGS APPLICATIONS
591
This type of covalent bond is called polar since the molecule resembles the positive and
negative poles of
a
magnet.
Naturally. polar molecules attract one another by aligning themselves via their
oppos-
ing poles (positive
to
negative and negative
to
positive). In the case
of
water, this type of
intermolecular attraction is known as hydrogen bonding. Therefore, when we examine a
water molecule in the bulk. we find
it
pulled
in
all directions with equal force and, as a
result. it is said
to
be
in
equilibrium. However, the molecules on the surface have no
molecules above
to
attract to and, therefore. the net effect is a downward force into the
bulk. This is surface tension.
The Ltbility of a surfactant
to
reduce surface tension in water, for example, results
from the combined hydrophilic and hydrophobic parts of its structure.
As
a result
of
the
hydrophobic part. these molecules tend
to
collect
at
the water/air interface. for example.
when added to an aqueous medium. The presence of surfactant molecules at this interface
results
in
a compressive force acting on the surface.
a
force that is known
as
surface
pressure.
A
simple experiment demonstrates surface pressure.
A
limp loop of thread on
water will snap into a circle when the water inside is touched by soap.
It
is this surface
compressive force, or surface pressure. that reduces the surface energy or surface tension.
Sometimes
it
is difficult to relate to the concept of surface tension. It is much easier
to think in terms of surface pressure. There is
a
relationship between the two terms: The
surface pressure of a surfactant solution at a certain concentration is equal to the surface
tension
of
the pure solvent minus the surface tension of the surfactant solution at the
same concentration. Therefore. as the surfactant concentration is increased, there is a
corresponding increase
in
surface pressure and. as a result, surface tension is reduced.
When the surface is completely saturated with surfactant molecules and the surface
pressure approaches its greatest value. the incremental addition of most surfactants will
result
in
the formation of micelles. This is known as the critical micelle concentration.
These micelles can take
on
different forms including spherical or cylindrical shapes.
At
high concentrations. most surfactants condense into a solid structured elastic film
as
a
result of the molecular attraction between the chains
of
methylene groups
in
neighbor-
ing molecules. This solid structured film determines the surface property
of
the surfactant
solution and helps explain how some surface active agents contribute
to
foam generation
and stabilization.
4.0
FOAM
CONTROL
In pure water. an air bubble rises attempting
to
achieve the same equilibrium state with
the denser fluid on the bottom.
It
should be noted that sustained foam. that is. foam in
which air or gas
is
firmly entrapped
in
a liquid for a considerable interval
of
time,
only
occurs
in
a
complicated colloidal system. Therefore. it is not possible for foam
to
exist
in a
puw
liquid.
The colloids
in
a foamy system are surfactants with varying degrees of hydrophillic/
hydrophobic balance. Here, the hydrophobic hydrocarbon tails protrude from the water,
both at the top surface and within the bubble, with the circular polar hydrophilic heads
engaged into the water phase.
As
mentioned earlier, most surfactants that concentrate at
this gas/liquid interface are capable
of
forming a solid structured elastic film. Therefore
gases that rise
in
these systems expand these elastic films without rupturing them. The
result is that entrapped air gradually rises
to
the surface
as
froth or bubbles.
