Satas D., Tracton A.A. (ed.). Coatings Technology Handbook
Подождите немного. Документ загружается.

This Page Intentionally Left Blank

Hydroxyethylcellulose
Lisa
A.
Burmeister
Aqualon
Company,
Wilmington.
Delawcrre
1
.O
INTRODUCTION
Hydroxyethylcellulose (HEC), is a nonionic, water-soluble polymer derived from cellu-
lose. It is used in water-borne paints for its action as a thickener, stabilizer, and/or suspend-
ing agent. HEC not only imparts mechanical and chemical stability to the paint system,
it plays a significant role in controlling the rheology of the paint during manufacture,
storage, and application. HEC is the most commonly used thickener in latex paints. In
fact, it is often referred to as the industry standard for thickening.
Physically, hydroxyethylcellulose is a white, free-flowing powder that dissolves
readily in hot or cold water. Available in a variety of types and grades, it can be used to
produce aqueous solutions with a wide range
of
viscosities and a pseudoplastic nature.
Upon drying, solutions of HEC form clear, gel-free films.
The nonionic nature of HEC is an advantage in latex paints. Nonionics can be used
over a wide pH range, and, unlike polyelectrolytes, are compatible with salts and charged
species such as surfactants, latex particles, and colorants.
More recently, a hydrophobically modified hydroxyethylcellulose (HMHEC) was
introduced to the latex paint industry. HMHEC is an associative cellulosic polymer de-
signed specifically for use in latex paints. It is a nonionic water-soluble polymer that
contains both hydroxyethyl and long chain alkyl groups. This unique dual substitution
differentiates the new-generation rheological modifier from traditional HEC. Like HEC,
hydrophobically modified hydroxyethylcellulose thickens the aqueous phase of the paint,
but substantial viscosity is built through association of the polymer’s hydroprobes with the
paint components. HMHEC combines the desirable properties of HEC with the enhanced
rheology control (particularly with respect to spatter resistance and film build) required
for today’s coatings. A detailed discussion of HMHEC’s chemical composition and rheo-
logical performance in latex paints is provided by Shaw and Leipold.
553

554
BURMEISTER
r--
“1
L”
-,J
n-2
Figure
1
Structurc
of
cellulose.
2.0
CHEMICAL COMPOSITION
OF
HYDROXYETHYLCELLULOSE
The cellulose molecule is a polymeric chain composed of repeating anhydroglucose units
(Fig.
I).
There is considerable intrachain and interchain hydrogen bonding, which results
in a highly ordered, highly crystalline structure.
It
is for this reason that cellulose is
not
soluble
in
water.
The reactions used
to
make derivations of cellulose are straightforward. Cellulose
is reacted with alkali, such
as
sodium hydroxide,
to
form alkali cellulose. The alkali
treatment is necessary to disrupt the cellulose crystallinity. The swollen chain is then ready
for the addition of appropriate substituents. Substitution on the cellulose chain causes
disorder and forces the chains apart
so
that water may enter and solvate the chain. Each
anhydroglucose unit in the cellulose molecule has three reactive hydroxyl groups. The
number
of
hydroxyl groups substituted
in
any reaction is known
as
the degree of substitu-
tion
(DS).
Technically,
all
three hydroxyls can be substituted. The product from such
a
reaction would have a
DS
of
3.
Hydroxyethyl groups can be introduced into the cellulose molecule
in
two ways.
First, ethylene oxide reacts with the hydroxyls in the cellulose chain. Second, ethylene
oxide, reacting with previously substituted hydroxyls, can polymerize to form
a
side chain.
The average number of moles of ethylene oxide that attach
to
each anhydroglucose
unit in cellulose
in
the two ways described is called “moles
of
substituent combine,” or
(MS).
Solubility
in
water is achieved as the degree
of
substitution is increased. By selecting
appropriate reaction conditions and moles
of
substituent, complete and quick solubility
in water is obtained. HEC with an MS of 2.5
is
most frequently used in latex paints
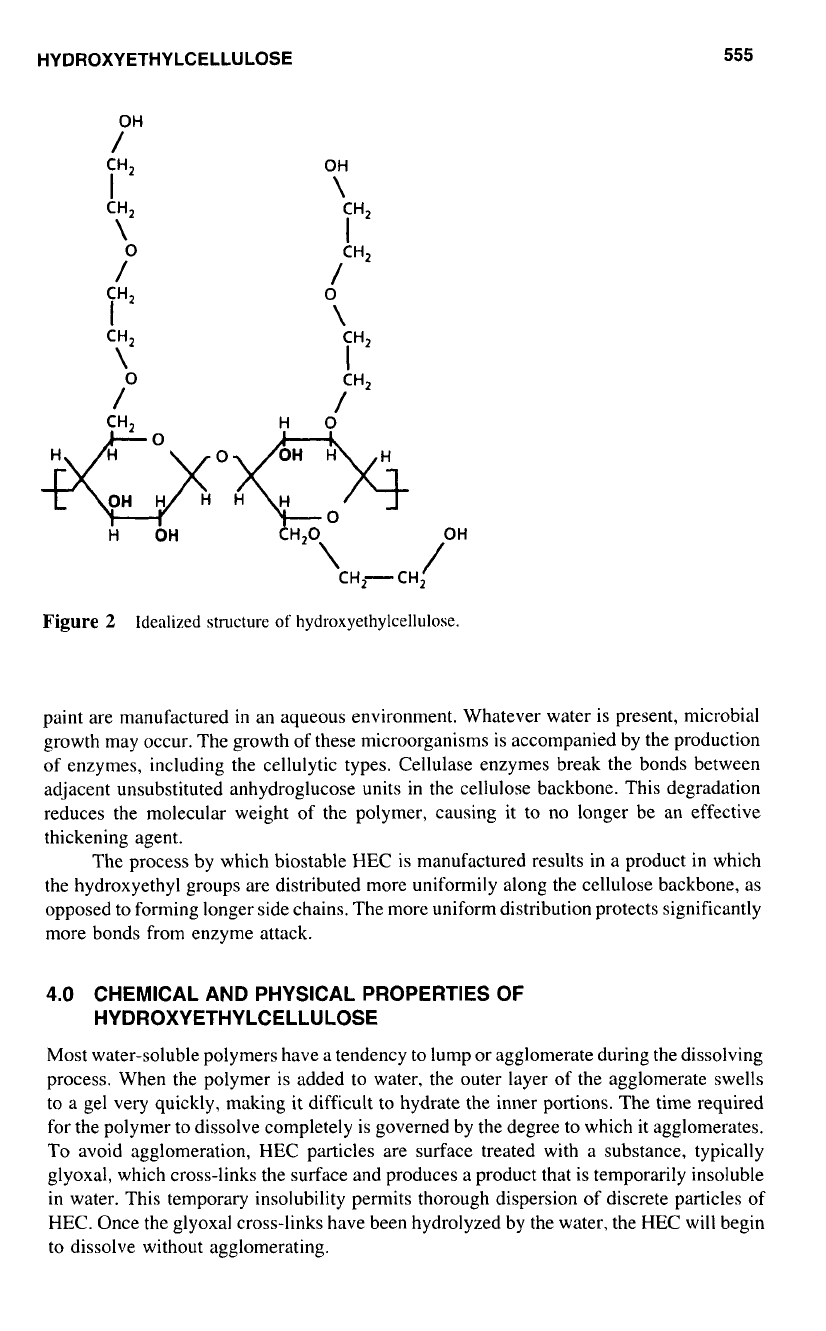
because of its optimal water solubility. An idealized structure of HEC is shown in Figure
2.
3.0
TYPES AND GRADES
OF
HYDROXYETHYLCELLULOSE
By controlling the chain length of the cellulose backbone, HEC can be produced in a
wide range of viscosity types. However, economics indicate that medium
(2%
Brookfield
viscosity
=
4500-6500
cPs (mPa.s at 25°C) or higher viscosity grades are used
to
prevent
sagging), and they can improve stability
of
some latex paint formulations. Most paints
require
0.1-1%
by weight of HEC to be thickened to the desired viscosity.
Hydroxyethylcellulose is also produced in
a
grade having superior biostability. Water
is the continuous phase in latex paints. In addition, many of the components of
a
latex
HYDROXYETHYLCELLULOSE
555
OH
CH,
I
CH,
\
0
/
iH2
CH,
\
Go
H
OH
CH,- CH, /OH
Figure
2
Idealized
structure
of
hydroxyethylcellulose.
paint are manufactured
in
an aqueous environment. Whatever water is present, microbial
growth may occur. The growth
of
these microorganisms is accompanied by the production
of enzymes, including the cellulytic types. Cellulase enzymes break the bonds between
adjacent unsubstituted anhydroglucose units
in
the cellulose backbone. This degradation
reduces the molecular weight
of
the polymer, causing
it
to no longer be an effective
thickening agent.
The process by which biostable HEC is manufactured results in
a
product
in
which
the hydroxyethyl groups are distributed more uniformily along the cellulose backbone, as
opposed to forming longer side chains. The more uniform distribution protects significantly
more bonds from enzyme attack.
4.0
CHEMICAL AND PHYSICAL PROPERTIES
OF
HYDROXYETHYLCELLULOSE
Most water-soluble polymers have
a
tendency to lump or agglomerate during the dissolving
process. When the polymer is added to water, the outer layer of the agglomerate swells
to
a
gel very quickly, making it difficult to hydrate the inner portions. The time required
for the polymer to dissolve completely is governed by the degree to which it agglomerates.
To
avoid agglomeration, HEC particles are surface treated with a substance, typically
glyoxal, which cross-links the surface and produces a product that
is
temporarily insoluble
in water. This temporary insolubility permits thorough dispersion
of
discrete particles of
HEC. Once the glyoxal cross-links have been hydrolyzed by the water, the HEC will begin
to
dissolve without agglomerating.
556
1000
.G
100
E
1
BURMEISTER
2345678910
pH
of
Buffered
Water
Figure
3
Effect
of
pH
and
temperature
on
the
hydration
time
of
HEC.
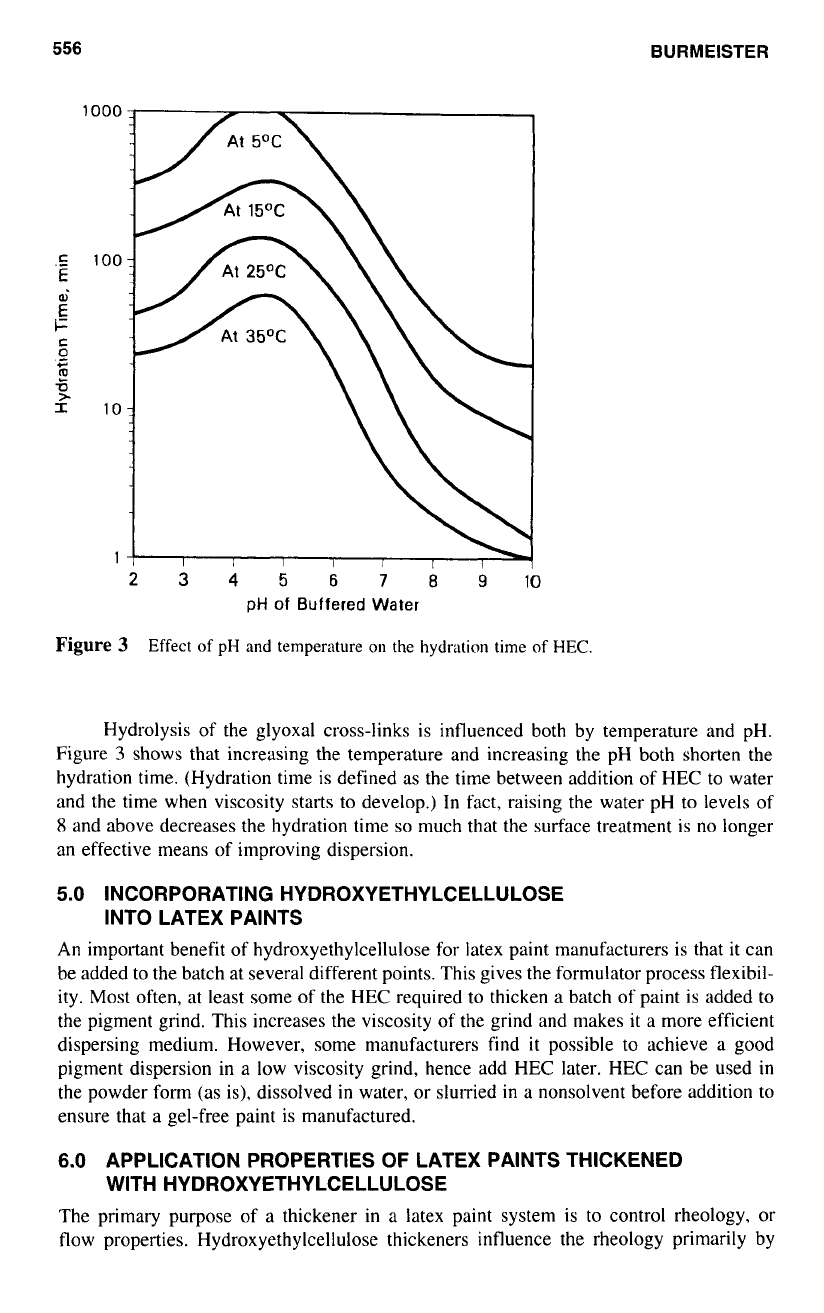
Hydrolysis of the glyoxal cross-links is influenced both by temperature and pH.
Figure
3
shows that increasing the temperature and increasing the pH both shorten the
hydration time. (Hydration time is defined
as
the time between addition of HEC to water
and the time when viscosity starts
to
develop.) In fact, raising the water pH
to
levels of
8
and above decreases the hydration time
so
much that the surface treatment is no longer
an effective means of improving dispersion.
5.0
INCORPORATING HYDROXYETHYLCELLULOSE
INTO LATEX PAINTS
An
important benefit
of
hydroxyethylcellulose for latex paint manufacturers is that
it
can
be added
to
the batch at several different points. This gives the formulator process flexibil-
ity. Most often, at least some of the HEC required to thicken
a
batch of paint is added
to
the pigment grind. This increases the viscosity
of
the grind and makes
it
a
more efficient
dispersing medium. However, some manufacturers find
it
possible to achieve a good
pigment dispersion in a low viscosity grind, hence add HEC later. HEC can be used in
the powder form (as is), dissolved in water, or slulried in
a
nonsolvent before addition to
ensure that
a
gel-free paint is manufactured.
6.0
APPLICATION PROPERTIES
OF
LATEX PAINTS THICKENED
WITH HYDROXYETHYLCELLULOSE
The primary purpose of
a
thickener in a latex paint system is
to
control rheology, or
flow properties. Hydroxyethylcellulose thickeners influence the rheology primarily by
HYDROXYETHYLCELLULOSE
557
thickening the aqueous phase. Fairly strong hydrogen bonds are formed between the poly-
mer chain and water molecules. The resulting chain association forms
a
concentrated
network. Because HEC thickens the water
only.
its efficiency is independent of the latex,
surfactant, or dispersant type. Formulation insensitivity is the greatest benefit
of
HEC.
Also,
very little viscosity change or phase separation seen in shelf stability, heat stability. or
freeze-thaw stability testing is attributable due to the aqueous phase thickening mechanism.
Sections
6.1-6.3
describe the effect of molecular weight or viscosity type
of
HEC on the
important properties of leveling, sag resistance, film build, and spatter resistance.
6.1
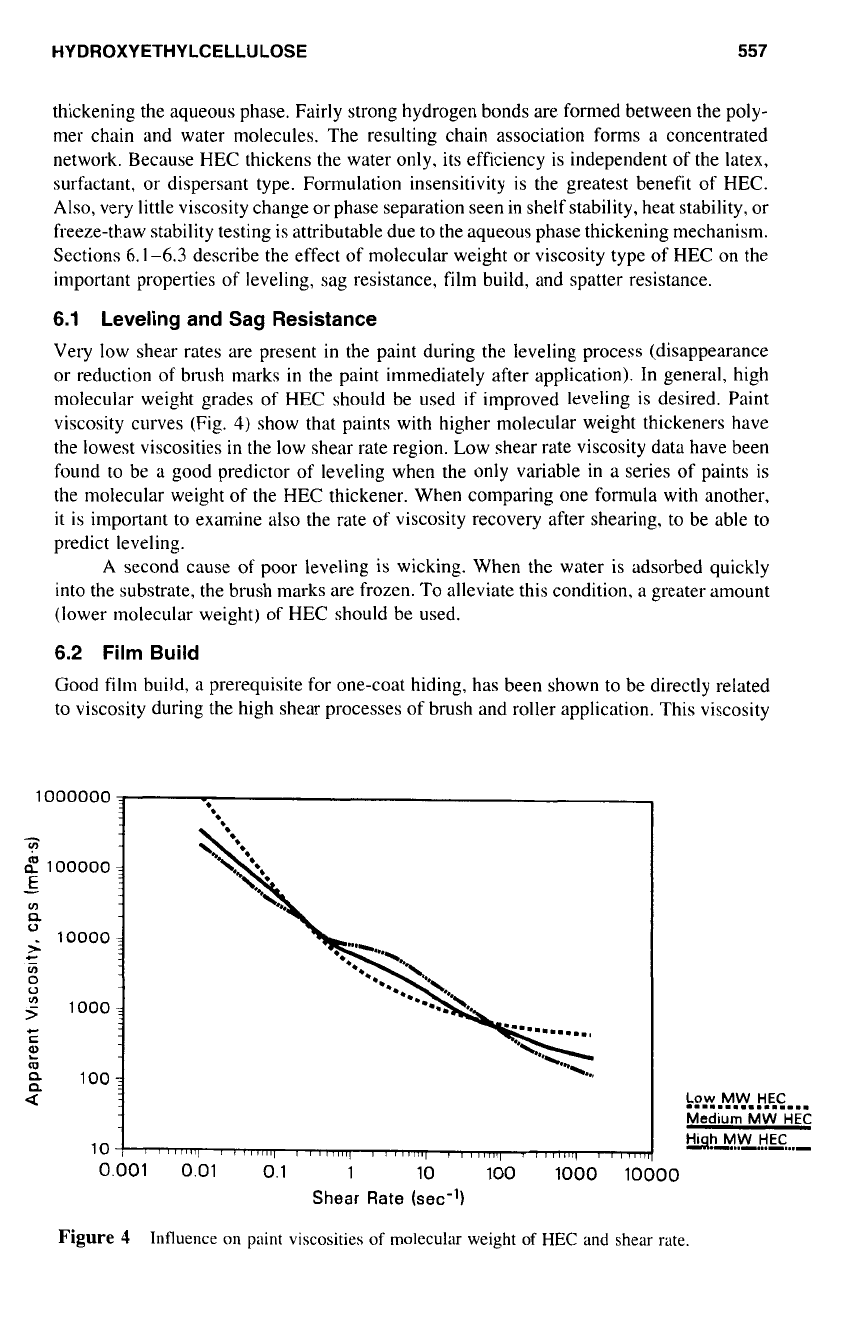
Leveling and Sag Resistance
Very low shear rates are present in the paint during the leveling process (disappearance
or reduction
of
brush marks in the paint immediately after application). In general, high
nlolecular weight grades of HEC should be used
if
improved leveling is desired. Paint
viscosity curves (Fig.
4)
show that paints with higher molecular weight thickeners have
the lowest viscosities in the low shear rate region. Low shear rate viscosity data have been
found to be
a
good predictor
of
leveling when the only variable
in
a
series
of
paints is
the molecular weight of the HEC thickener. When comparing one formula with another,
it is important
to
examine
also
the rate of viscosity recovery after shearing, to be able
to
predict leveling.
A
second cause
of
poor leveling is wicking. When the water is adsorbed quickly
into the substrate, the brush marks are frozen.
To
alleviate this condition,
a
greater amount
(lower molecular weight) of HEC should be used.
6.2
Film
Build
Good film build,
a
prerequisite for one-coat hiding, has been shown to be directly related
to viscosity during the high shear processes of brush and roller application. This viscosity
0.001
0.01
0.1
1
10
100
1000
10000
Shear Rate (sec")
Figure
4
Influence on paint viscosities
of
molecular weight
of
HEC
and shear rate.

558
BURMEISTER
is controlled by thickener concentration. Therefore, to increase the film build of a formula
while maintaining the Stormer viscosity, a greater amount of
a
low molecular weight
HEC
should be used.
6.3 Spatter
Resistance
Spatter resistance,
also
a
high shear application property, does not depend on thickener
viscosity, but on the elasticity
of
the aqueous phase. Like film build, spatter resistance
can be predicted from the polymer solution rheology. Higher molecular weight thickeners
are more elastic, and consequently impart elasticity to the paint. Paint made from lower
molecular weight grades of
HEC
is more resistant to spatter during roller application.
During roller application, threads of paint are pulled off the nip of the roller. When
the thickener has some degree of elasticity, these threads are stabilized and can be stretched
farther before breaking. More spatter is created when these threads are stabilized and
stretched than if they break close to the nip of the roller.
REFERENCE
1.
K.
G.
Shaw
and
D.
P.
Leipold,
J.
Corrtirlgs
Teclmol.,
57
(727), 63-72.

Antistatic and Conductive Additives
1.0
CONDUCTIVITY ADDITIVES FOR SPRAY PAINTS
In
the electrostatic spray technique, the atomized paint particles receive
a
high voltage in
the “gun.” Subsequently the paint particles are attracted to the grounded workpiece,
resulting in highly efficient paint utilization with good coverage
of
any awkward areas
(Fig.
1).
Paints formulated largely from nonpolar solvents have an electrical resistance that
is far too high for satisfactory application in this way. It is necessary to reduce the paint
resistance below
1
Ma,
so
very large proportions
of
polar solvent would be necessary;
therefore a search for more efficient chemical additive revealed that some quaternary
ammonium compounds (Fig.
2)
are particularly effective alternatives.
Choosing the best quaternary ammonium compound involved the investigation of
various chemical structures, molecular weights, and anions. Using commercially available
blends
of
raw materials and avoiding potentially corrosive ions, it has been possible to
develop
a
particularly effective product. namely Catafor CA. Bearing in mind the customer
preference for pourable liquids, an
80%
solution in butanol is normally used to give very
low resistivities with small additions, as illustrated with xylene in Figure
3.
When using Catafor CA in paints, it is advantageous
to
add
a
modest amount
of
polar solvent because
a
synergistic effect is usually observed (Table
1).
The advantages of using quaternary ammonium salts
as
a
conductivity additive can
be summarized
BS
follows:
I.
Catafor CA, containing quaternary ammonium salts. is compatible with most
2.
The fluid
80%
solution is quickly and easily dissolved.
3.
Only low dosage is needed to produce
a
large drop in resistivity
(0.5-3%
in
types of paint thus avoiding reformulation.
practice).
559
560
DAVIS
Figure
1
Schematic diagram
of
a electrostatically charged spray.
Figure
2
Structure
of
quaternary ammonium compounds.
a,
V
c
m
m
m
LT
a,
c
.-
0.5.
0.4
0.3
0.2
'
0.1
'
12345
O/!oCATA
FOR
CA80
Figure
3
Effcct
of
quaternary ammonium salt
011
electrical resistancc in paint.
ANTISTATIC AND CONDUCTIVE ADDITIVES
561
Table
1
Effect of Catafor CA
in
a Long Oil Alkyd Paint
Resistance
Pam-solvent combination
(Mn)
Paint
+
hydrocarbon solvent
20
Paint
+
hydrocarbon solvent
+
1%
Catafor CA80
1.8
Paint
+
hydrocarbon solvcnt
+
10%
mcthyl cthyl ketone
+
1%
Catafor CA80 0.29
4.
The same low dosage will reduce surface tension to a suitably low value.
5.
Yellowing effects are not usually observed. even in sensitive white paints.
6.
Effects on the paint film properties (evenness. general appearance, hardness.
corrosion resistance. etc.) are minimal.
2.0
ANTISTATIC ADDITIVES
FOR
PLASTIC MATERIALS
The many adverse effects of static electricity
on
insulating plastic materials are well
recorded.'.' These include textile processing difficulties. poor sound reproduction from
records. excessive dust and dirt collection on surfaces, and dust or solvent explosion
hazards. One
of
the most important hazards associated with modern technology is electro-
static discharge, which can cause critical damage to microchip circuits.
so
essential in
electronics.
Various types of antistatic agent are reviewed in the literature,' and
it
appears that
surfactant molecules are particularly effective. Nonionic, anionic, cationic, and even am-
pholytic types have been used as internal antistatic agents. depending on the type
of
polymer. For example, nonionics are recommended for polyolefins, but cationics are partic-
ularly effective
in
the more polar polymers.' such as polyvinyl chloride, polystyrenes. and
polyurethanes.
The large intluence
of
relative humidity on the effectiveness of antistatic agents
suggests that a film of water is held on the plastic surface by the presence
of
the ionized
or polar groups of the surfactant molecular projecting from the surface, with the hydropho-
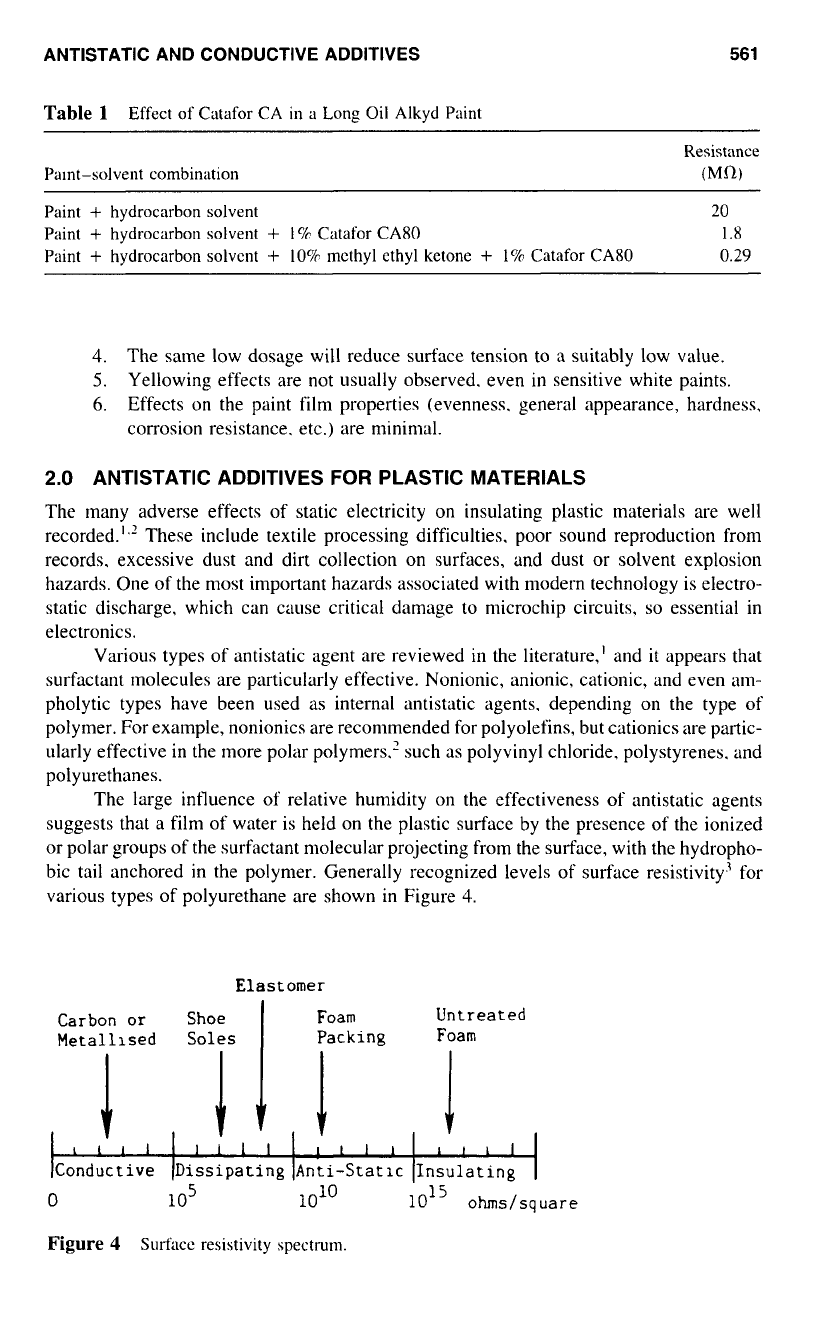
bic tail anchored in the polymer. Generally recognized levels of surface resistivity3 for
various types of polyurethane are shown
in
Figure
4.
Elastomer
Carbon
Meta,lsed
or
Shoe
Sol,
I
,king
Foam
Untreated
F,
1111
Insulating
Dissipating \Anti-Statlc
Conductive
IIII
1111
I111
0
lo5
1o1O
otms/square
Figure
4
Surfacc resistivity spectrum
