Satas D., Tracton A.A. (ed.). Coatings Technology Handbook
Подождите немного. Документ загружается.


61
2
VERNARDAKIS
Figure
2
Scanning electron photomicrograph showing primary particles: (a)
individual
crystals
and
(b)
associated crystals
of
micronized NaCl.
sodium chloride (although this is not a pigment) is used only to illustrate the individual
and associated crystals that make up the primary particles of a compound.
Aggregates are collections
of
primary particles that are attached to each other at
their surfaces
or
crystal faces and show a tightly packed structure. Agglomerates consist
of primary particles and aggregates joined at the comers and edges in a looser
type
of
arrangement. Aggregates are formed during the manufacturing process in the course
of
the ripening period of the precipitates. Agglomerates, most often, are formed during the
drying of the presscakes and the subsequent dry milling of the pigment lumps. Figure
3
shows typical arrangements of aggregated and agglomerated pigment particles.
Flocculates consist
of
primary particles, aggregates, and agglomerates, generally
arranged in a fairly open structure, as shown in Fig.
4.
Flocculates may be broken down
easily under shear, but they will form again when such shear forces are removed and the
dispersion is allowed to stand undisturbed.
3.0
THE DISPERSION PROCESS
The primary purpose of dispersion
is
to break down pigment aggregates and agglomerates
to their optimum pigmentary particulate size (down to individual single particles, if possi-
ble) and to distribute these pigment particles evenly throughout a medium (i.e., the carrier).
Usually the carrier is a liquid
or
a solid polymeric material that is deformable at high
temperatures during processing.
To
achieve the optimum benefits of a pigment, both visual
and economic, it is necessary to obtain
as
full a reduction as possible to the primary
particle size. After all, the color strength of a pigment depends on its exposed surface

PIGMENT DISPERSION
61
3
!
c
!
Figure
3
Transmission electron photomicrograph showing (a) aggregated and
(b)
agglomerated
pigment particles. D&C Red
No.
30,
Vat Red
1.
p:
i
Figure
4
Transmission electron photomicrograph showing flocculated pigment particles. Dimeth-
ylquinacridone magenta, Pigment Red
122.

61
4
VERNARDAKIS
area: the smaller the particle size, the higher the surface area, and thus the stronger the
color. Furthermore, the pigment is generally the most expensive constituent of any pig-
mented system; therefore the user normally wants to obtain optimum performance with
the smallest possible amount of pigment. Ideally. a good pigment dispersion consists
chiefly
of
primary particles, with only a minimum of loose aggregates and agglomerates.
In
practice, reduction to the primary particle size is largely determined by the nature of
the pigment (i.e., its dispersibility). by the dispersion system and processing equipment,
and by the end-use requirements of the product.
Dispersion should not be confused with pulverization. The latter is simply a commi-
nution process whereby large pigment lumps are broken down to smaller units, which
constitute the powder form. Pulverization does not break down the aggregated, agglomer-
ated. and flocculated particles into primary particles. Dispersion, however, accomplishes
this effectively.
3.1 Pigment Wetting
It is generally recognized that the dispersion process consists of three distinct stages:
wetting,
deaggregation-deagglomeration,
and stabilization. The wetting stage involves
the removal from the surface
of
the pigment particles of adsorbed molecules
of
gas, liquid,
and other materials and their replacement with molecules of the vehicle. In other words,
the pigment-air interface in dry pigment powders or the pigment-water interface in
presscakes is replaced by the pigment-vehicle interface. This is accomplished through
preferential adsorption. The efficiency of wetting depends primarily on the comparative
surface tension properties
of
the pigment and the vehicle, as well as the viscosity of the
resultant mix.
3.2 Particle Deaggregation and Deagglomeration
After the initial wetting stage, it is necessary to deaggregate and deagglonlerate the pigment
particles. This is usually accomplished by mechanical action with devices such as ball
mills, bead mills, and two-roll mills. As the pigment powder is broken down to the
individual particles. higher surface areas become exposed
to
the vehicle and larger amounts
of
it
are required to wet out newly formed surfaces. During this stage of deaggregation,
the amount of free vehicle
in
the bulk diminishes; therefore, the viscosity
of
the dispersion
increases. At higher viscosities, shear forces are greater and the breaking down and separa-
tion of particles become more efficient. It is this process of mechanical breakdown of the
aggregates and agglomerates that demands a high energy input and can become quite
costly. Some easily dispersible pigments have been developed lately to aid
in
the reduction
of
energy requirements. Such pigments are produced by surface treatment of the pigment
during manufacture, with the purpose of reducing or inhibiting
agglomeration-aggregation
formation. In many cases, such treatments are highly specific to
a
single ink, paint, coating,
or plastic medium.
3.3 Dispersion Stabilization
The third stage
of
great importance in the dispersion process is the stabilization
of
the
pigment dispersion. This ensures that complete wetting and separation of the particles has
been reached, and also that the pigment particles are homogeneously distributed
in
the
medium. If the dispersion has
not
been stabilized, flocculation may occur
as
a result of

PIGMENT DISPERSION
61
5
clumping together of the pigment particles. Flocculation is generally a reversible process.
Flocculates typically break down when shear is applied and will form again when the
shear is removed. Where a pigment dispersion is not stabilized by the action of resin
molecules in the vehicle, the use of surfactants or polymeric dispersants can be considered.
Such additives may be used directly during pigment manufacture, or they may be incorpo-
rated
in
the vehicle.
4.0
THE
ROLE
OF SURFACE
ENERGY
It is well known that molecular forces at the surface of a liquid are
in
a state of imbalance.
The same is true of the surface of a solid, where the molecules or ions on the surface are
subject
to
unbalanced forces of attraction normal to the surface plane. Such atoms do not
have
all
their forces satisfied by union with other atoms.
As
a result. there is a net force.
which tends to pull the surface molecules into the bulk. The opposing force, which resists
this inwardly pulling force, is known as the surface tension or surface energy.
All
liquids
and solids have surface energies
to
a greater or lesser degree.
To
satisfy these surface
forces, liquids and solids tend
to
attract and retain
on
their surfaces dissolved substances
in
the solution or gasses from the surrounding atmosphere. These forces are short-ranged
attractive forces. known as van der Waals or London forces, and they play a very important
role in particle aggregation. wetting. and dispersion stabilization.
4.1
Surface Energy and Surface Area
Pigments having a very small particle size exhibit high surface area and consequently
high surface energies.
As
large pigment particles are broken down into several smaller
particles, new surfaces are constantly created, contributing to a higher surface area and
thus a higher surface energy.
Let
us
assume that a pigment powder has a surface area
S
of 60 m'/g and a density
p
of 1.0 g/cm3. Its basic particle diameter
D
from
will be
0.1
pm. If these particles are cubic in structure. and if, for the sake of simplicity,
we assume that a
1
cmz
of
pigment is broken down into particles
0.1
pm
in size. then
I
X
1015
particles will be produced. We assume
also
that the particles are
in
perfect cubic
packing.
To
get an idea of the area created by the new surface, we need only compare
the surface area of
6
cm' for the
1
cm cubic particle to the surface area of 600,000 cm'
(60 m') for the
1
X
10"
cubic particles that are
0.1
pm in size. The increase in surface
area is 100.000-fold. The new surfaces produced are tremendously large. The surface
energies associated with these new surfaces are
also
quite large. These van der Waals
surface energies create the attraction between the submicrometer particles that come to-
gether to form the aggregates and agglomerates.
4.2
Surface Energy and Pigment Wetting
Surface energies play an important role
in
the wetting and stabilization of pigment disper-
sions. For wetting
to
be effective, the wetting energies of the pigment-vehicle interface
must be greater than the sum of the adsorption energy (this is because of substances

61
6
VERNARDAKIS
Figure
5
Partial wetting
of
a
solid surface
by
a
liquid in accordance
with
the Young-DuprC
equation.
adsorbed
on
the pigment surface) and the attractive energy that holds the pigment particles
together. Generally, lower energy (low surface tension) liquids, such as aliphatic and
aromatic hydrocarbons, will spread over, or wet, higher energy surfaces. Quite often, it
happens that a liquid does not spread over a pigment surface completely. This occurs
when a high-energy liquid (high surface tension), such as water, will not entirely wet out
a high-energy surface.
In
this case, the wetting energy is equal to or less than the sum of
the adsorption and interparticle attraction energies, and wetting may be either partial or
nonexistent. The liquid will
not
spread entirely over the surface, as shown
in
Fig.
5.
The
relationship that describes such a system is given by the Young-Duprt equation as
ysv
=
YSL +
yr*v.cos
0
where ysv,
ysL,
and
yr.,,
are the interfacial energies at the solid-vapor, solid-liquid, and
liquid-vapor interfaces, respectively, and
0
is the contact angle. For complete wetting,
the contact angle is zero (cos
0
becomes unity), and the liquid spreads entirely over the
solid surface. For
0
>
0,
wetting either is incomplete or does not occur at all.
4.3
Surface Energy and Destabilization
of
the Dispersion
Surface energies play an important role in the destabilization of the dispersion. Particles
dispersed
in
luqid media are in constant motion (thermal or Brownian movement).
As
they move through the medium, they collide with other pigment particles. The frequency
of these collisions depends
on
the size
of
the particles and on the viscosity of the medium.
During such collisions, the particles will attract and may join with other particles because
of the powerful short-range London-van der Waals attractive forces, which in effect are
surface energies. These forces are electrical and are due
to
the interaction of the dipoles
that are present in the particles, as permanent dipoles (polar particles) or inducted dipoles
(nonpolar but polarized particles).
Once the particles have come together, they may reaggregate or form flocculates if
their surface is not protected, and they will settle to the bottom
of
the container. This is
an undesirable effect for the ink, paint, or coatings manufacturer. Therefore, to prevent
reaggregation or flocculation, such dispersions must be stabilized.
4.4
Surface Energy and the Acid-Base Concept
The idea of surface energy in pigments has been closely related
to
the acid-base concept,
advanced by Sorensen," who has used it
to
describe the interaction between pigments,

PIGMENT
DISPERSION
61
7
binders, and solvents, to obtain optimally stable pigment dispersions having the best appli-
cation properties
in
fluid
ink
systems. Such interrelation between the surface energies of
these three components
in
a dispersion can be characterized by their acid-base properties.
Pigments can be classified
as
acidic (electron acceptors), basic (electron donors), ampho-
teric (electron acceptors and donors), or neutral. Binders and solvents can be similarly
characterized.
Acidic pigments should be used with basic resins (polyamide, melamine. alkyd).
while basic pigments should be used with acidic resins (vinyl, acrylic, maleic). Ampho-
terics can be used with both resins. Neutral pigments should be surface treated to improve
their dispersion characteristics. The solvent must have the same acid-base character as the
pigment, whereby the interaction between the solvent and the pigment surface is minimized
and at the same time the interaction between the resin binder and the pigment surface is
maximized.
In
other words, there should be no competition between solvent and binder
for the pigment surface; to obtain maximum dispersion and stability. only the binder should
adsorb.
5.0
MECHANISMS
FOR
THE STABILIZATION
OF
DISPERSION
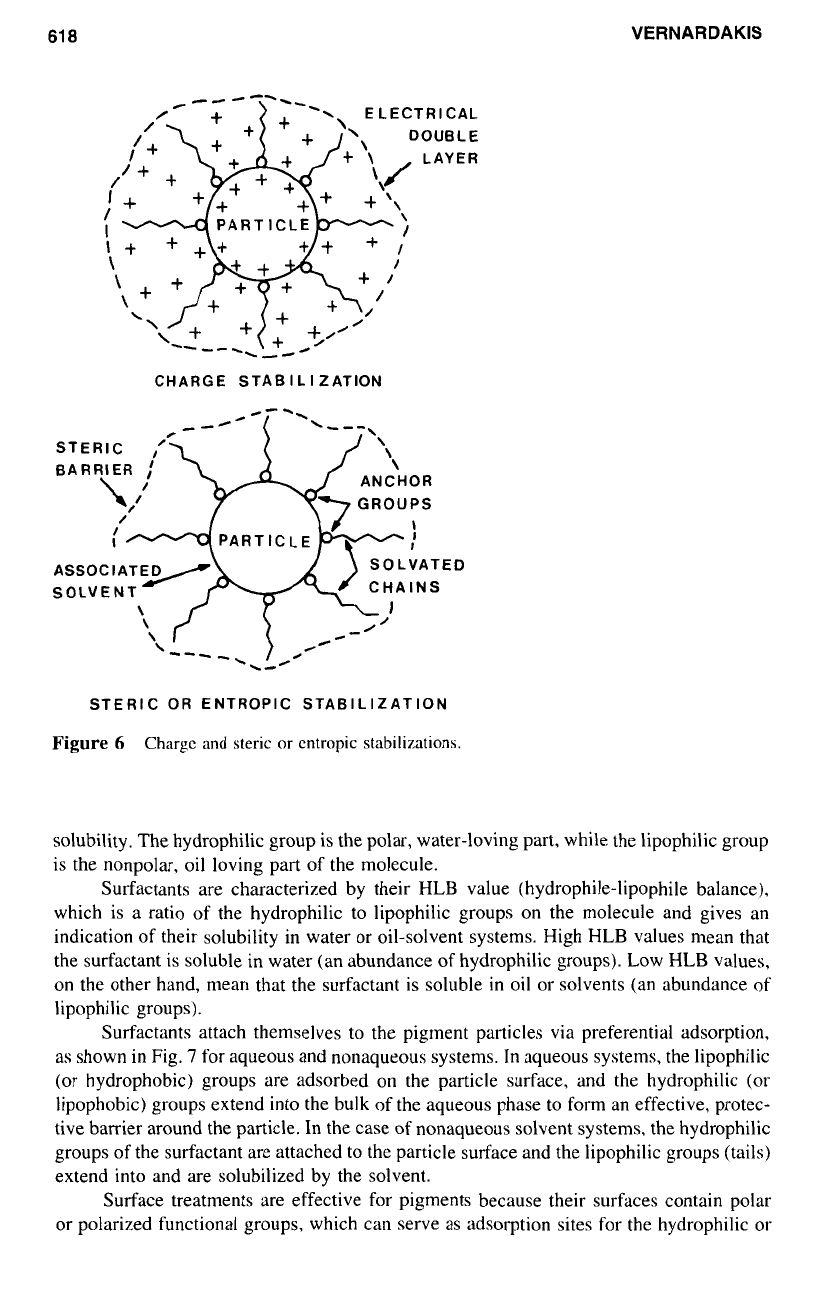
5.1 Charge Stabilization
Dispersions may become stable through two generally accepted mechanisms: charge stabi-
lization and steric or entropic stabilization. Charge stabilization is due to electrical repul-
sion forces, which are the result
of
a
charged electrical double layer surrounding the
particles as shown
in
Fig.
6.
The charged electrical double layer developed around the
particles extends well into the liquid medium, and since all the particles are surrounded
by the same charge (positive or negative), they repel each other when they come into
close proximity.
5.2
Steric or Entropic Stabilization
Steric stabilization is due to steric hindrance resulting from the adsorbed dispersing agent,
the chains
of
which become solvated in the liquid medium, thus creating an effective steric
barrier that prevents the other particles from approaching too close. This phenomenon is
also called entropic stabilization because, as the coated particles approach each other, the
solvated chains
of
the adsorbed dispersant lose some of their degrees of freedom, resulting
in
a
decrease
in
entropy. Such lowering in entropy gives rise to repulsive forces, which
keep the particles away from each other. This type of steric or entropic stabilization is
also represented
in
Fig.
6.
6.0 SURFACE TREATMENT
6.1 Surfactants
Surface active agents or, simply, surfactants are substances that are used to lower the
interfacial tension between
a
liquid and
a
solid. Such is the case for pigments
in
fluid
media, with the expressed purpose of improving pigment dispersibility by improving pig-
ment wetting characteristics, preventing reaggregation, and increasing the stability
of
the
dispersion. A surfactant molecule typically contains two groups of opposite polarity and
61
8
VERNARDAKIS
”-”
-\
ELECTRICAL
CHARGE STAB
ILI
ZATION
‘E
D
STERIC
OR
ENTROPIC STABILIZATION
Figure
6
Chnrgc
and
steric or cntropic stabilizations.
solubility. The hydrophilic group is the polar, water-loving part, while the lipophilic group
is the nonpolar, oil loving part of the molecule.
Surfactants are characterized by their HLB value (hydrophile-lipophile balance),
which is a ratio of the hydrophilic to lipophilic groups on the molecule and gives an
indication of their solubility in water or oil-solvent systems. High HLB values mean that
the surfactant is soluble in water (an abundance
of
hydrophilic groups). Low HLB values,
on
the other hand, mean that the surfactant is soluble
in
oil or solvents (an abundance of
lipophilic groups).
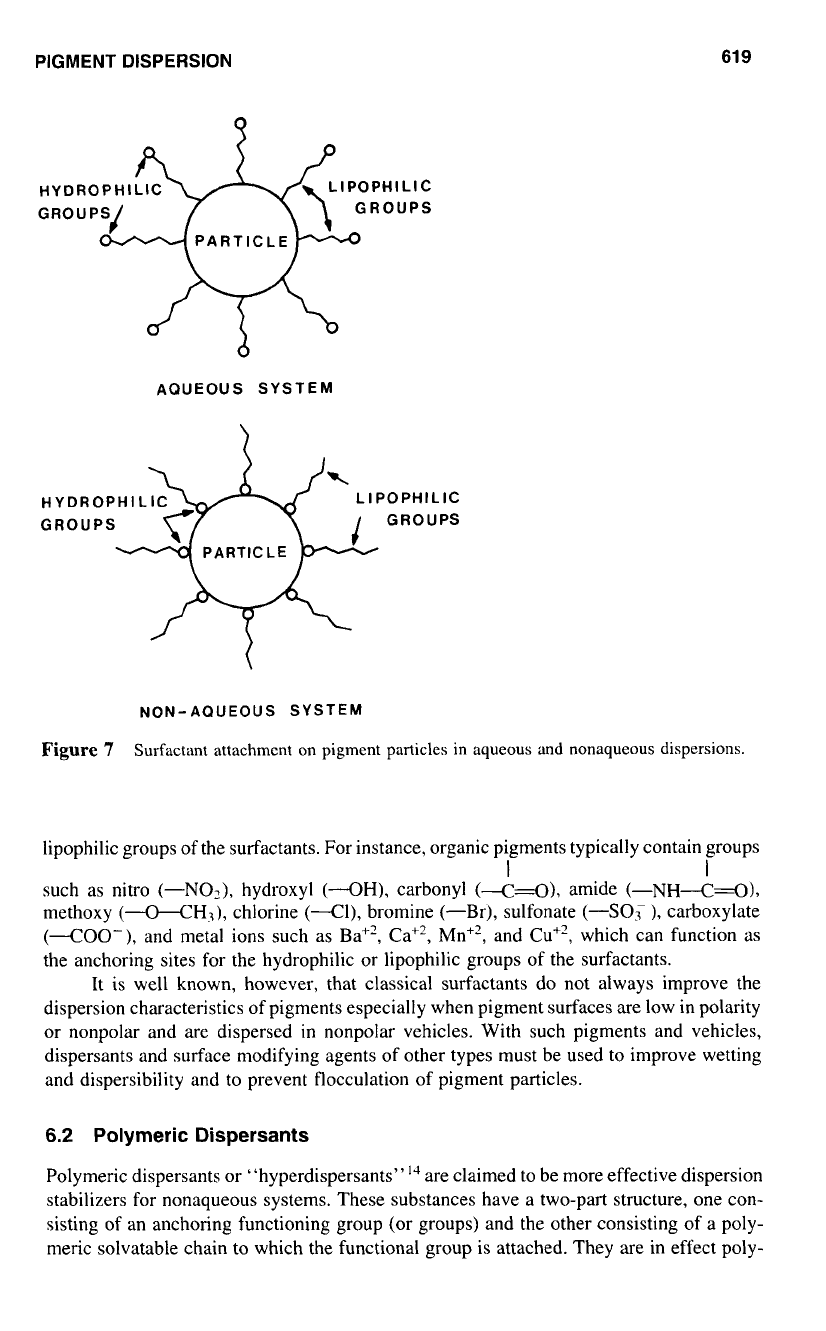
Surfactants attach themselves to the pigment particles via preferential adsorption,
as shown in Fig.
7
for aqueous and nonaqueous systems. In aqueous systems, the lipophilic
(or hydrophobic) groups are adsorbed
on
the particle surface, and the hydrophilic (or
lipophobic) groups extend
into
the bulk of the aqueous phase to form an effective, protec-
tive barrier around the particle. In the case
of
nonaqueous solvent systems, the hydrophilic
groups of the surfactant are attached to the particle surface and the lipophilic groups (tails)
extend into and are solubilized by the solvent.
Surface treatments are effective for pigments because their surfaces contain polar
or polarized functional groups, which can serve as adsorption sites for the hydrophilic
or
PIGMENT
DISPERSION
Q
61
9
LIPOPHILIC
GROUPS
PARTICLE
d
AQUEOUS SYSTEM
HYDROPHILIC LIPOPHILIC
GROUPS GROUPS
PARTICLE
NON-AQUEOUS SYSTEM
Figure
7
Surfactant attachment on pigment particles in aqueous
and
nonaqueous dispersions.
lipophilic groups
of
the surfactants. For instance, organic pigments typically contain groups
such as nitro
(“NO?),
hydroxyl
(”OH),
carbonyl
(.-C=O),
amide
(-NH-C=O),
methoxy
(“CH3),
chlorine
(-Cl),
bromine (-Br), sulfonate
(“SO,
),
carboxylate
(“COO-),
and metal ions such
as
Ba+’, Ca+*, Mn+’, and Cu+*, which can function
as
the anchoring sites for the hydrophilic or lipophilic groups of the surfactants.
It
is
well known, however, that classical surfactants do not always improve the
dispersion characteristics of pigments especially when pigment surfaces are low in polarity
or nonpolar and are dispersed in nonpolar vehicles. With such pigments and vehicles,
dispersants and surface modifying agents of other types must be used
to
improve wetting
and dispersibility and to prevent flocculation
of
pigment particles.
I
l
6.2
Polymeric
Dispersants
Polymeric dispersants or “hyperdispersants”’4 are claimed to be more effective dispersion
stabilizers for nonaqueous systems. These substances have a two-part structure, one con-
sisting of an anchoring functioning group (or groups) and the other consisting of a poly-
nleric solvatable chain to which the functional group is attached. They are in effect poly-

620
VERNARDAKIS
meric surfactants or dispersants but were developed for use
in
specific nonaqueous systems,
where classical surfactants have limitations. When they are used as dispersants for organic
pigments, it is preferable that they have multiple anchoring groups on one polymeric chain,
because organic particles are not
as
strongly polar
as
inorganic particles. Such dispersants
may be of
a
fatty polyester type, containing
a
carboxy group at the end [e.g.. poly(l2-
hydroxystearic acid)] with the carboxy group functioning as the anchor and the polyester
group as the solvated chain. Others with multiple anchor groups are fatty polyureas and
polyurethanes, which may even contain polymeric solvatable groups instead of the long
fatty chains.
6.3
Surface Modifying Agents
Surface modifying agents are another group of additives that can be used to aid the disper-
sion of organic pigments in organic media. These agents are often pigment derivatives
(e.g., large flat dye molecules), which provide improved resistance to flocculation and
greater stability to the dispersion. The pigment derivative is adsorbed onto the pigment
surface via the van der Waals attractive forces, which act over
a
large area, because such
large planar dye molecules lie flat on the pigment surface. They may be used either alone
or
in
conjunction with a polymeric dispersant. When used alone, they introduce or increase
on
the surface
of
nonpolar or low polarity pigments, the number of polar sites, which are
necessary to interact with the resin in the vehicles, to stabilize the dispersion. When used
together with the polymeric dispersant, they provide anchoring sites
on
which the anchor
groups of the dispersant will become attached. In this context. they can be used synergisti-
cally with dispersing agents, at which time they are called colored synergists.
7.0
SURFACE TREATMENT DURING PIGMENT MANUFACTURE
Generally, surface-treated pigments are more easily dispersible, produce more stable dis-
persions in fluid media with improved flow, and impart higher strength and gloss to the
printed films, when compared with untreated pigments. Surface treatments can be carried
out at different stages of pigment manufacture. Some pigments are prepared directly
as
finished products, while others are in the form
of
a
crude pigment that must be conditioned
into the pigmentary state.
Use of surfactants is typically made at the initial stage of pigment manufacture.
During the precipitation of the intermediate (e.g., diazo in the preparation of azo pigments),
surfactants are used to wet out and control the fineness of the precipitate, and they may
also
act
as
promoters to accelerate the
azo
coupling reaction. At the second stage, during
the precipitation of the pigment (e.g., in the azo coupling reaction), surfactants may be
used in the dispersion of the pigment particles
as
they are being formed-for example,
in
azo
yellows, which are precipitated in the pigmentary state, or
in
the dispersion of the
precursor (dyestuff),
as
in the case of metallized azo reds (which are first formed
as
sodium
salts), to control the salt formation (barium, calcium, etc.), and thus produce the final
pigment. At the third stage, during the conditioning of the pigment, surface treatments
are used for pigment particle dispersion, for coating the pigment surface to prevent aggrega-
tion, and for controlling the growth
of
crystal particles. If particles are too difficult to
filter, use of
a
specific additive (flocculant) sometimes induces controlled flocculation and
facilitates filtration. Complex formation with additives may also be carried out during this
conditioning stage to stabilize the particles and increase dispersibility,
as
is the case with
PIGMENT DISPERSION
621
diarylide yellows. which may be surface treated with fatty amines to produce Schiff base
stabilization and result
in
easily dispersible pigments.
8.0
SURFACE TREATMENT
OF
PIGMENTS: APPLICATION
8.1
Organic Pigments
The published literature dealing with surface treatments of organic pigments. patented or
otherwise, is
so
extensive that no attempt is made to review
it.
although
it
may be referred
to
occasionally. Readers are urged however. to consult the review by Hayes,15 which
covers the role
of
classical surfactants. polymeric dispersants, and pigment derivatives in
surface treatments. Other reviews of interest are those by Topham.'" Merkle and Schafer."
and Hampton and McMillan.'J the latter dealing specifically with polyrneric dispersants.
Further examples will be presented here.
It
is
well known
to
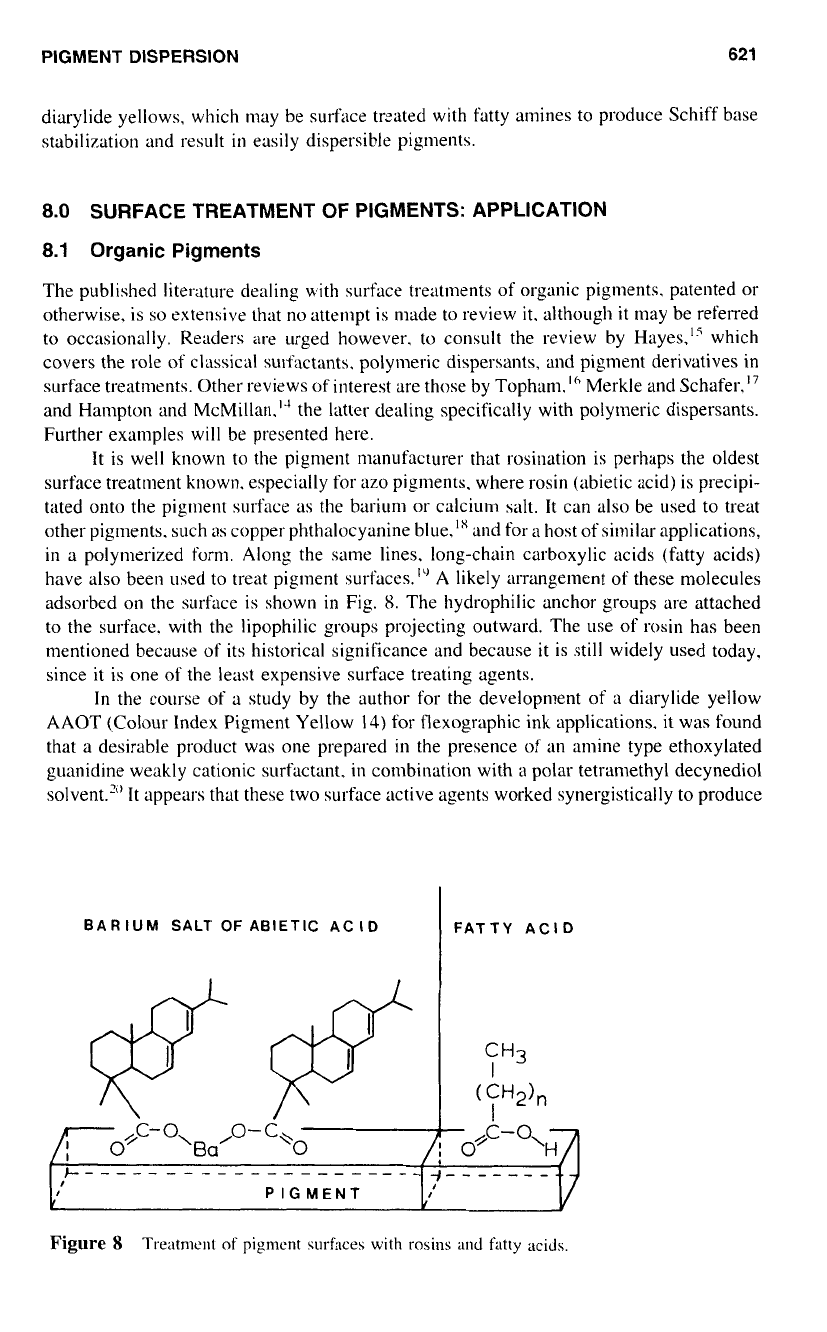
the pigment manufacturer that rosination is perhaps the oldest
surface treatment known. especially for
azo
pigments. where rosin (abietic acid) is precipi-
tated onto the pigment surface as the barium or calcium salt. It can also be used to treat
other pigments. such
as
copper phthalocyanine blue,ls and for
a
host
of
similar applications,
in a polymerized form. Along the same lines. long-chain carboxylic acids (fatty acids)
have also been used to treat pigment SUI-faces.'" A likely arrangement of these molecules
adsorbed
on
the surface is shown
in
Fig.
8.
The hydrophilic anchor groups are attached
to
the surface. with the lipophilic groups projecting outward. The use
of
rosin has been
mentioned because of its historical significance and because
it
is still widely used today,
since it is one
of
the least expensive surface treating agents.
In
the course of a study by the author for the development of a diarylide yellow
AAOT (Colour Index Pigment Yellow
14)
for tlexographic ink applications. it was found
that a desirable product was one prepared
in
the presence
of
;m
amine type ethoxylated
guanidine weakly cationic surfactant,
in
combination with
a
polar tetramethyl decynediol
solvent.'"
It
appears that these two surface active agents worked synergistically
to
produce
BARIUM SALT OF ABIETIC ACID
FATTY ACID
Figure
8
Treatment
of
pigmcnt
surfaces
with
rosins
and
fatty
acids.
