Satas D., Tracton A.A. (ed.). Coatings Technology Handbook
Подождите немного. Документ загружается.

LEWIS
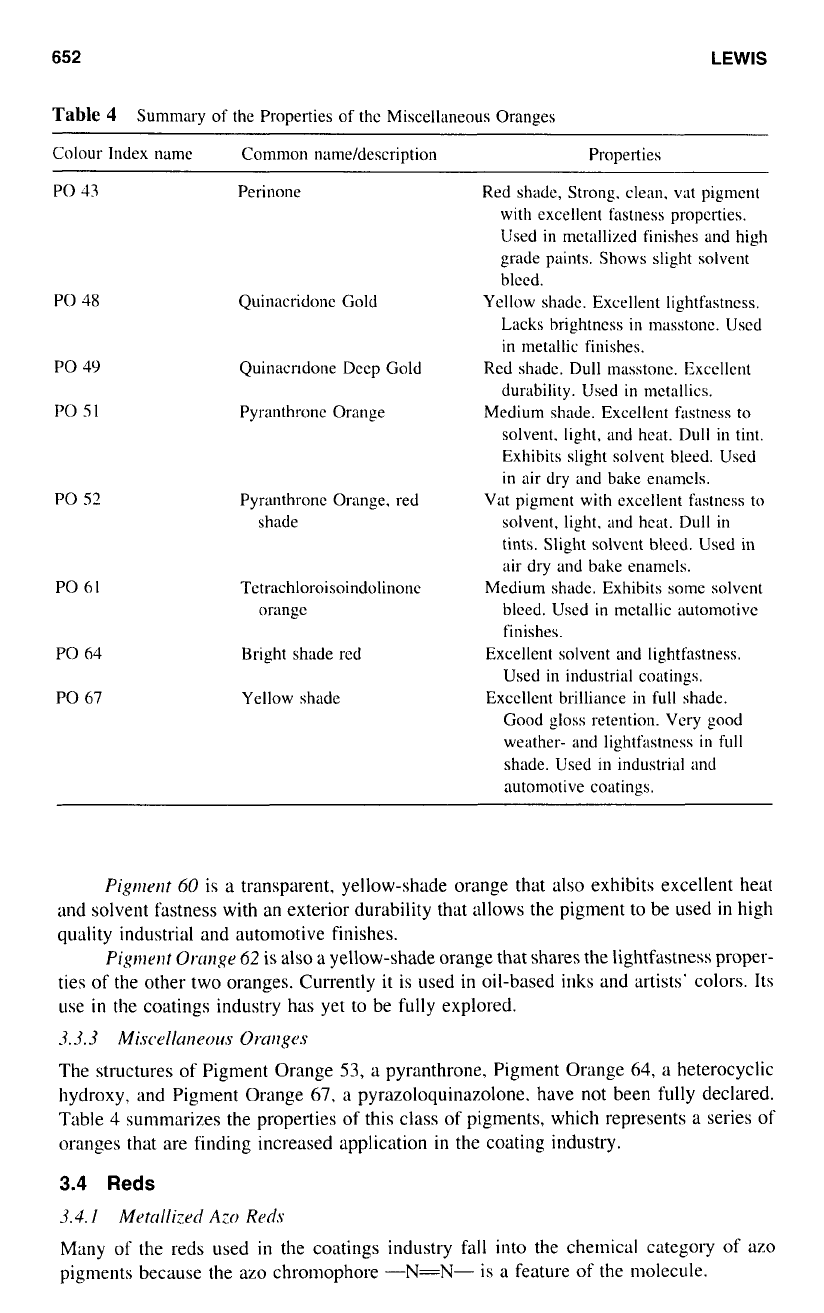
Table
4
Summary
of
the Properties of the Miscellaneous Oranges
Colour
Index name
Common
nnmeldcscription Properties
PO
48
PO
49
PO
SI
PO
52
PO
61
PO
64
PO
61
Quinacridonc
Gold
Quinacrtdone
Dccp
Gold
Pyranthrone Orange
Pyranthronc Orange. red
shade
Tctrachloroisoindolinonc
orangc
Bright shade
red
Yellow shade
PO
13
Perinone Red shadc, Strong.
clean.
vat pigment
with excellent fastness properties.
Used in mctallized finishes
and
high
grade paints. Shows slight solvent
blccd.
Ycllow
shadc.
Excellent lightfastncss.
Lacks brightness
in
masstonc. Used
in metallic finishes.
Red shade.
Dull
nmstonc. Exccllcnt
Medium shade. Excellcnt
fastncss
to
durability. Used in mctallics.
solvent. light,
and
heat.
Dull
in tint.
Exhibits slight solvent bkd. Used
in air dry and bake
enamels.
Vat pigment with excellent
fastncss
to
solvent, light. and heat.
Dull
in
tints. Slight solvcnt bleed. Used
in
air
dry
and
bake enamels.
Medium shade. Exhibits some solvcnt
bleed. Used in mctallic automotivc
finishes.
Exceknt solvent and lightfastness.
Used in industrial coatings.
Exccllcnt brilliance in
full
shade.
Good gloss retention. Very good
weather-
and
lightfastncss in
full
shade. Used in industrial
and
automotive coatings.
Pigmrrlt
60
is a transparent, yellow-shade orange that
also
exhibits excellent heat
and solvent fastness with an exterior durability that allows the pigment to be used
in
high
quality industrial and automotive finishes.
Pigment
Omngr
62
is
also
a yellow-shade orange that shares the lightfastness proper-
ties of the other two oranges. Currently it is used
in
oil-based inks and artists' colors. Its
use
in
the coatings industry has yet to be fully explored.
3.3.3
Mi.sce1lmeou.s
0muge.s
The structures of Pigment Orange
53,
a pyranthrone, Pigment Orange
64,
a heterocyclic
hydroxy, and Pigment Orange
67,
a
pyrazoloquinazolone. have
not
been fully declared.
Table
4
summarizes the properties of this class of pigments, which represents a series of
oranges that are finding increased application
in
the coating industry.
3.4
Reds
3.4.
l
Mctcrllizrd
Azo
Rds
Many of the reds used
in
the coatings industry fall
into
the chemical category of azo
pigments because the
azo
chromophore
-N=N-
is
a
feature of the molecule.

ORGANIC PIGMENTS
653
A further subdivision may be made into acid, monazo metallized pigments such as
Manganese Red 2B (PR 48:4) and Calcium Lithol (PR 49: 2) and nonmetallized azo reds
such as the Naphthols (e.g. PR
17
and PR
23)
and Toluidine Red (PR
3).
Typically, each
ofthe acid, monoazotnetallized pigments contains an anionic grouping such
as
a carboxylic
(XOOH)
or sulfonic acid
(-S03H)
group, which will ionize and react with a metal
cation such
as
calcium or manganese to form an insoluble. metallized pigment.
Nonlnetallized pigments do not contain an anionic group
in
their structure and. as
such, will not complex with a metal cation.
All
azo reds contain one or more azo groups and are produced by similar reaction
sequences. The initial reaction sequence. described as diazotization, involves reacting an
aromatic primary amine with nitrous acid, formed in situ by reacting sodium nitrite with
hydrochloric acid
at
low temperatures to yield a diazonium salt. Invariably the diazonium
salt that is formed by this process is unstable and should be kept cold to avoid any
decomposition.
The diazonium salt is reacted quickly with the second half of the pigment, which
is called the coupler. The coupling reaction takes place rapidly
in
the cold to yield the
sodium salt of the pigment. This sodium salt is all but useless as a pigment for the coatings
industry because of its marked tendency
to
bleed even in the weakest
of
solvent systems.
The pigment is, therefore, metallized to confer improved properties on the product. The
pigment suspension is then filtered and washed
to
remove any residual inorganics derived
from the reaction.
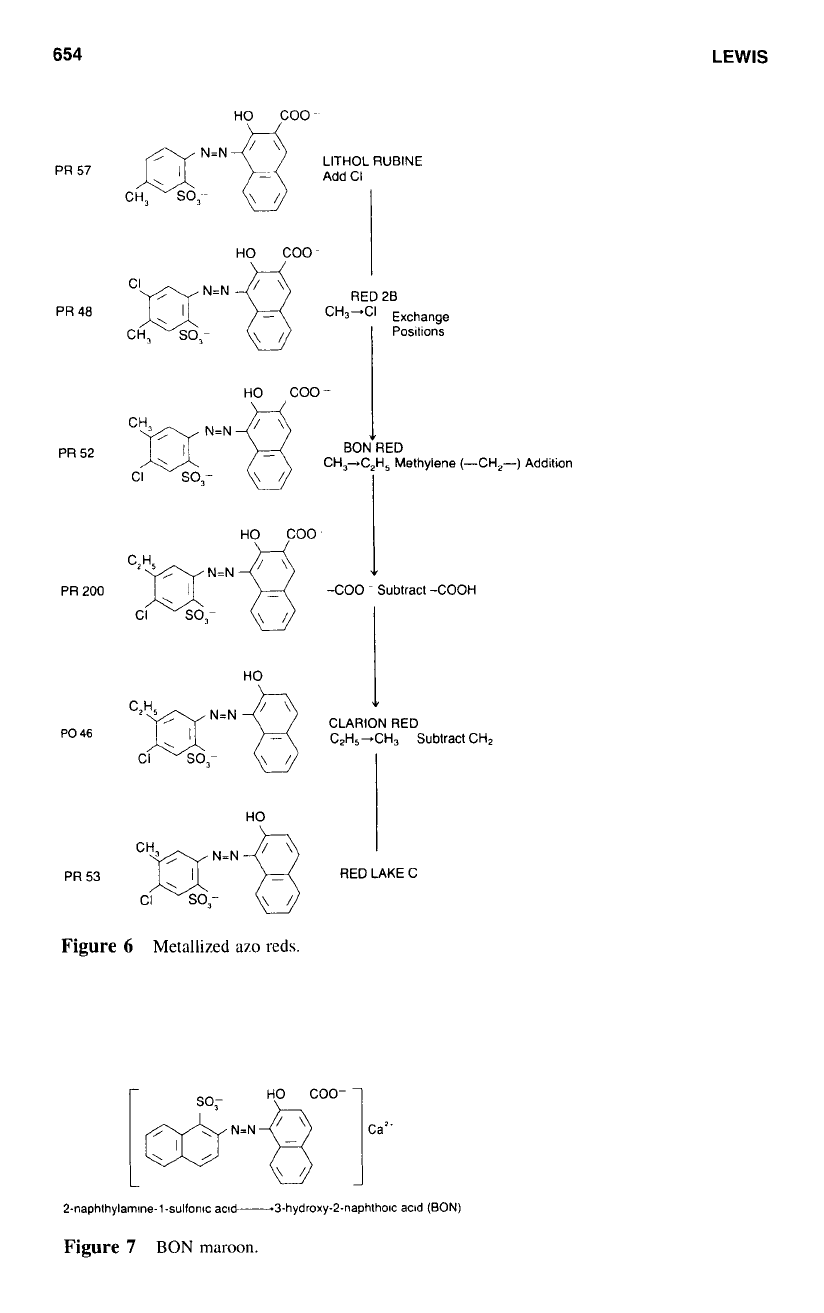
Figure
6
illustrates the structures
of
the different metallized azo reds that are readily
available.
The
Litllol
Rrtls
are primarily Barium Lithol (PR 49:
I)
and Calcium Lithol (PR
49
:
2). Although limited in their application
in
the coating industry, these pigments do
find some use at masstone levels-that is. the pigment is not tinted with a white
tint
base-where their fastness properties are acceptable.
The pigments are bright reds with high
tint
strength and good dispersion characteris-
tics. The barium salt is lighter and yellower
in
hue conlpared to the calcium salt.
Perrrwlerlt
Red 2B
is the generic name that encompasses Barium Red 2B (PR
48
:
I
),
Calcium Red 2B (PR
48
:
2). and Manganese Red 2B (PR
48
:
4). The major use
of
the
calcium and barium salts is in baked industrial enamels, which are
not
required to be fast
to outdoor exposure. Use
in
alkaline systems is severely restricted because of the poor
alkaline fastness of these salts.
The barium salt is characterized by
a
clean, yellow hue and, although slightly poorer
than the bluer calcium salt
in
lightfastness and tinting strength, it does possess a slight
advantage in bake stability.
Manganese Red 2B has a sufficiently improved lightfastness to be used
in
implement
finishes. The manganese salt is slightly bluer. dirtier. and less intense when compared to
the calcium salt.
Ruhinr Red
also
known as Calcium Lithol Rubine (PR
57
:
1)
is a clean. blue-shade
red pigment exhibiting the high tinting ability typical of the
azo
reds
of
this class. Its
major use
in
coatings is
in
interior systems that call for an inexpensive red with good
solvent and heat resistance. Again,
to
maintain maxin~um fastness properties. use of the
pigment at near
to
masstone levels is recommended.
BON
ml,
used as calcium BON Red (PR 52:
1)
or Manganese BON Red (PR 52: 2).
is characterized by outstanding cleanliness. brightness, and purity of color. The manganese
salt offers a very blue-shade red with improved lightfastness compared to the calcium
salt. As such. this salt is suitable for exterior coatings applications.
BON
Mnroorl,
(PR
63:
1)
is illustrated
in
Figure
7;
the manganese
salt
of BON
654
LEWIS
PR
57
PR
48
PR
52
PR
200
PO
46
PR
53
H0
COO
a
N=N
g
LI-l::;
RUBlNE
CH,
so,-
\
/
H0
COO-
“B
N=N
g
RED
28
Exchange
Poslltons
CH,
so,-
\
/
H0
COO-
”
EON
RED
CH,-C,H,
Methylene
(“CH,”)
Addition
H0
COO
l
J
-COO
~
Subtract
-COOH
H0
Figure
6
Metallized
azo reds.
CLARION
RED
C2H,+CH,
Subtract
CH2
I
l
RED LAKE
C
2-naphlhylam1ne-l-sulfonc
ac1d~-3~hydroxy-2-naphtho1c
acld (BON)
Figure
7
BON
maroon.

ORGANIC PIGMENTS
655
Maroon is of considerably more importance than either the calcium or barium salts. Its
lightfastness is such that the pigment can be used at masstone levels for implement finishes.
3.4.2
Norl-Metcrli:ecJ
Am
Reds
As
implied by their classification, the nonmetallized azo reds do not contain a precipitating
metal cation and, as such. offer increased stability against hydrolysis in strongly acidic
or alkaline environments.
Synthesis of this class of pigment follows the previously described classical method
of diazotization of a primary aromatic amine followed by coupling
of
the resultant diazo-
nium salt. No anionic groups capable of accepting a metal cation are present in the mole-
cule; thus metallization is not a factor in their synthesis. Typical nonnletallized reds are
Toluidine Red (PR
3)
and the wide range of Napthol Reds as represented by Pigment
Reds
17,
22.
and
23.
Toluidine Red is used in full shade
in
such coatings applications as farm implements,
lawn and garden equipment. and bulletin paints, where a bright, economical red of adequate
lightfastness is required. Because of the pigment's poor durability
in
tint shades, it is rarely
used at any level other than a full shade.
The individual properties of the Napthol Reds depend on the specific composition
of the product
as
well as the conditioning steps used during pigment manufacture.
As
a
class. they are a group
of
pigments that exhibit good tinctorial properties combined with
moderate fastness
to
heat. light, and solvents.
Unlike the metallized azo reds. the Napthol Reds are extremely resistant to acid,
alkali. and soap. These properties lead to their use in latex emulsion systems and masonry
paint.
In terms of performance and economic characteristics. the Napthols form a link
between the toluidine reds at the lower end of the scale and the perylene and quinacridone
reds at the higher end.
3.4.3
High
Pe,lfi,rrlm1ce
Reds
Pigments for the exacting standards of today's automotive coatings are required to show
satisfactory durability to outdoor exposure in such states as Arizona and Florida for
2
and
possibly
5
years before being approved for use
in
automotive finishes. Similar requirements
are placed
on
pigments chosen for use in automotive repair systems and marine coatings.
High performance reds fall into four basic classes; quinacridone reds and violets,
reds based on vat dyestuffs known to include the perylene reds. reds derived from the
benzimidazolone diazonium salts, and the disazo condensation reds.
Quirlctcridone
Reds
Quinacridones Inay be described
as
heterocyclic pigments in
that their structure comprises
a
fused ring structure in which the ring atoms are dissimilar.
h
the case of quinacridones, the ring atoms are carbon and nitrogen (Fig.
8).
Addition of
0
OyJD
\
N
H
0
/I
\
c'
Figure
8
Structure
of
translincnr
quinacridone.
656
LEWIS
A.
Cyclization
of
2,5-diarylaminoterephthalic
acid
CH,COOC,H,
I
I)
cycllzalton
Rlng
Closure
In polyphosporc acld
B.
Oxidation
of
dihydroquinacridone
H
II
0
CH,-COOC,H,
I
CH,-COOC,H,
COOC,H,
I
->
trans
linear
qumacrldone
0
Figure
9
Routcs
to
quinacridone.
differing auxochromic groups such as methyl
(--CH3)
and chlorine
(“cl)
gives Pigment
Red 122 and Pigment Red 202, respectively.
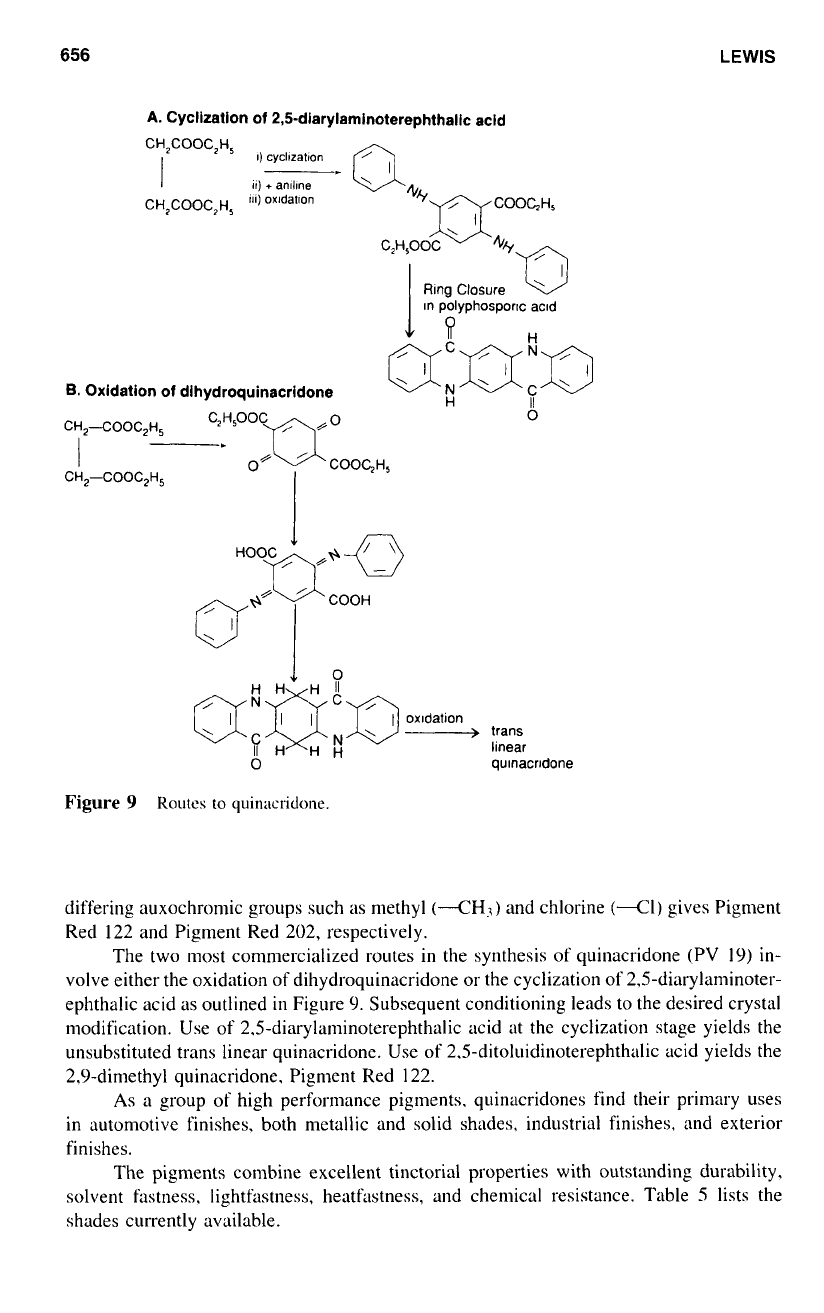
The two most commercialized routes in the synthesis of quinacridone (PV
19)
in-
volve either the oxidation of dihydroquinacridone or the cyclization
of
2.5-diarylaminoter-
ephthalic acid as outlined
in
Figure
9.
Subsequent conditioning leads to the desired crystal
modification. Use of 2.5-diarylaminoterephthalic acid at the cyclization stage yields the
unsubstituted trans linear quinacridone. Use of
2.5-ditoluidinoterephthalic
acid yields the
2,9-dimethyl quinacridone. Pigment Red 122.
As
a group
of
high performance pigments. quinacridones find their primary uses
in automotive finishes. both metallic and solid shades. industrial finishes, and exterior
finishes.
The pigments combine excellent tinctorial properties with outstanding durability,
solvent fastness. lightfastness, heatfastness, and chemical resistance. Table
5
lists the
shades currently available.

ORGANIC
PIGMENTS
657
Table
5
Types
of
Quinncridone
Colour
Index name Hue Comments
PO 48
PO 49
PR
122
PR 192
PR
202
PR 206
PR
207
PR 209
PV 19
PV
42
Gold
Deep
Gold
Magenta-yellow
Red-yellow
Magenta-blue
Maroon
Scarlet
Yellow-shade red
Violet-blue
Rcd-yellow
Maroon
Quinacridone
quinone
Quinacridone
quinone
2.9-Dimethyl quinacridone
Unsymmetrical monomethyl quinacridonc
2.9-dichloroquinacridone
Mixed solid solution
4.1
1
-dichloroquinacridolle
3.1
0-dichloroquinacridone
p-quinacridone
y-quinncridone
Mixed
solid
solution
Vc~t
Rrcls
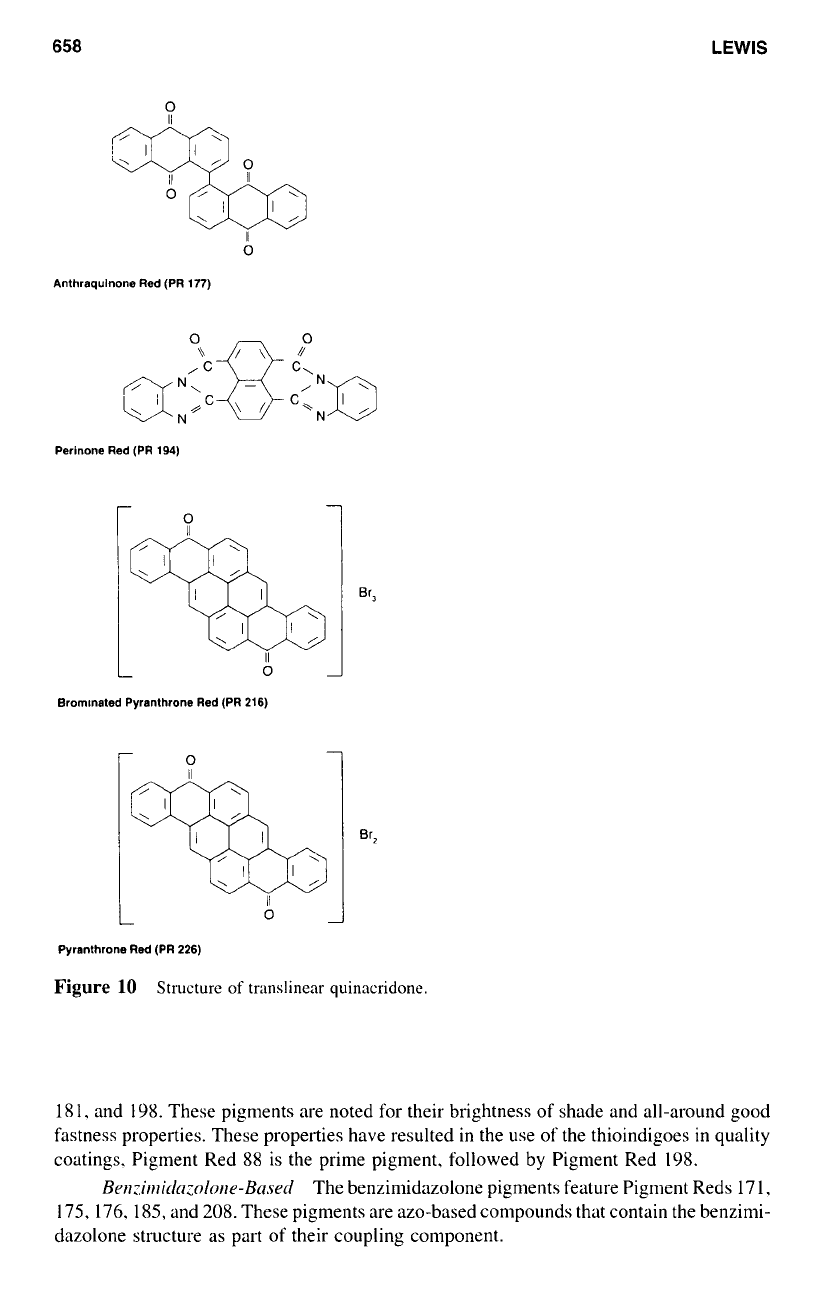
The vat red pigments. based on anthraquinine. include such structures
as Anthraquinone Red (PR
177),
Perinone Red (PR 194). Brominated Pyranthone Red
(PR 216). and Pyranthone Red (PR 226). as illustrated in Figure
IO.
These anthraquinone-derived pigments are manufactured via a series of complex
reactions
to
include such processing as sulfonation. nitration. halogenation, condensation.
and substitution.
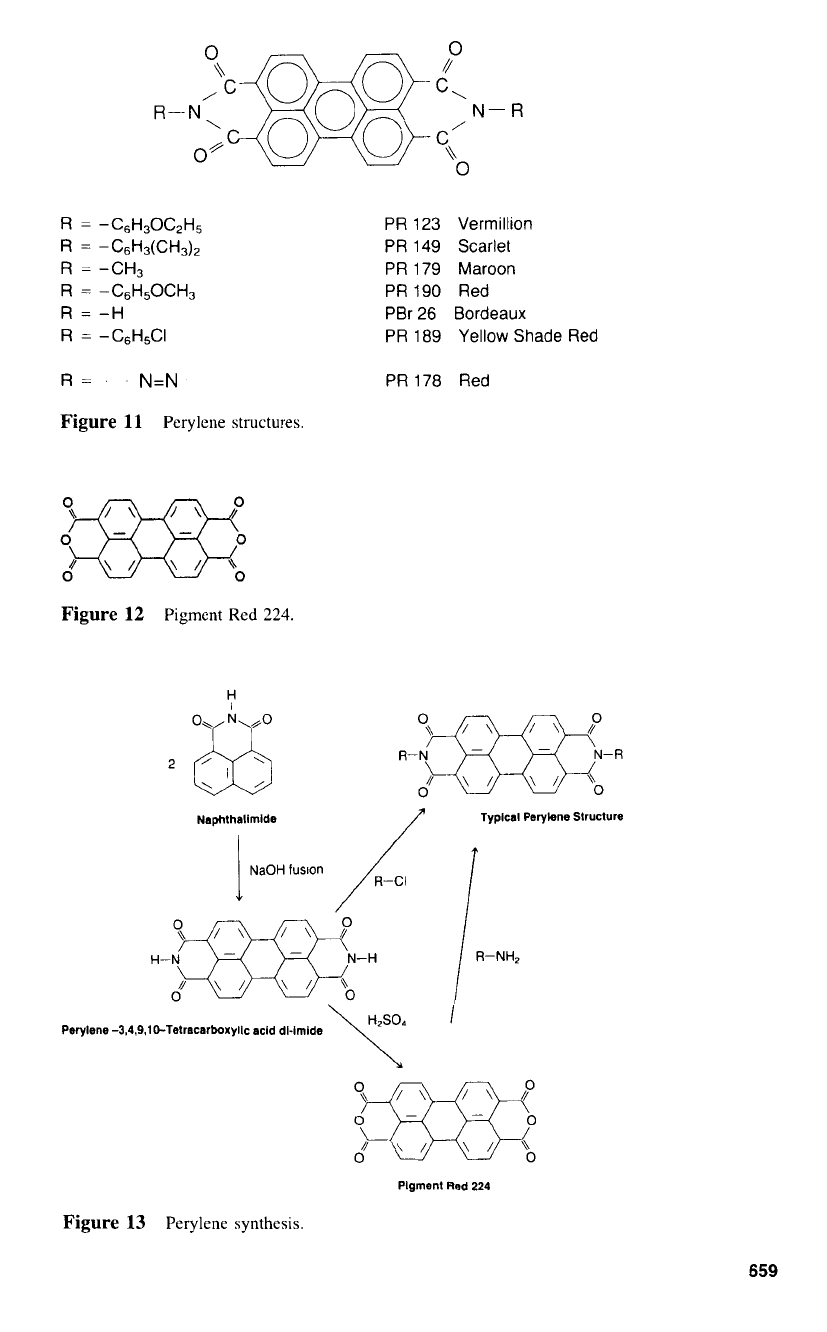
Perylerw
Reds
Perylene reds provide pure, transparent shades and novel styling
effects when used in metallic finishes. These pigments offer improved flow characteristics
when used in highly pigmented coatings formulations such
as
those required for high
solids base codclear coat systems. as well as high tranparency and good bleed resistance.
The perylenes possess high color strength, good thermal stability. excellent light-
and weather-fastness and. with the exception
of
Pigment Red 224, excellent chemical
resistance.
Perylenes may also be described as vat pigments and
in
fact are the only class of
vat pigments to be specifically developed
as
pigments rather than as dyestuffs. Almost
all
the perylene pigments have a structure as shown by the generic formula illustrated
in
Figure
1
I
;
that is; they are based
on
NN-substituted perylene-3,4.9, IO-tetracarboxylic
diimide.
A
notable exception is Pigment Red 224 (Fig. 12). which
is
actually derived from
the perylene tetracarboxylic dianhydride.
Acenaphthene is oxidized
to
1.X-naphthalic anhydride followed by ammonation
to
yield the naphthalimide. The napthalimide is condensed in a fused caustic medium to yield
the
perylene-3,4.9,lO-tetracarboxylic
acid diimide. The diimide can then be conditioned
to convert the crude into pigment and achieve Pigment Violet 26. or methylated to give
Pigment Red
179.
The diimide may be hydrolyzed to produce the dianhydride. Pigment Red 224. or
condensed with various aromatic
or
aliphatic amines
to
give such pigments as those fea-
tured
in
Figure
13.
As with many of the pigments already described. the perylenes have
to
be conditioned to obtain the compounds in a pigmentary form.
Thioitdigo
R&
The thioindiogoid chronlophore serves as a nucleus for
a
wide
range of red
to
violet pigments. The thioindigo reds include Pigment Reds 36,
87. 88,
658
LEWIS
%
0
01
\
\
/
I/
0
Anthraquinone
Red
(PR
177)
Perinone
Red
(PR
194)
0
-
Brominated
Pyranthrone
Red
(PR
216)
[%-
\
0
/l
/
-
Pyrenthrone
Red
(PR
226)
Figure
10
Structure
of
translinear quinncridone.
18
1.
and
198.
These pigments are noted for their brightness
of
shade and all-around good
fastness properties. These properties have resulted in the use of the thioindigoes
in
quality
coatings. Pigment Red
88
is the prime pigment, followed by Pigment Red
198.
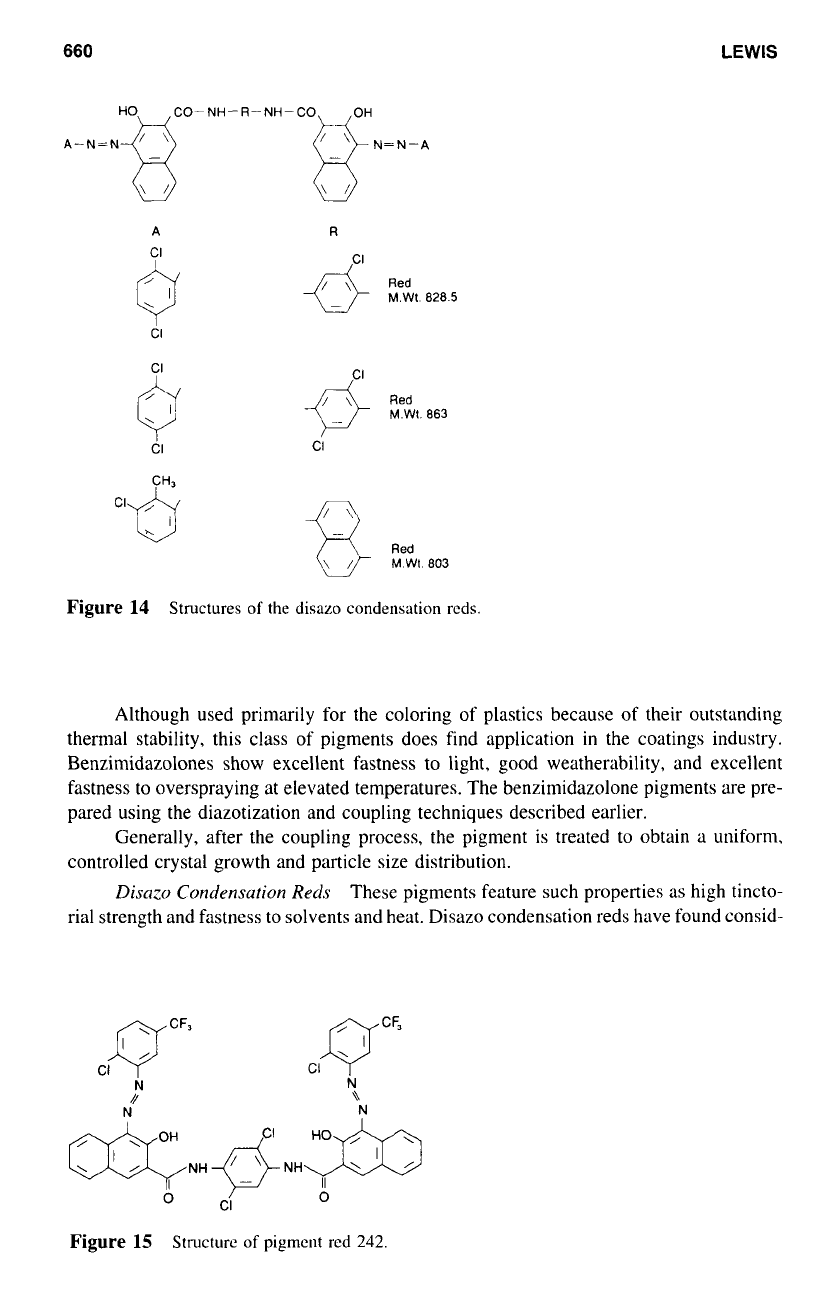
Brn~irwirkr~olnr~e-Bnserl
The benzimidazolone pigments feature Pigment Reds
17
1.
175,
176,
185,
and
208.
These pigments are azo-based compounds that contain the benzimi-
dazolone structure as part
of
their coupling component.
R-
PR
123 Vermillion
PR 149 Scarlet
PR
179 Maroon
PR
190 Red
PBr
26
Bordeaux
PR 189 Yellow Shade Red
Figure 11
Perylene
structures.
Figure 12
Pigment
Red
224.
H
2
o&
Naphthallmlda
,,/'
T~~IC~I
Paryhne
Structure
1
NaOH
fuslon
/,
1
Plgment
Rad
224
Figure 13
Perylene
synthesis.
659
660
LEWIS
A
Cl
Cl
Red
M
WI
803
Figure
14
Structures
of
the
disazo
condensation rcds.
Although used primarily for the coloring of plastics because
of
their outstanding
thermal stability, this class of pigments does find application
in
the coatings industry.
Benzimidazolones show excellent fastness
to
light, good weatherability, and excellent
fastness
to
overspraying at elevated temperatures. The benzimidazolone pigments are pre-
pared using the diazotization and coupling techniques described earlier.
Generally, after the coupling process, the pigment is treated
to
obtain
a
uniform,
controlled crystal growth and particle size distribution.
Disn:o
Condensation
Reds
These pigments feature such properties as high tincto-
rial strength and fastness
to
solvents and heat. Disazo condensation reds have found consid-
"
ci
U
Figure
15
Structure
of
pigrncnt
rcd
242.

ORGANIC
PIGMENTS
661
Figure
16
Structure
of
pigment red
214.
erable use as replacement pigments for lead containing pigments. Their outstanding fast-
ness properties have resulted in their use in high quality industrial finishes.
Figure 14 illustrates three typical structures
of
the disazo condensation reds. Colour
Index names for these three pigments have not been assigned. The synthesis sequence
generally is similar for each
of
the disazo condensation pigments. The
azo
components
are initially coupled
to
yield monoazo dyestuff carboxylic acids, which are converted to
acid chlorides before final conversion to the disazo by condensation with the arylide
component
to
yield the pigment in question.
Miscel1meou.s
High
Petfbrtmnce
Reds
In recent years a number
of
novel high
performance reds have been commercialized by such companies as Sandoz and BASF.
New from Sandoz are Pigment Reds 242, 214, and 257.
Pigment Red
242
is
shown in Figure 15. The product is a yellow-shade red with a
clean bright shade and very good all-around fastness properties.
It
is recommended for
lead-free coatings formulations for the production
of
high quality finishes and bright red
shades.
Pigment Red 214 (Fig.
16)
is a bluish red with properties similar to those
of
Pigment
Red 242.
Pigment Red 257, (Fig.
17)
is a nickel complex pigment with a red violet masstone
and a magenta undertone; its fastness properties are similar to those
of
the quinacridone
pigments.
Cl
Cl
H-N
Cl
Cl
Cl
Figure
17
Structure
of
pigment red
257.
