Satas D., Tracton A.A. (ed.). Coatings Technology Handbook
Подождите немного. Документ загружается.

672
VAUGHN
“Methylene Ether Link”
\
formed
&
water evolved
“20
“Methylene Bridge”
Hoyc
formed
&
water evolved
/N\
-
CH,OH
HoyC
-\
H
H
/
N\
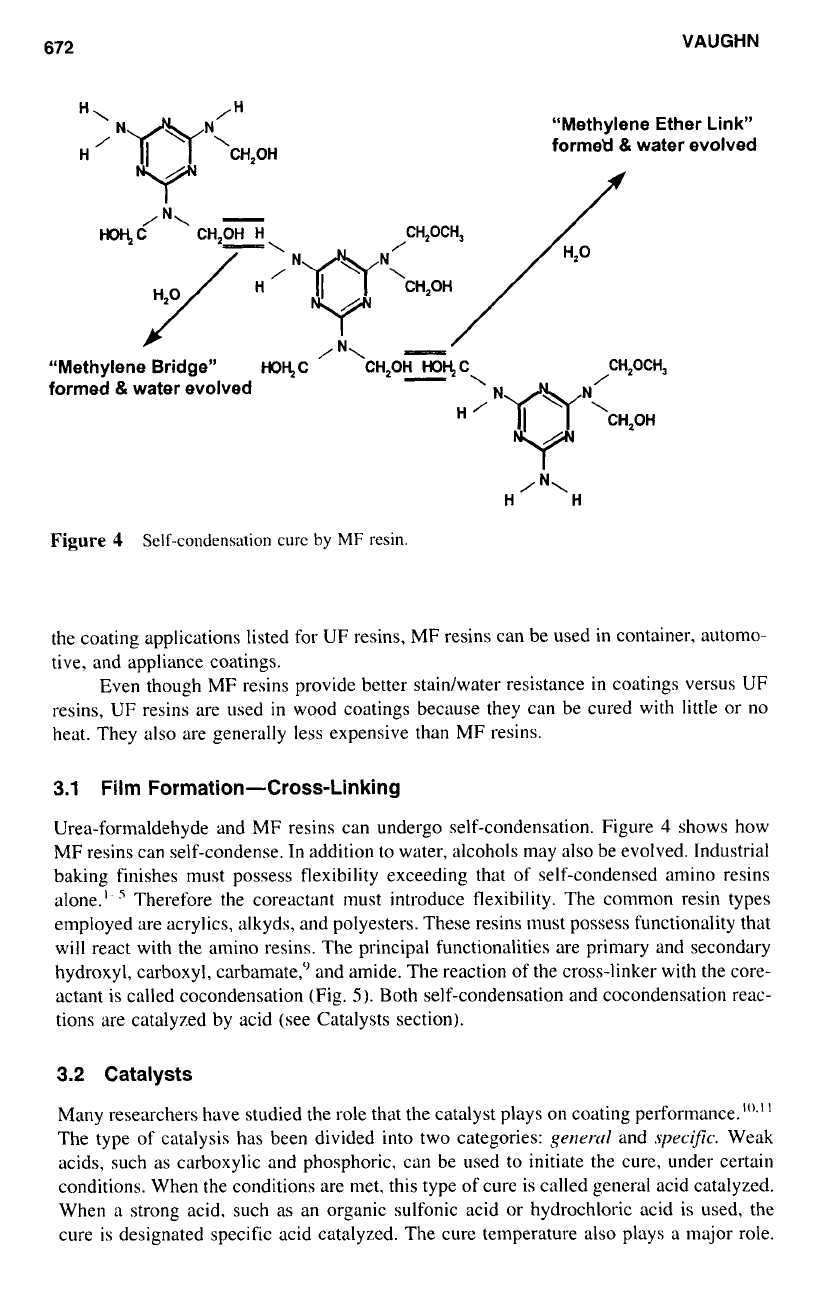
Figure
4
Self-condensation curc
by
MF
resin.
the coating applications listed for UF resins, MF resins can be used in container, automo-
tive. and appliance coatings.
Even though MF resins provide better staidwater resistance
in
coatings versus UF
resins, UF resins are used
in
wood coatings because they can be cured with little
or
no
heat. They also are generally less expensive than MF resins.
3.1
Film Formation-Cross-Linking
Urea-formaldehyde and MF resins can undergo self-condensation. Figure
4
shows how
MF resins can self-condense. In addition
to
water, alcohols may also be evolved. Industrial
baking finishes must possess flexibility exceeding that of self-condensed amino resins
alone.’ Therefore the coreactant must introduce flexibility. The common resin types
employed are acrylics, alkyds, and polyesters. These resins must possess functionality that
will react with the amino resins. The principal functionalities are primary and secondary
hydroxyl, carboxyl, carbamate,” and amide. The reaction
of
the cross-linker with the core-
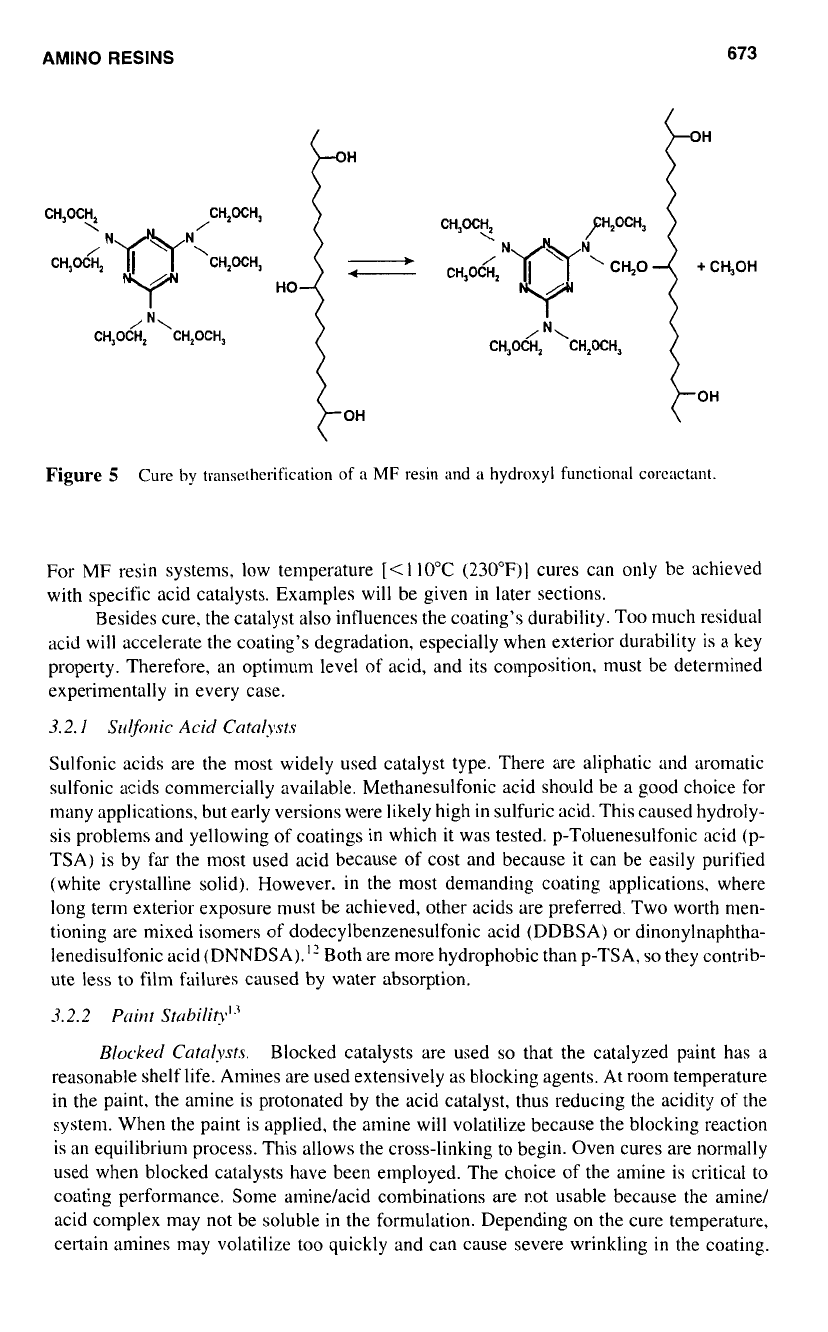
actant is called cocondensation (Fig.
5).
Both self-condensation and cocondensation reac-
tions are catalyzed by acid (see Catalysts section).
3.2
Catalysts
Many researchers have studied the role that the catalyst plays
on
coating performance.’”.”
The type of catalysis has been divided
into
two categories:
gerrenrl
and
specific.
Weak
acids, such as carboxylic and phosphoric, can be used to initiate the cure, under certain
conditions. When the conditions are met. this type
of
cure is called general acid catalyzed.
When a strong acid, such as an organic sulfonic acid or hydrochloric acid is used, the
cure is designated specific acid catalyzed. The cure temperature also plays a major role.
AMINO
RESINS
673
H
CH,OCH
CH,OCH,
-,NW'
CH,OCH,
Y
-
CH,OCH,
CH,OCH, CH,OCH,
/
N\
CH,OCH, CH,OCH,
Figure
5
Cure
by
transctherification
of
a
MF
resin
and
a
hydroxyl
functional
corcactant.
For MF resin systems, low temperature
[<
1
10°C (230"F)I
cures can
only
be achieved
with specific acid catalysts. Examples will be given in later sections.
Besides cure. the catalyst also influences the coating's durability. Too much residual
acid will accelerate the coating's degradation, especially when exterior durability is a key
property. Therefore, an optimum level of acid, and its composition, must be determined
experimentally in every case.
3.2.
I
Slrlfonic
Acid
Catalysts
Sulfonic acids are the most widely used catalyst type. There are aliphatic and aromatic
sulfonic acids commercially available. Methanesulfonic acid should be a good choice for
many applications, but early versions were likely high in sulfuric acid. This caused hydroly-
sis problems and yellowing of coatings
in
which it was tested. p-Toluenesulfonic acid (p-
TSA) is by far the most used acid because
of
cost and because it can be easily purified
(white crystalline solid). However.
in
the most demanding coating applications, where
long term exterior exposure must be achieved, other acids are preferred. Two worth men-
tioning are mixed isomers of dodecylbenzenesulfonic acid (DDBSA) or dinonylnaphtha-
lenedisulfonic acid (DNNDSA)." Both are more hydrophobic than p-TSA,
so
they contrib-
ute less
to
film failures caused by water absorption.
3.2.2
Ptrirlt
StcrDi/ih'3
Blocked
Catalysts.
Blocked catalysts are used
so
that the catalyzed paint has a
reasonable shelf life. Amines are used extensively as blocking agents. At room temperature
in the paint, the amine is protonated by the acid catalyst, thus reducing the acidity
of
the
system. When the paint is applied, the amine will volatilize because the blocking reaction
is an equilibrium process. This allows the cross-linking to begin. Oven cures are normally
used when blocked catalysts have been employed. The choice of the amine is critical
to
coating performance. Some aminehcid combinations are not usable because the amine/
acid complex may not be soluble
in
the formulation. Depending on the cure temperature,
certain amines may volatilize too quickly and can cause severe wrinkling
in
the coating.

674 VAUGHN
This effect is due
to
a differential cure throughout the thickness
of
the coating. The concen-
tration of the amine is lowest at the surface,
so
it cures faster than the interior, thus leading
to
a wrinkled appearance.lJ,” Other amines, such as
2-amino-2-methylpropanol
(AMP)
can cause auxiliary cross-linking.’”
These amine blocked catalysts are ionic (charged species) in character. Another type
is covalently blocked.” The advantage of this type is that it can be used
in
electrostatic
spray equipment where the paint should have low conductivity. However, covalently
blocked catalysts require a cure temperature
2
110°C (230°F) to deblock. Certain amine
blocked systems deblock at
2
65°C (149°F).
PI-irmny
Alcokols.
Note in Fig.
5
that the byproduct of the cross-linking is alcohol
formation. A few percent of a primary alcohol in the formulated paint can prolong the
stability of the system. The added alcohol effect can be explained by Le Chltelier’s princi-
ple (when an equilibrium system is perturbed, the equilibrium will always be displaced
in such a way as to oppose the applied change). Methanol would be the best choice because
it would volatilize the fastest, but it is also flammable. Most formulators choose butanol
as the retarder. Added alcohol, along with a blocked catalyst, provides systems with the
highest stability. It is worth noting that four times more n-butanol and more amine were
required
to
stabilize a paint made with a
55%
solids partially butylated MF resin than was
required when a high-solids
hexamethoxymethylmelamine
(HMMM) was used.Ix
3.3
Automotive Coatings-Clear Coats
This topic could
fill
a book in itself. Melamine-formaldehyde resins impart characteristics
such as superior distinctness-of-image
(DOI),
high gloss, and excellent mar resistance to
automotive clear coats. There has been some partial replacement of MF resins with isocya-
nate cross-linkers because coatings made with MF resins do not achieve the same level
of acid-etch This test is supposed
to
mimic a coating exposed to acid
rain. However, coatings made with MF resins substantially outperform isocyanate systems
where mar resistance is concerned.”.”
4.0
OTHER AMINO RESINS
Benzoguanamine resins impart flexibility and superior resistance to alkali and alkaline
detergent resistance versus MF resins. They also produce tlexible coatings. They cost
more than MF resins and have poorer
UV
durability,
so
they are used only when the above
properties dictate their use. Benzoguanamines have been used significantly in can coatings.
Glycoluril resin formulations require higher cure temperatures
or
higher catalyst levels,
but have excellent corrosion and humidity resistance. High cost limits their use. The
acrylamide-type amino resins have had very limited use. Some applications include coil
coatings for exterior usez3 and self-cross-linkable coatings.’4
5.0
FUTURE TRENDS
Amino resins will be with us for quite a while because,
as
a class of cross-linker, they
are very economical and give excellent performance. Government regulations have already
forced amino resin producers to make high-solids resins. Now these resins can be supplied
at lower free formaldehyde levels. A new low free formaldehyde MF resinX was introduced
that can cure at 82°C (180°F) with as little as
0.5%
acid catalyst.

AMINO
RESINS
675
Some paint suppliers have developed new vehicle resins
so
that
MF
resins can
continue to be used in high performance applications (again mainly cost driven). Carba-
mate-”.” and silane-containing’” vehicle resins provide enhanced exterior durability bene-
fits when cross-linked with
MF
resins. The desire to use amino resins in paint will keep
formulators busy for years to come.
REFERENCES
1.
2.
3.
4.
5.
6.
7.
8.
9.
IO.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
F.
N.
Jones and P.
S.
Ramachandran,
Polyrwric
Mcrferials
Science
Efrgirwerirlg,
77, 89
(I
997).
D.
E.
Erickson and T. P. Golden,
Pcrirrt
Coatir~gs
/ndusfry,
August 1995, p.
50.
P. Swaraj, ed.,
Surfirce
CONI~II~S:
Scieuce
cmd
Technology.
2d
Chichester: John Wilcy, 1997,
G.
D.
Vaughn,
PaitIt
Cocltirlgs
Irlrlustry
August 1998, p. 58.
J.
0.
Santer,
Progress
in
Urpnic
Coatings,
12, 309 (1984).
“Standard Test Method for Volatile Content of Coatings”, ASTM D2369-98.
J. J. Gummeson,
J.
Comings
Tech.,
June 1990, p. 43.
G.
D.
Vaughn and J. D. Jacquin, Presented at the 77th Annual Meeting of the FSCT, Dallas,
TX, October 20-22, 1999.
H. P. Higginbottom,
G.
R.
Bowers,
P.
E.
Ferrell, and L. H. Hill,
J.
Corrtirtgs
Tech..
July 1999,
W. J. Blank,
J.
Cocctings
Tech..
April 1982, p. 26.
A.
Berge,
B.
Kvacvcn, and J. Ugelstad,
Eur.
Po/vnwr
J.,
6, 981 (1970).
L. Calbo,
J.
Coatings
Tech.,
January 1980, p. 75.
“Standard Test Method for Package Stability of Paint”, ASTM D1849-95.
Z.
W. Wicks, Jr. and G.-F. Chen,
J.
Cor/tings
Tech.,
March 1978, p. 39.
L. W. Hill and
Z.
W. Wicks, Jr.,
Progress
in
Urgcrnic
Corrtiqs.
8,
161 (1980).
P.
E.
Ferrell, J. J. Gummeson, L. W. Hill, and L.
J.
Truesdell-Snider. Presented at the
73d
Annual Meeting of the FSCT, St. Louis, MO, October 9-1
I,
1995.
W. J. Blank, U.S. Patent
5
102 961, 1992.
J. C. Brogan and
N.
Albrecht,
Pmc.
4th
Internntionol
Prrint
Congress,
Sao Paulo, Brazil, 1995,
p. 839.
J. W. Holubka, P. J. Schmitz, and L.-F. Xu,
J.
Contings
Tech.,
February 2000,
p.
77.
P.
J. Schmitz, J. W. Holubka, and L.-F.
Xu,
J.
Coutitzgs
Tech.,
May 2000, p. 39.
B. Pourdeyhimi,
X.
Wang, and F. Lee,
Eur.
Corctings
J.,
4,
100
(1999).
J. L. Courter and Kamenetzky,
Eur.
Coatings
J.,
7-8, 24 (1999).
M.
T.
Keck, R. J. Lewarchik, and J. C. Allman,
U.S.
Patent
5
688 598, 1997.
S.
Swamp and M. A. Mayo,
US.
Patent
5
618
586,
1997.
J. D. McGee and
B.
D. Bammel, Presented at the 8th Annual ESD Advance Coatings Technol-
ogy Conference, Detroit, MI, September 28-29, 1998.
I.
Hazan, Presented at the 8th Annual ESD Advance Coatings Technology Conference, Detroit,
MI, September 28-29, 1998.
pp. 861-865.
p. 49.
This Page Intentionally Left Blank

Part
4
Surface
Coatings
This Page Intentionally Left Blank

76
Flexographic Inks
Sam Gilbert
Sur1
Cltemic~rl
Corportrtiort,
Northltrkr.
lllirtois
1
.O
INTRODUCTION
Flexographic inks, like most other inks, are based on colorants (pigment or dye), various
combinations of resins, and various combinations of solvents (organic and water). The
particular combination
of
materials that is necessary for
a
given ink is determined by the
flexographic process, its physical and mechanical attributes (e.g. press applications and
plates), and by the end-use applications, which incorporate considerations of substrates
and finished product requirements.
2.0
PROCESS
For printing any given package we must first look at the process. If,
in
fact, that package
will be printed by flexography, then certain ink attributes are predetermined.
The flexographic inking system is based on either
a
three-roll system or
a
system
that uses enclosed doctor blades. The three-roll system uses
a
rubber pickup roll that picks
up the ink and transfers it to an anilox roll (engraved metal or ceramic roll). The ink fills
the engraved cells and is then transferred to plates (either rubber or plastic), where the
image is raised in relief from the background, nonimage area.
A
final transfer then occurs
from the plate to the substrate.
In the enclosed doctor blade system, ink is applied directly to the anilox roll, with
the doctor blade being used to remove excess ink. Ink is then transferred through the
balance of the process
as
described above.
In both cases the amount of color (ink) to be applied
to
the substrate is determined
by the viscosity of the ink. The amount of the ink carried by an anilox roll is dependent
on the volume
of
the engraved cells in that roll. Higher viscosity with any given anilox
will generally provide more color.
679

680
GILBERT
To
provide easy ink transfer through the inking system, the actual viscosity of most
inks is very low
(0.1
-
1
.O
poise). The solvents used to make the inks and to adjust the
viscosity of the finished ink at the press are very volatile. Flexo presses can run at speeds
from
50
to
2000
ft/min. The solvent used will vary in volatility from very fast (ethyl
acetate) to very slow (glycol ethers), depending on press speed and ink formulation. The
major solvents used are alcohols (ethyl, N-propyl), acetates (ethyl, N-propyl), and alipathic
hydrocarbons (heptane). Water containing inks constitute
a
large percentage of the inks
used.
Another press consideration is the printing plate type that will be used. Rubber plates
demand certain solvents, and photopolymer plates demand different solvent combinations.
The solvent system used in the ink must be one that will not attack the plates but will
provide good ink transfer from the plate
to
the substrate.
3.0
SUBSTRATE
The next consideration is the substrate to be printed: paper, polyethylene, polypropylene,
foil,
or
some other material.
The attributes desirable
in
the package will determine the substrate. What is to be
packaged?
Is
it
a
solid'? A liquid? Is it perishable, corrosive, moisture sensitive,
or
heat
sensitive'? Does it need protection from light. oxygen, or grease'? Will the product be
processed in the package'? Must it withstand cooking temperatures or storage
as
a frozen
food'? Will the Environmental Protection Agency (EPA), the Food and
Drug
Administra-
tion (FDA)
or
other government body be involved? How will the package be formed,
filled, and sealed'?
These conditions determine the package type and the substrate to be used. The
chosen substrate will partly define the proper composition of ink vehicles.
4.0
COLORANTS
The choice
of
colors will complete the process
of
putting together an ink. There are two
kinds of colorants available for use in printing inks-dyes and pigments. For the most
part dyes do not offer the light and product resistance that pigments offer, and their use
in flexographic inks has declined substantially.
The pigments used in manufacturing most flexo inks are classed as either organic
or inorganic. They are colored, largely insoluble compounds. Each compound
as
found
in
nature
or
as
manufactured produces its own unique color.
5.0
VEHICLE
The varnish portion of the ink, more properly referred to
as
the vehicle, is a composite
of
resins, various additives, and solvents.
Basically, the resin may be thought of
as
a
virtually transparent component that
suspends and supports the pigment on the printed surface. It is the vehicle that provides
adhesion to the substrate and provides most of the properties required. For the proper
formulation of inks, the resins must be soluble in the desired solvents, offer good transfer,
and have good, fast solvent release.
When the ink is being manufactured, sufficient pigment is added
to
the resin system
to obtain the desired color strength. The pigment must be dispersed
so
that agglomerates

FLEXOGRAPHIC
INKS
681
are eliminated and all pigment surfaces are wetted with the vehicle; otherwise adhesion
of
the ink will be poor and ink properties will suffer.
To obtain the correct color strength on the substrate, the viscosity of the ink must
be controlled. High viscosity produces high color strength but poor ink properties. Low
viscosity produces low color strength. The solvent
or
water used
to
adjust viscosity is
only the carrier. It
is
added to the mixture
of
pigment and resin to make the combination
flow. After the ink has been applied to the surface to be printed, the solvent must be
removed. This is done by evaporation, normally in hot
air
ovens. The air volume and
velocity are more important than temperature level.
6.0
FORMULATIONS
A typical formula for use on polyethylene film might be:
Pigment
Nitrocellulose resin
Polyamide resin
Wax
additive
Ethyl alcohol
N-Propyl acetate
Heptane
15.0%
5.0%
20.0%
1
.O%
3S.096
9.0%
15.0%
This formula is, of course, solvent based. Formulas largely based on water also are
quite common for use on paper. A typical formula might be:
Pigment
Acrylic resin
Acrylic emulsion
Solvent
Wax
Antifoaming agent
Water
15.0%
4.0%
60.0%
5.0%
1
.O%
0.5%
14.5%
Water systems for use on many different substrates are now being developed and
will be quite common in the future.
