Satas D., Tracton A.A. (ed.). Coatings Technology Handbook
Подождите немного. Документ загружается.

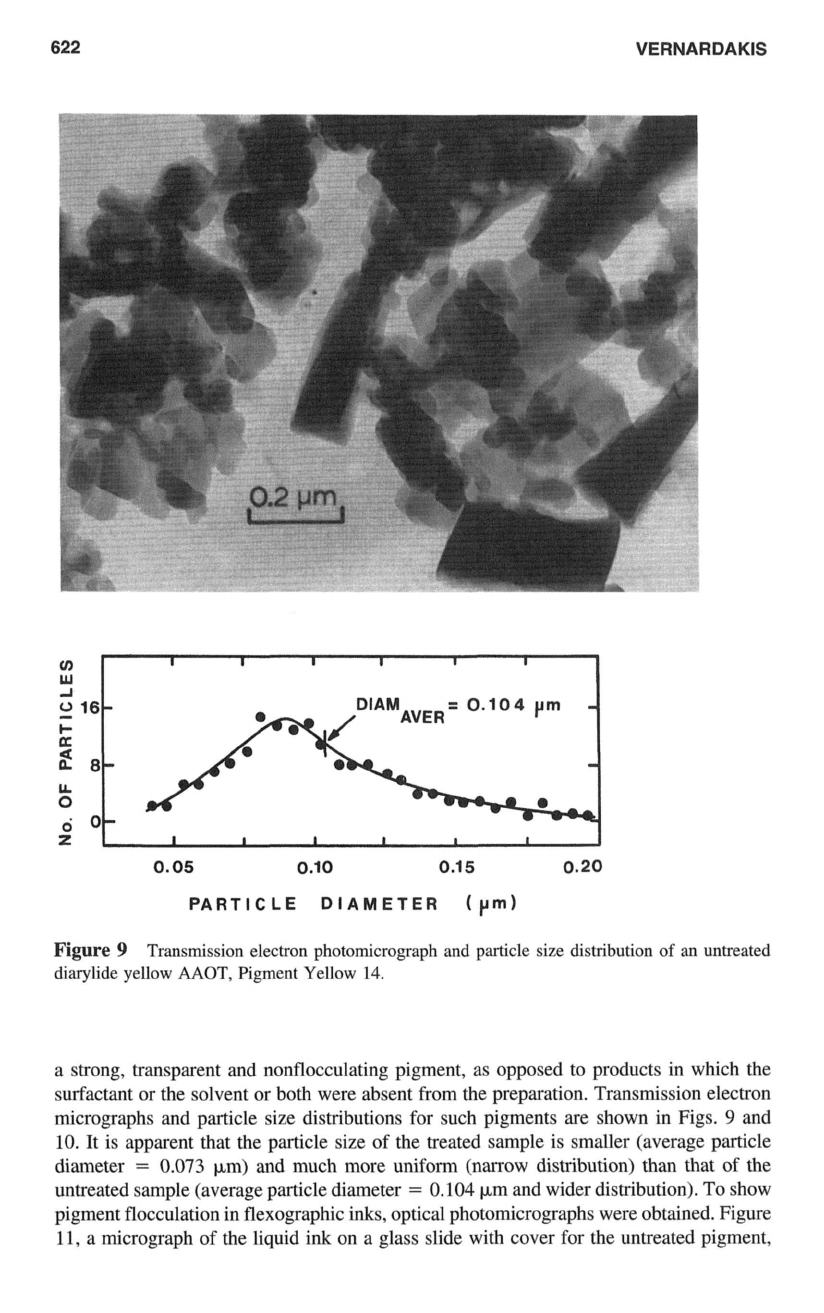
622
VERNARDAKIS
I
W
..l
0
-
16-
DIAMAVER=
0.104
pm
-
c
a
2
8-
0
zi
I
I
I
I
I
I
LL
0
0-
0.05
0.10 0.1
5
0.20
PARTICLE DIAMETER
(pm)
k;
Figure
9
Transmission electron photomicrograph and particle size distribution
of
an untreated
diarylide yellow
AAOT,
Pigment Yellow
14.
a strong, transparent and nonflocculating pigment, as opposed to products in which the
surfactant
or
the solvent
or
both were absent from the preparation. Transmission electron
micrographs and particle size distributions
for
such pigments are shown in Figs.
9
and
10.
It is apparent that the particle size
of
the treated sample is smaller (average particle
diameter
=
0.073
pm) and much more uniform (narrow distribution) than that of the
untreated sample (average particle diameter
=
0.104
pm and wider distribution).
To
show
pigment flocculation in flexographic inks, optical photomicrographs were obtained. Figure
11,
a micrograph
of
the liquid ink
on
a glass slide with cover
for
the untreated pigment,
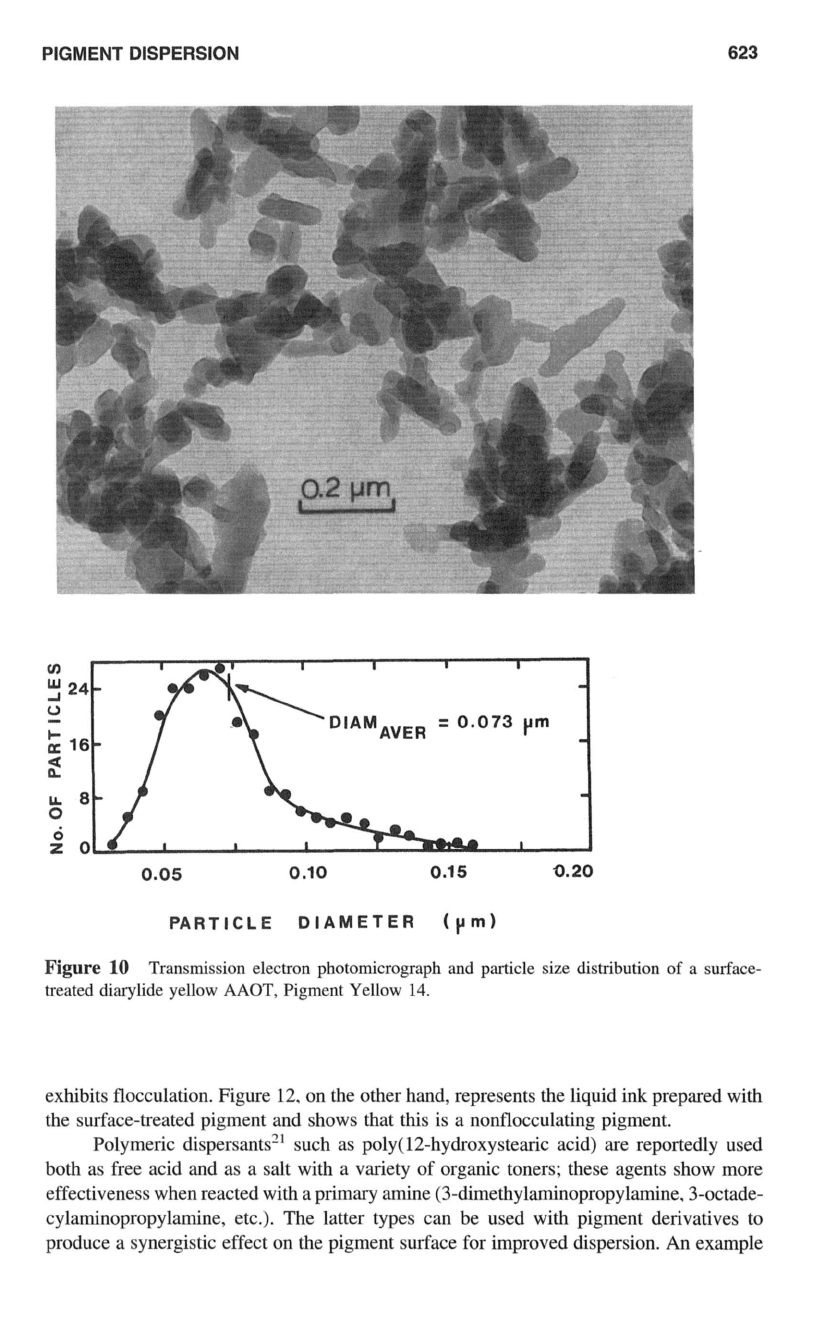
PIGMENT DISPERSION
623
”I
0.05
0.10
0.1
5
0.20
Figure
10
Transmission electron photomicrograph and particle size distribution
of
a surface-
treated diarylide yellow
MOT,
Pigment Yellow
14.
exhibits flocculation. Figure
12.
on
the other hand, represents the liquid ink prepared with
the surface-treated pigment and shows that this is a nonflocculating pigment.
Polymeric dispersants” such as poly( 12-hydroxystearic acid) are reportedly used
both as free acid and as a salt with a variety of organic toners; these agents show more
effectiveness when reacted with a primary amine
(3-dimethylaminopropylamine.
3-octade-
cylaminopropylamine, etc.). The latter types can be used with pigment derivatives to
produce a synergistic effect
on
the pigment surface for improved dispersion.
An
example

624
VERNARDAKIS
Figure
11
Optical photomicrograph
of
liquid ink prepared with untreated diarylide yellow
AAOT.
Shows flocculation
of
pigment particles. Same pigment as that
of
Fig.
9.
Figure
12
Optical photomicrograph
of
liquid ink prepared with surface-treated diarylide yellow
AAOT.
Does not show flocculation
of
pigment particles. Same pigment as that
of
Fig.
10.
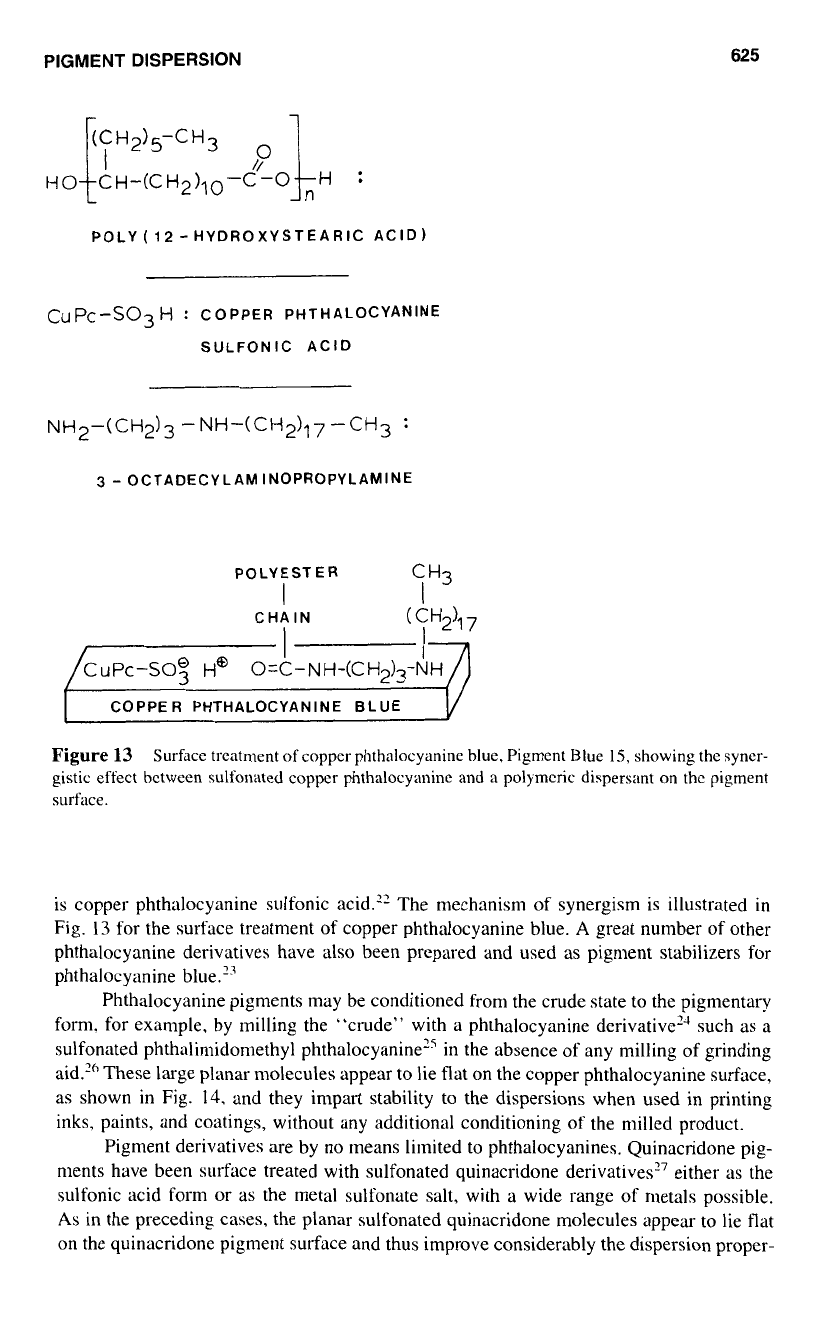
PIGMENT DISPERSION
625
H
:
POLY (12 -HYDROXYSTEARIC ACID)
CUPC-SO~H
:
COPPER PHTHALOCYANINE
SULFONIC ACID
NH*-(CH2)3 -NH-(CH2)17 “CH3
:
3
-
OCTADECYLAM INOPROPYLAMINE
POLYESTER
l
CH3
l
‘“I
CHAIN
CUPC-SO; H@ O=C-NH-(CH&-NH
COPPER PHTHALOCYANINE BLUE
Figure
13
Surface treatment of copper phthalocyanine blue, Pigment Blue
15,
showing the syner-
gistic effect bctwecn sulfonated coppcr phthalocyanine and a polymcric dispersant on thc pigment
surface.
is copper phthalocyanine sulfonic acid.” The mechanism of synergism is illustrated
in
Fig.
13
for the surface treatment of copper phthalocyanine blue.
A
great number of other
phthalocyanine derivatives have also been prepared and used as pigment stabilizers for
phthalocyanine blue.’3
Phthalocyanine pigments may be conditioned from the crude state to the pigmentary
form. for example, by milling the “crude” with
a
phthalocyanine derivative” such as
a
sulfonated phthalimidomethyl phthalocyanine’5 in the absence
of
any milling of grinding
aid.’” These large planar molecules appear to lie flat on the copper phthalocyanine surface,
as shown in Fig.
14.
and they impart stability to the dispersions when used in printing
inks, paints, and coatings, without any additional conditioning
of
the milled product.
Pigment derivatives are by no means limited to phthalocyanines. Quinacridone pig-
ments have been surface treated with sulfonated quinacridone derivatives” either
as
the
sulfonic acid form or as the metal sulfonate salt, with
a
wide range
of
metals possible.
As
in the preceding cases, the planar sulfonated quinacridone molecules appear
to
lie flat
on the quinacridone pigment surface and thus improve considerably the dispersion proper-

626
VERNARDAKIS
1
SULFONATED PHTHALIMIDOMETHYL
COPPER PHTHALOCYANINE
I
I
I
COPPER PHTHALOCYANINE BLUE
Figure
14
Surface treatment
of
copper phthalocyanine blue, Pigment Blue
1
S,
with
a
sulfonated
copper phthalocyanine additive, surface modifying agent.
ties
of
the pigment, especially when used in coating applications. Figure
15
represents the
arrangement of sulfonated quinacridone derivative
on
the pigment surface.
Pigment derivatives of azo reds,'x oranges, and yellows" have also been used for
surface treating the corresponding pigments. With azo yellows, treatments can be carried
out in situ with fatty amines to produce easily dispersible products through a Schiff base
reaction between the
-c=o
(carbonyl) groups
of
the pigment and the "NH2 groups of
primary amines, to form <=N- Schiff Derivatives
of
monoarylide and
diarylide yellow pigments can also be prepared by reacting the pigment with
a
primary
diamine and a glycidyl ether" to produce
a
Schiff base. The structure of one of these
derivatives is shown in Fig.
16
for Pigment Yellow
12,
AAA yellow. Again. the planar
pigment molecule appears to lie
on
the pigment surface, and the long chains project
outward into the vehicle to produce stabilization
of
the dispersion.
I
I
8.2
Inorganic
Pigments
Titanium dioxide, in the two naturally occurring crystal forms, anatase and rutile, is the
most important white pigment, which provides maximum opacifying power. Normally,
TiO, pigments are not used in their pure form because
of
their poor dispersibility in
a
variety of resins and solvents. Generally, they are surface coated with small amounts of
alumina, silica, or both (up
to
3%
total, on TiO?)
to
increase the functionality of the
surface (active adsorption sites for the resin molecules) and to improve dispersibility and
impart stability to the dispersion, especially in alkyd resin paint systems. For alumina-
coated titanium dioxide,3J the highly basic sites on the alumina surface, which are much
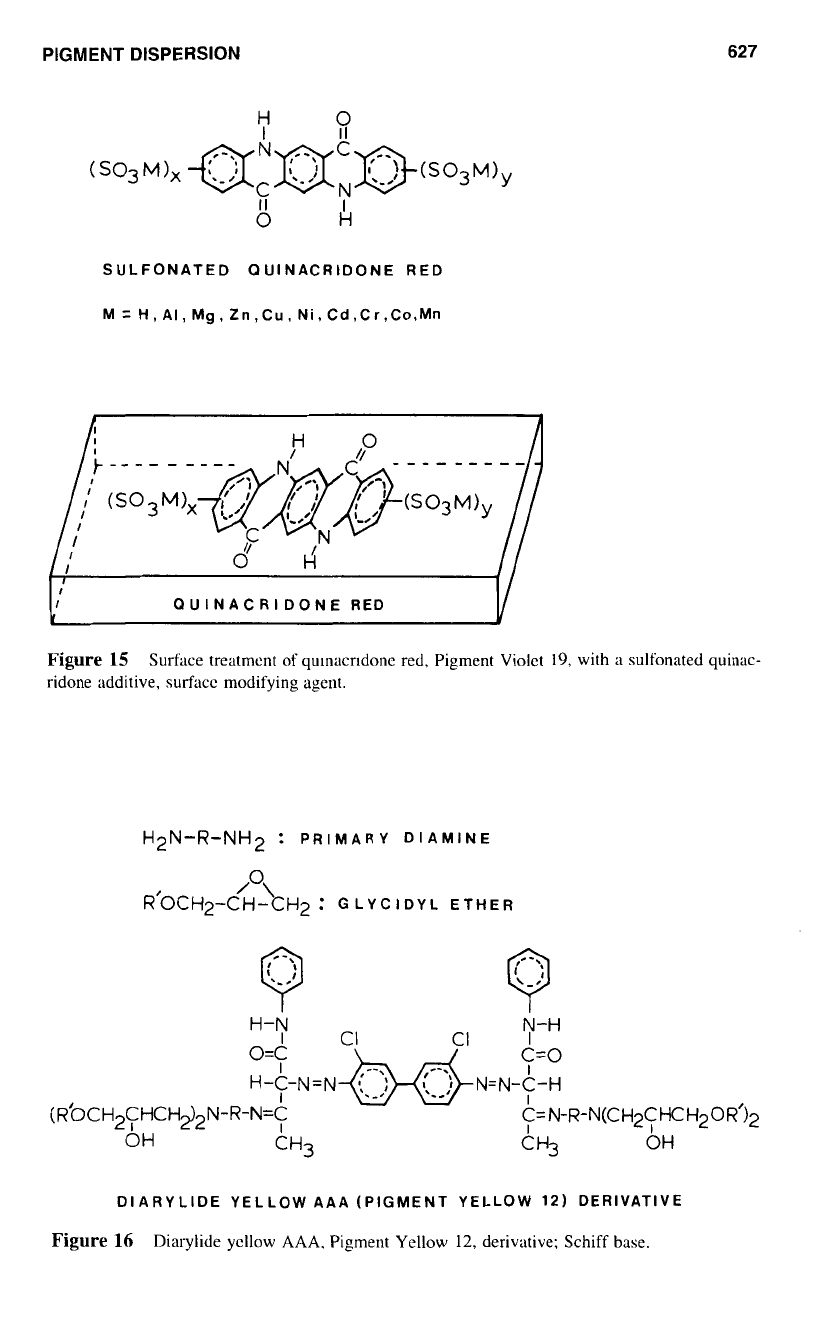
PIGMENT DISPERSION
627
SULFONATED OUINACRIDONE RED
M
=
H, AI,
Mg,
Zn,Cu, Ni, Cd,Cr,Co,Mn
Figure
15
Surface treatment
of
qulnacrldonc red, Pigment Violet
19,
with
a
sulfonated quinac-
ridone additive, surface modifying agent.
H2N-R-NH2
:
PRIMARY DIAMINE
/O\
R’OCH2-CH-CH2:
GLYCIDYL ETHER
DlARYLlDE YELLOW
AAA
(PIGMENT YELLOW
12)
DERIVATIVE
Figure
16
Diarylide yellow AAA, Pigment Yellow
12,
derivative; Schiff base.

628
VERNARDAKIS
more basic than the sites on the TiO? surface, cause specific adsorption of the acidic
functional groups of the alkyd resin molecules. The remaining parts
of
the resin molecules
(long chains) extend away from the surface. creating a considerable amount
of
steric
hindrance around each pigment particle, thus resulting in steric stabilization of the disper-
sion.
Alumina-coated titanium dioxide, iron oxide red. and other inorganic pigments and
fillers can be surface treated with alkanolamines (aminoalkanols). having the general for-
mulas
RI-CH-CH,-NH~, R2-CH-
CH
-R3, etc.
l
II
OH OH NHZ
where
R,,
R?, and
R3
are alkyl groups containing from
1
to
22
carbon atoms in the
chain.j5 The dispersibility of these pigments is increased considerably when used in paint
formulations containing air drying resin vehicles. The stability of the dispersion is similarly
improved because of the steric stabilization imparted
to
the pigment particles by the R
I,
R?, and R3 long chain alkyl groups.
Organic isocyanate adducts" are used as effective dispersing agents for several
classes
of
inorganic pigments, including zinc oxide, iron oxides. Prussian Blue, cadmium
sulfide, ultramarine, vermilion. and chrome pigments (zinc, barium. and calcium chro-
mates). These agents improve the dispersion characteristics and the flocculation resistance
of
the above-listed pigments when incorporated into conventional alkyd paint vehicles
with organic solvents, where these systems also contain a substantial amount of titanium
dioxide.
9.0
THE CHARACTERIZATION AND ASSESSMENT
OF
DISPERSION
The extent
to
which
a
pigment is dispersed in the medium or the degree of dispersion is
normally assessed in terms of color strength. gloss, brightness, and transparency, and it
also has an effect
on
the rheological properties of the ~ystem.~"~" Since all these properties
are governed by the size and distribution of the pigment particles in the dispersion. one
can. today, measure these properties using any of the latest particle size analyzers based
on the light scattering principle of the dispersed particles." With these instruments.
a
very
dilute suspension is required, and it is necessary to know the refractive index and viscosity
of
the suspending medium. The average particle diameters and the particle size distribu-
tions obtained are those of individual particles. aggregates, agglomerates, and flocculates
in the dispersion. The advantages of these instruments are that they are quite easy
to
operate, they give results rapidly, and they allow the dispersion process to be followed at
different times and at different stages.
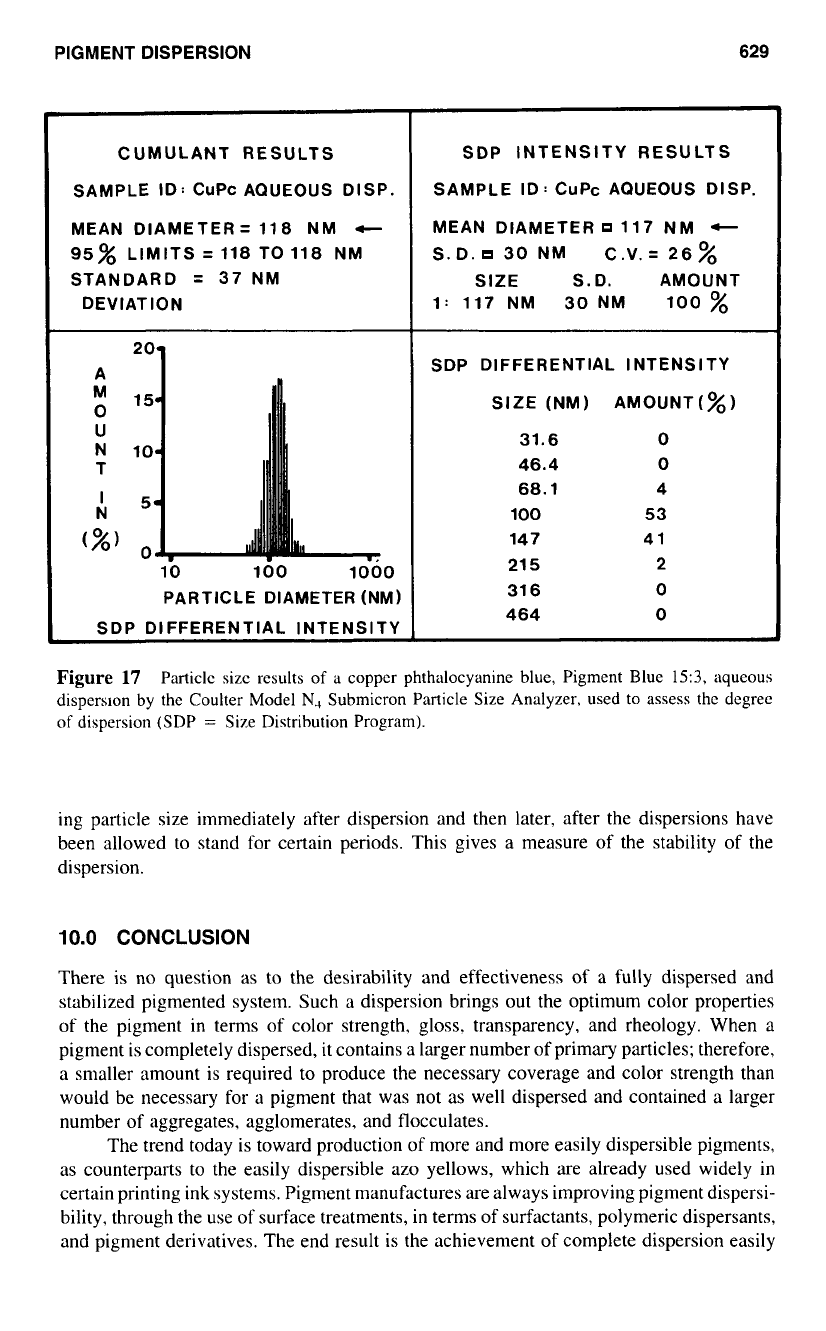
One such instrument is the Coulter model N4 Submicron Particle Analyzer. Figure
17
represents the particle size results for a green-shade phthalocyanine blue, C.I. Pigment
Blue
15
:
3.
in an aqueous dispersion. The distribution is quite narrow, and the mean particle
diameter is
0.1
17
pm. These results are very similar
to
those obtained from inspection of
the transmission electron micrographs of Fig.
1
for the same phthalocyanine blue pigment
in the dry powder form. showing that very little aggregation exists in the dispersion.
Such particle size analyzers, based on light scattering, can be used very effectively
to
study particle size changes that occur during the dispersion of pigments in fluid systems.
Furthermore, time studies may be carried out on the flocculation of pigments by determin-
PIGMENT
DISPERSION
629
CUMULANT RESULTS
SAMPLE ID: CUPC AQUEOUS DISP.
MEAN DIAMETER
=
118
NM
c
95%
LIMITS
=
118
TO
118
NM
STANDARD
=
37
NM
DEVIATION
A
0
U
N
io
T
15
15
N
(%I
0
l0
l00
1000
PARTICLE DIAMETER (NM)
SDP DIFFERENTIAL INTENSITY
SDP INTENSITY RESULTS
SAMPLE ID: CuPc AQUEOUS DISP.
MEAN DIAMETER
11
7
NM
-
S.D.
30
NM C.V.=
26%
1:
117
NM
30
NM
100
%
SIZE
S.
D. AMOUNT
SDP DIFFERENTIAL INTENSITY
SIZE
(NM) AMOUNT(%)
31.6
0
46.4
0
68.1 4
100
53
147 41
21
5
2
31 6
0
464
0
Figure
17
Particle size results
of
a
copper phthalocyanine blue, Pigment Blue
15:3,
aqueous
dispersion
by the Coulter Model
N.,
Submicron Particle Size Analyzer, used
to
assess the degree
of
dispersion (SDP
=
Size Distribution Program).
ing particle size immediately after dispersion and then later, after the dispersions have
been allowed to stand for certain periods. This gives
a
measure
of
the stability of the
dispersion.
10.0
CONCLUSION
There is no question as to the desirability and effectiveness of a fully dispersed and
stabilized pigmented system. Such
a
dispersion brings out the optimum color properties
of the pigment in terms of color strength, gloss, transparency, and rheology. When a
pigment is completely dispersed,
it
contains a larger number of primary particles; therefore,
a smaller amount is required to produce the necessary coverage and color strength than
would be necessary for a pigment that was not as well dispersed and contained a larger
number of aggregates, agglomerates, and flocculates.
The trend today is toward production of more and more easily dispersible pigments,
as counterparts to the easily dispersible azo yellows, which are already used widely in
certain printing ink systems. Pigment manufactures are always improving pigment dispersi-
bility, through the use of surface treatments, in terms
of
surfactants, polymeric dispersants,
and pigment derivatives. The end result is the achievement
of
complete dispersion easily

630
VERNARDAKIS
and quickly. Since this is an energy-intensive process,
in
terms
of
the dispersion equipment
utilized, less energy
is
required, which results in greater economic benefits for the pigment
user.
REFERENCES
I.
2.
3.
‘4.
5.
6.
7.
8.
9.
IO.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29,
30.
31.
32
33
34
35.
36.
37.
38.
G.
D.
Parfitt, ed..
Disprrsiorl
of’Poudrrs
in
Liquids,
2d cd. New York: John Wiley,
1973.
T. C. Patton,
Ptrir1f
Flobv
cwl
Pigrrlerlt
Disprsiorl.
2d ed. New
York:
John Wiley,
1979.
V.
T.
Crowl,
J.
Oil
Colour Cherr~.
Assoc..
55,
388 (1972).
0.
Hafncr.
J.
Oil
Colour
Chem.
Assoc.,
57, 268 (1974).
W. Can,
J.
Oil
Colour
ClwtI.
Assoc..
61, 397 (1978).
D. M. Varlcy and H. H. Bower,
J.
Oil
Colour
Chortr.
Assoc..
62. 401 (1979).
H.
M.
Smith.
f‘olyrr~
P~ir~t
Color
J.,
175, 660 (1985).
P. A. Lcwis,
cd..
Pi<yrnrrlf
Hrmlhook.
Vol.
1,
2d
ed. New York: John Wilcy,
1988.
G.
D. Parfitt and K.
S.
W. Sing, cds.,
Clrcrrrrc.lPrizcrtior1
o~Po~~~~ler-Sur~tcp.s.
London: Academic
Prcss.
1976.
H.
P. Preuss,
Pigrrrerffs
irf
Ptrirft.
Park Ridge. NJ: Noyes,
1974.
W.
M. Morgans,
01trlir1r.s
of
Prtirlt
Trc~lrrrology
Vols.
l
and
2,
2d cd. London: Charles Griffin,
1982.
T.
G.
Vernardakis,
Am.
Ink
Mrtkrr,
62(2), 24 (1984).
P. Sorensen,
J.
Pnir~t
Tochr~ol..
47, 3
1
(1975).
J.
S.
Hamptom and J. F. MacMillan,
Am.
hk
Mtrkrr,
63(
l),
16 (1985).
B.
G.
Hays,
AHI.
hk
MLrker,
62(6). 28 (1984).
A Topham,
Prog.
Org.
Cotrtirr~q.~.
5,
237 (1977).
K. Merkle and
H.
Schafer. in
Pigrtlerff
Htrrfdhook.
Vol.
111
(T.C. Patton, ed.).
New
York: John
Wiley,
1973,
pp.
157-167.
A.
E.
Ambler and R. W. Tomlinson,
US.
Patent
3,296,001
(Jan.
3, 1967),
ICI.
T. C. Rees and R. J. Flores,
U.S.
Patent
4,032,357
(June
28, 1977),
Sherwin-Williams.
T.
G.
Vernardakis,
Dyes
Pipents.
2, 175
(
1981
).
J. F. Stansfield and A. Topham.
U.S.
Patent
3,996,059
(Dcc.
7. 1976).
ICI.
P. K. Davies, L. R.
Rogers.
J. F. Stansfield, and A. Topham,
U.S.
Patent
4,057.436
(Nov.
8,
1977.
ICI.
Anon., British Patent
1,544,839
(Apr.
25, 1979),
BASF.
W. H. McKellin, H.
T.
Lacey, and V. A. Giambalvo,
U.S.
Patent
2,855,403
(Oct.
7, 1958),
American Cyanamid.
V. A. Giambalvo and W. Berry,
U.S.
Patcnt
3,589,924
(Junc
29, 1971).
American Cyanamid.
S.
L.
Johnson. G. McLaren and
G.
H. Robcrtson,
U.S.
Patent
4,448,607
(May
15,
1984),
Sun
Chemical.
E. E.
Jaffe
and W.
J.
Marshall,
U.S.
Patent
3,386,843
(Junc
4,
1968).
DuPont.
J. Mitchell and A. Topham,
U.S.
Patent
3,446,641
(May
27, 1969).
ICI.
J. Mitchell and A. Topham, British Patent
1,139,294
(Jim.
8,
1969),
ICI.
Anon., Britlsh Patcnt
1,080,115
(Aug.
23, 1967).
KVK.
F. Dawson, J. Mitchcll. L. R. Rogers, W. Todd, and A. Topham, British Patcnt
1,096.362
(Dec.
29. 1967).
ICI.
G.
H. Robertson,
U.S.
Patent
4,220,473
(Sep.
2,
1980),
Sun Chemical.
R.
J.
Schwartz and
T.
Sulzbcrg.
U.S.
Patent
4,468,255
(Aug.
28, 1984),
Sun Chemical.
M.
J.
B. Franklin. K. Goldsbrough,
G.
D.
Parfitt. and J. Pcacock.
J.
Pni/rt
Trchrfol..
42, 740
(
1970).
H. Lindcn, H. Rutzen, and B. Wcgemund,
US.
Patcnt
4,167.421
(Sept.
11,
1979),
Hcnkel.
F.
Hauxwcll, J.
F.
Stansfield, and A. Topham,
U.S.
Patent
4.042,413
(Aug.
16. 1977).
ICI.
W.
Cm,
J.
Oil
Colour
C1wm.
A.ssoc~..
65, 373 (1982).
K.
Tsutsui and
S.
Ikeda,
Pro,?.
Or,y
Cocrtiyqs,
IO.
235 (1982).
39.
R. Polkc.
Am.
Olk
Mrrker,
61(6),
15
(1983).
73
Colored Inorganic Pigments
This chapter describes the chemistry, manufacture, and properties associated with the
major classes of colored inorganic pigments
as
used in the coatings industry. Thus pigmen-
tary inorganic whites such as titanium dioxide are not covered.
1.0
THE COLOUR INDEX SYSTEM
The Colour Index System is
a
coding system developed under the joint sponsorship of
the Society of Dyers and Colourists (SDC) in the United Kingdom and the Association
of Textile Chemists and Colorists (AATCC)
in
the United States. The system is referred
to
as
the “Colour Index.” In referring to pigments using the Colour Index System. we
may describe, for example, Molybdate Orange
as
Pigment Red
104,
Colour Index number
77605.
The Colour Index names for pigments are abbreviated
as
follows:
PB
=
Pigment Blue
PBr
=
Pigment Brown
PM
=
Pigment Metal
PV
=
Pigment Violet
PW
=
Pigment White
2.0
PIGMENT SELECTION
PBk
=
Pigment Black
PG
=
Pigment Green
PO
=
Pigment Orange
PR
=
Pigment Red
PY
=
Pigment Yellow
Once
a
formulator has decided on the shade required for a particular application, the next
most important criteria
of
any pigment are its fastness properties. It is pointless
to
formulate
a
coating with a pigment that will not withstand the exposure specifications of the coatings
end use. Such specifications can extend
to
requiring
as
much
as
5
years
of
outdoor exposure
in states such as Florida and Arizona.
631
