Satas D., Tracton A.A. (ed.). Coatings Technology Handbook
Подождите немного. Документ загружается.


492
BLOMSTROM
both acids and bases. although they are slowly attacked by strong aqueous acids. Their
resistance
to
hydrocarbons and mineral oils is outstanding. Because
of
the residual hydro-
xyl groups
in
the polymers (Fig.
l),
they are readily cross-linked with a variety
of
widely
available crosslinking reagents that react with hydroxyl. Examples of the more common
cross-linking reagents are dialdehydes and phenolic, amino, isocyanate. and epoxy func-
tional resins. The butyral shows limited compatibility with other resins (e.g, alkyd. polyvi-
nyl
chloride, silicones. urea- and melamine-formaldehyde. cellulose acetate butyrate, and
ethyl cellulose) and excellent compatibility with nitrocellulose. epoxy. phenol-fonnalde-
hyde. isocyanate, and some rosin ester derivatives. The formal. being more polar than the
butyral. shows more limited compatibility with other resins.
It
is fully conlpatible with
most common isocyanate and epoxy resins, and shows limited compatibility with alkyd,
phenolic. melamine- and urea-formaldehyde resins. and silicones.
3.2
Physical and Chemical Properties
The physical and chemical properties of Butvar resins are shown in Table
I.
and the
mechanical, thermal, and electrical properties are given
in
Table
2.
Table
3
gives solubility
data for the Butvar resins in
a
variety
of
solvents. The physical and chemical properties
of
the Formvar resins are shown in Table
4;
the mechanical. thermal. and electrical proper-
ties are listed in Table
5.
Solubility data for the Formvar resins are given
in
Table
6.’.
Properties of Butvar dispersion BR are shown in Table
74.
The data given
in
Tables
1-7
are typical properties, not the manufacturer’s specifications. For actual product specifica-
tions, the nmwfacturer should be consulted. The polyvinyl formal grades are all chemically
identical: they differ only in molecular weight. The polyvinyl butyrals fall
into
two chemi-
cal groups, high
(17.5-21
wt
9’0)
residual polyvinyl alcohol, and low
(10.5-13
wt
96)
polyvinyl alcohol. Within each
of
these groups, molecular weight is also varied.
In
general,
as the percentage of polyvinyl alcohol increases. toughness, modulus. and adhesion
to
polar surfaces will increase. while the range of useful solvents decreases (conversely,
solvent resistance increases).
As
the percentage
of
polyvinyl alcohol decreases. the resins
become more broadly soluble, softer. and lower in glass transition temperature.
Within
a
given chemical group. increasing molecular weight results
in
a modest
increase in tensile strength and modulus, increased solution viscosity. and a modest in-
crease
in
solvent and chemical resistance. In general, even the lowest molecular weight
grades
of
these resins are high enough in molecular weight to be well within the chain
entanglement region
of
the
E‘
spectrum such that physical properties do not change drasti-
cally as molecular weight increases. Molecular weight variations can be used effectively
to control the rheology/viscosity
of
coatings formulations more than the properties
of
the
final coating.
3.3
Solution Viscosity
Solution viscosity is a function
of
chemical composition. molecular weight. and solvent
composition. Within
a
given chemical class, solution viscosity in a given solvent will
increase with molecular weight. The effect
of
solvent composition on solution viscosity
is more complex and not within the scope of this discussion. Solvent systems are often
chosen for
a
variety of reasons other than viscosity: drying rate. toxicity and other environ-
mental concerns, utility with other resins needed in a formulation. etc. Usually by judicious
experimentation. a compromise solvent system can be found, that will give a reasonable
mix of the required properties.
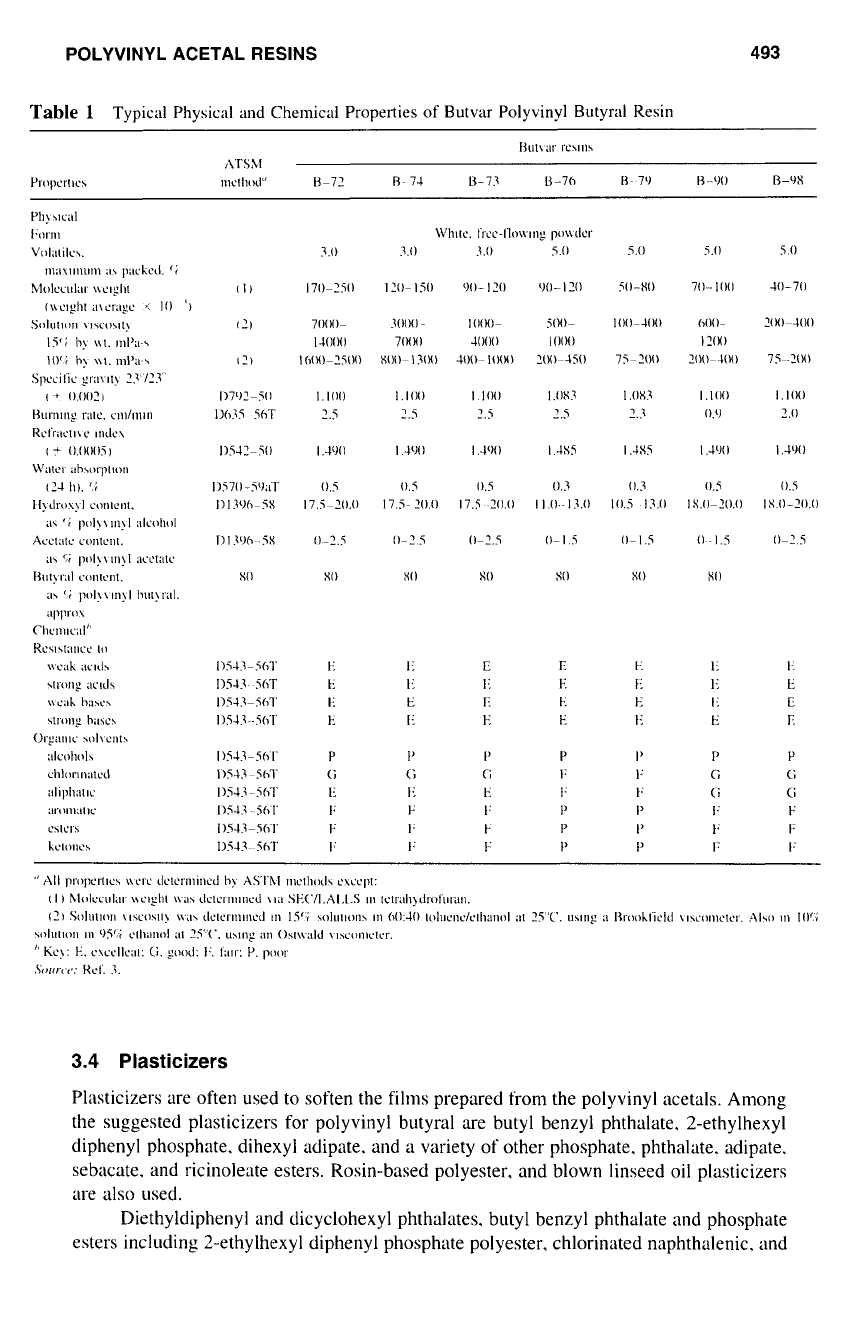
POLYVINYL ACETAL RESINS 493
Table
1
Typical Physical
and
Chemical Properties
of
Butvar Polyvinyl Butyral Resin
l3ut\:lr
rc\111\
,\.r S
M
Plopcrllc\
I11clllll'l"
13-72 7-1 B-73 B-70
1%
7')
H"J0
&')X
t.
I:
I:
1'.
E
I:
I:
E
3.4
Plasticizers
Plasticizers are often used to soften the films prepared from the polyvinyl acetals. Among
the suggested plasticizers for polyvinyl butyral are butyl benzyl phthalate, 2-ethylhexyl
diphenyl phosphate. dihexyl adipate, and
a
variety of other phosphate, phthalate. adipate.
sebacate, and ricinoleate esters. Rosin-based polyester, and blown linseed
oil
plasticizers
are
also used.
Diethyldiphenyl and dicyclohexyl phthalates. butyl benzyl phthalate and phosphate
esters including 2-ethylhexyl diphenyl phosphate polyester. chlorinated naphthalenic, and

494
BLOMSTROM
Table
2
Typical Mechanml. Thermal, and Electrical Properties
of
Bulvar Polyvinyl Butyral Resin
Properties
Bulvar resins
ASTM
method B-72 B-74 B-73 B-76 B-79 B-90 B-98
Mechanical"
Tensile strength. MPa
yield
break
Elongation.
(X-
yield
break
Modulus
of
elasticity
(apparnct). GPa
Flexural strength.
yleld. MPn
Hardness. Rockwell
M
E
Notched Izod Impact strength
(I
.25 cm
X
I
.25 cm), J/m
Thermal
("C)
Flow temperature,
Glass
temperature"
Heat distortion temperature
Heat-scaling temperature"
Dielectric constant
Electrical
50
Hz
1
kHz
I
MHz
10
MHz
Dissipatlon factor
X
IO3
50
Hz
1
kHz
1
MHz
IO
MHz
Diclectrlc strength. V/m
(3.2
mm
thlckness)
short titne
step by
step
D638-58T 47-54
D63X-58T 48-55
D638-58T
8
D638-58T 70
D638-58T 2.3-
2.4
D7Y0-59T 83-90
D785-51
115
D785-51 20
D256-56
59
D569-59 145-
155
72-78
D648-56 56-60
1
05
D 150-59T 3.2
D150-59T 3.0
D150-59T 2.8
D
150-59T 2.7
D150-59T 6.4
D150-59T 6.2
D 150-59T 27
D 150-59T
3
1
D149-59 17
D149-59
16
41-54
48-55
8
75
2.3-
2.4
83-90
I
15
20
59
135-
145
72-78
56-60
1
0s
3.2
3.0
2.8
2.1
6.4
6.3
27
31
17
16
45-52
4 1-48
8
80
2.2-
2.3
79-86
115
20
55
125-
I30
72-78
55-59
99
3.0
2.7
2.6
2.5
5.8
5.5
22
22
19
16
40-47
32-39
8
1
10
I
.9-
2.0
72-79
100
5
43
1
10-
I
15
62-72
50-54
93
2.7
2.6
2.6
2.5
5.0
3.9
13
15
I9
15
40-47
32-39
8
110
1.9-
2.0
72-79
100
5
43
110-
115
62-72
50-54
93
2.7
2.6
2.6
2.5
5
.0
3.9
13
15
19
15
43-50
49-46
8
100
2.1-
2.2
76-83
115
20
48
125-
130
72-78
52-56
205
3.2
3.0
2.8
2.7
6.6
5.9
22
23
18
l5
43-50
39-46
8
110
2.1-
2.2
76-83
1
10
20
37
1
05-
110
72-78
45-55
200
3.3
3.0
2.8
2.8
6.4
6.1
3-3
74
16
15
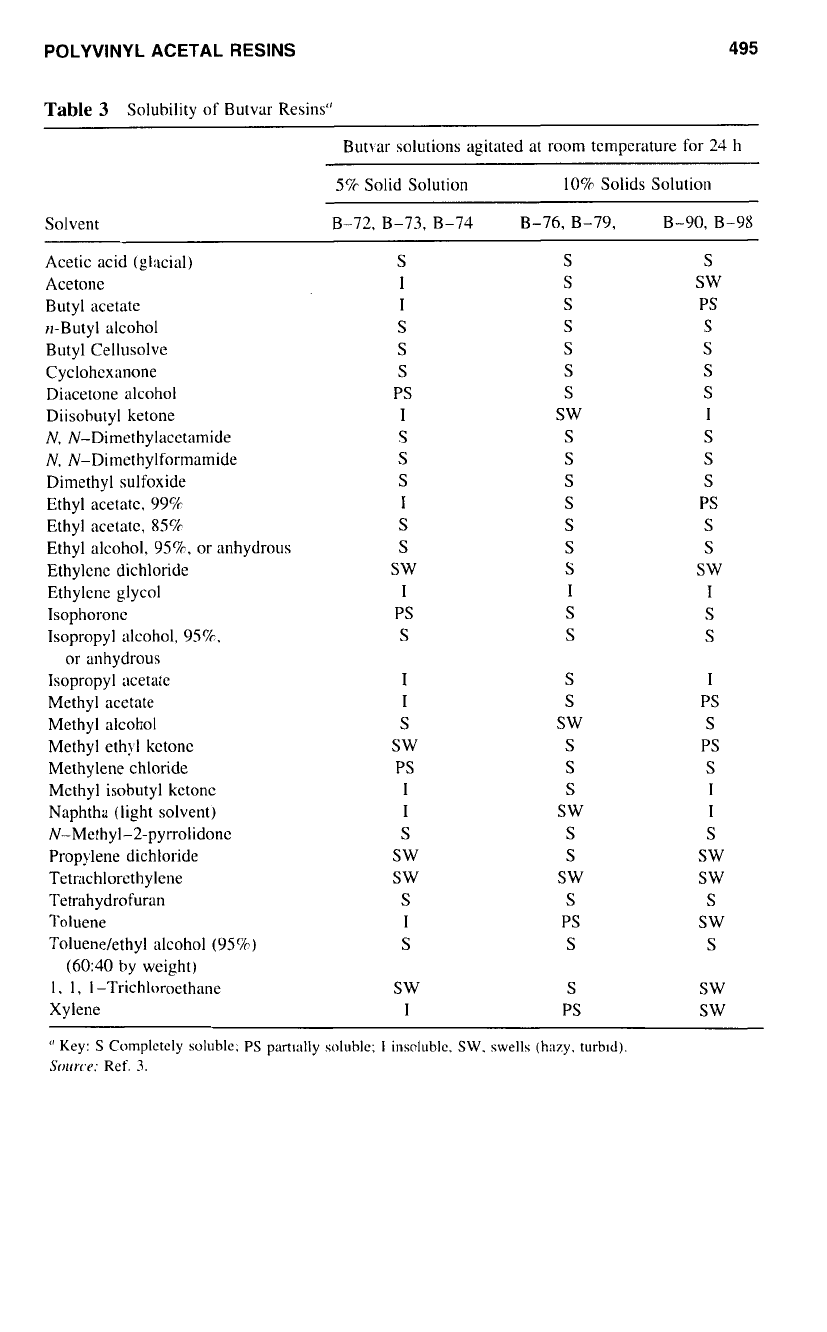
POLYVINYL ACETAL RESINS
495
Table
3
Solubility of Butvar Resins"
Butvar solutions agitated at room temperature for 24 h
5%
Solid Solution 10% Solids Solution
Solvent B-72, B-13, B-l4
B-16,
B-19, B-90, B-98
Acetic acid (glacial)
Acetonc
Butyl acetate
rt-Butyl alcohol
Butyl Cellusolve
Cyclohexanone
Diacetone alcohol
Diisobutyl ketone
N. N-Dimethylacetamide
N. N-Dirnethylformamide
Dimethyl sulfoxide
Ethyl acetate,
09%
Ethyl acetate,
85%
Ethyl alcohol,
959,
or
anhydrous
Ethylene dichloride
Ethylene glycol
Isophoronc
Isopropyl alcohol, 95%.
or
anhydrous
Isopropyl acetate
Methyl acetate
Methyl alcohol
Methyl ethyl kctonc
Methylene chloride
Mcthyl isobutyl kctonc
Naphtha (light solvent)
N-Mcthyl-2-pyrrolidonc
Propylene dichloride
Tetrnchlorcthylene
Tetrahydrofuran
Toluene
Toluene/ethyl alcohol
(95%)
(60:40 by weight)
1.
l,
1
-Trichloroethane
Xylene
S
1
I
S
S
S
PS
I
S
S
S
I
S
S
SW
I
PS
S
I
I
S
SW
PS
I
I
S
SW
SW
S
I
S
SW
I
S
S
S
S
S
S
S
SW
S
S
S
S
S
S
S
I
S
S
S
S
SW
S
S
S
SW
S
S
SW
S
PS
S
S
PS
S
SW
PS
S
S
S
S
I
S
S
S
PS
S
S
SW
I
S
S
I
PS
S
PS
S
I
I
S
SW
SW
S
SW
S
SW
SW
~ ~~ ~~
~
l'
Key:
S
Completely
soluble:
PS
part~ally
soluble;
I
insoluhlc,
SW.
swells
(hazy. turbld).
Soutrr:
Ref.
3.
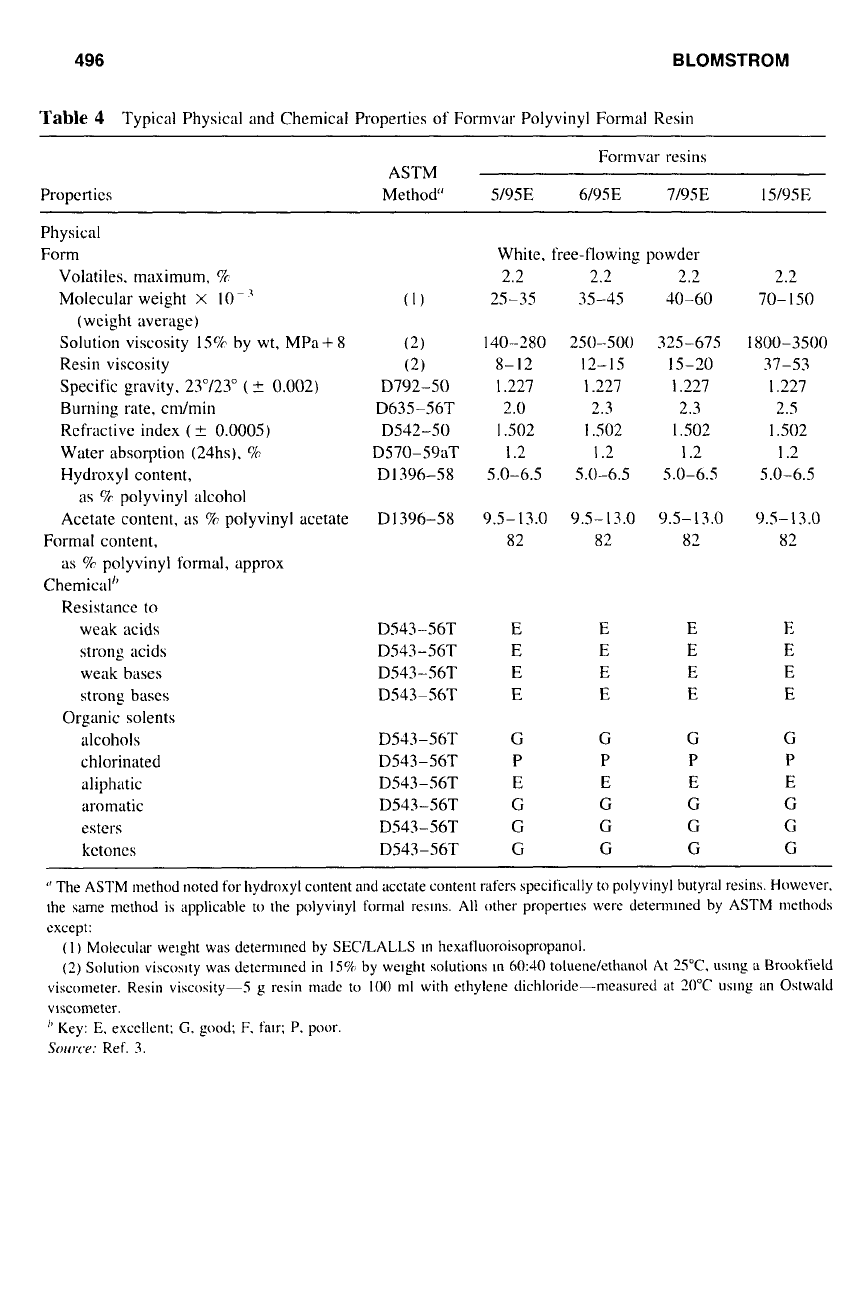
496
BLOMSTROM
Table
4
Typical Physical and Chemical Properties of Formvar Polyvinyl Formal Resin
Properties
Formvar resins
ASTM
Method"
Y95E
6/95E
7/95E
I
5/95E
Physical
Form
Volatiles. maximum,
'7c
Molecular weight
X
10-
'
(weight average)
Solution viscosity
15%
by wt, MPa+8
Resin viscosity
Specific gravity,
23"/23"
(2
0.002)
Burning rate, cnhin
Refractive index
(
2
0.0005)
Water absorption (24hs).
p/r
Hydroxyl content,
as
7r
polyvinyl alcohol
Acetate content,
;IS
%
polyvinyl acetate
as
Yr
polyvinyl formal, approx
Resistance
to
weak acids
strong acids
weak bases
strong bases
Organic solents
alcohols
chlorinated
aliphatic
aromatic
esters
kctones
Formal content,
Chemical"
(2)
(2)
D792-50
D635-56T
D542-50
D570-59aT
Dl
396-58
D 1396-58
DM-56T
D543-56T
D543-56T
D543-56T
D.543-56T
D543-56T
D543-56T
D543-56T
D543-56T
D543-56T
White. free-flowing powder
2.2
2.2
2.2
25-35
35-45
40-60
140-280
250-500
325-675
8-12
12-15
15-20
1.227
I
.227
1.227
2.0
2.3
2.3
I
,502
1.502
I
,502
1.2
1.2
1.2
5.0-6.5
5.0-6.5
5.0-6.5
9.5-13.0
9.5-13.0
9.5-13.0
82
82
82
E
E
E
E
E
E
E
E
E
E
E
E
G
G
G
P P P
E
E
E
G G G
G G G
G G
G
2.2
70-
I
50
1x00-3500
37-53
1.227
2.5
1
.so2
1.2
5.0-6.5
9.5-13.0
82
E
E
E
E
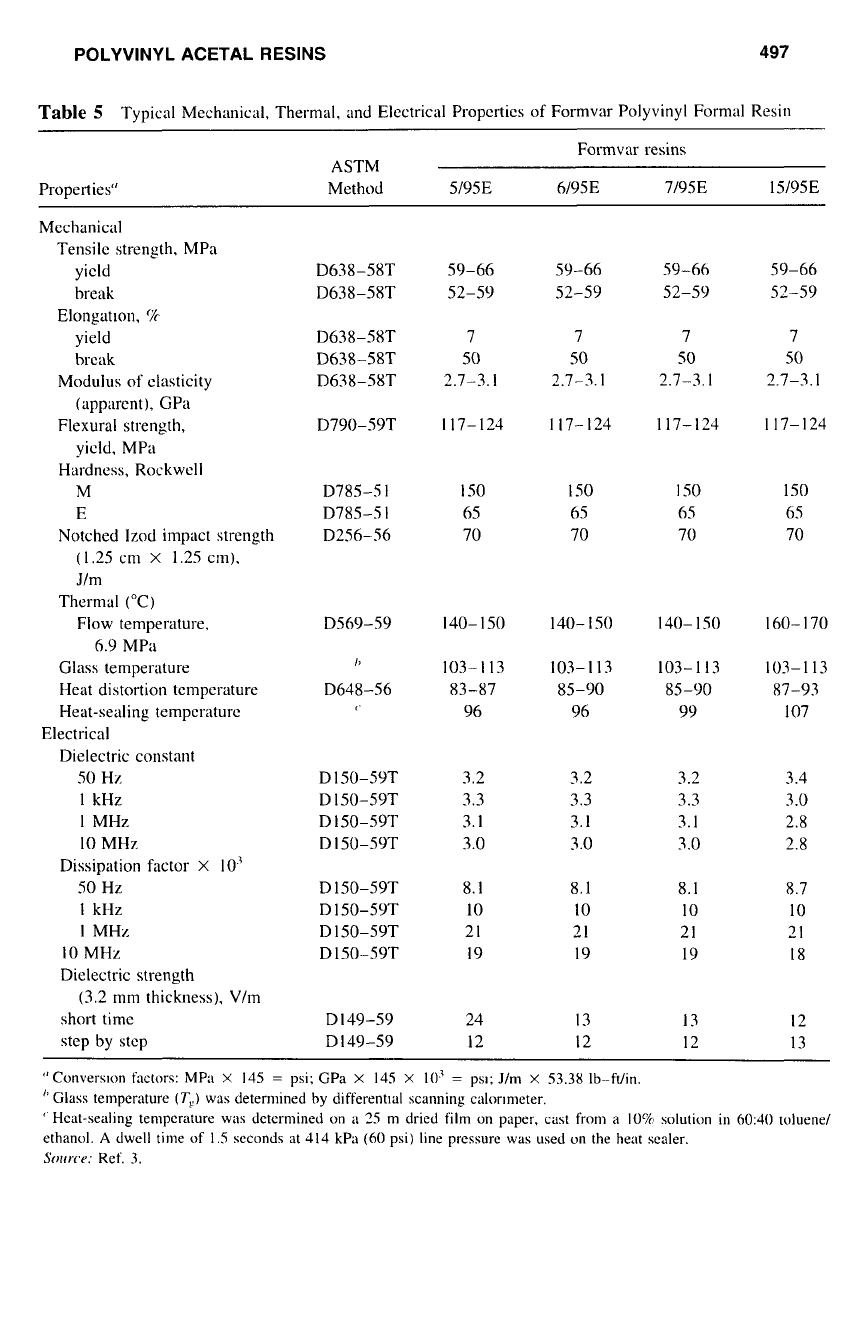
POLYVINYL ACETAL RESINS 497
Table
5
Typical Mechanical, Thermal. and Electrical Properties
of
Formvar Polyvinyl Formal Resin
Formvar resins
ASTM
Properties" Method 5195E 6195E 7195E
1
5195E
Mechanical
Tensile strength. MPa
yield
break
Elongation.
9
yield
break
Modulus of elasticity
(apparent). GPa
Flexural strength,
yield. MPa
Hardness, Rockwell
M
E
Notched lzod impact strength
(1.25 cm
X
1.25 cm).
Jlm
Thermal
("C)
6.9
MPa
Glass temperature
Heat distortion temperature
Heat-sealing temperature
Dielectric constant
Flow temperature.
Electrical
SO
Hz
1
kHz
I
MHz
IO
MHz
Dissipation factor
X
50
Hz
1
kHz
I
MHz
IO
MHz
Dielectric strength
short time
step by stcp
(3.2
mm thickness), Vlm
D638-58T
D638-58T
D638-58T
D638-58T
D638-58T
D790-59T
D785-5
1
D78.5-5
I
D256-56
D569-59
I2
D648-56
D 150-59T
D 150-59T
D
I
50-59T
D 150-59T
D 150-59T
D 150-59T
D
1
50-59T
D 150-59T
D 149-59
D 149-59
59-66
52-59
7
50
2.7-3.
I
117-124
I
50
65
70
140-
1
50
103-1
13
83-87
96
3.2
3.3
3.1
3.0
8.1
IO
21
19
24
12
59-66
52-59
7
50
2.7-3.1
117-124
1
50
65
70
140-150
103-113
85-90
96
3.2
3.3
3.1
3.0
8.1
IO
21
19
13
12
59-66
52-59
7
50
2.7-3.
I
117-124
1
50
65
70
140-
1
50
103-1
13
85-90
99
3.2
3.3
3.
I
3.0
8.1
IO
21
19
13
12
59-66
52-59
7
50
2.7-3.1
117-124
1
50
65
70
160-
170
103-1
13
87-93
I07
3.4
3
.o
2.8
2.8
8.7
IO
21
18
12
13
"Converston factors:
MPo
X
145
=
psi;
GPa
X
145
X
IO'
=
psi;
Jlm
X
53.38
Ib-ft/in.
"
Glass
temperature
CT,)
was
determined
by
differentlal
scanning
calorlmeter.
'
Heat-sealing temperature
was
determined
on
a
3-5 m
dried
film
on
paper,
cast
from
a
10%'
solution
in
60:4O
ethanol.
A
dwell
time
of
1.5
seconds
at
414
kPa
(60
psi)
line
pressure
was
used
on
the
heat
sealer.
Sorrrcr:
Ref.
3.

498
BLOMSTROM
Table
6
Solubility
of
Formvar Resins"
Solvent
Formvar resins
15195E. 71958. 61958, 5I95E
Acetic acid (glacial)
Acetone
Aniline
Benzene
Butyl alcohols
Butyl acetate
Carbon disulfide
Cresylic acid
Cyclohexanone
Diacctone alcohol
Diisobutyl ketone
Dimethyl sulfoxide
N. N-Dimethylacetamide
N,
N-Dirncthylformamide
Ethyl acetate, 99%
Ethyl acetate, 85%
Ethyl nlcohol,
95%.
or anhydrous
Furfural
Hexane
Isopropyl alcohol. 95%, or anhydrous
Methyl acetate
Methyl alcohol
Methyl benzoate
Methyl butynol
Methyl Cellosolve acetate
Methyl ethyl ketone
Methyl isobutyl ketone
Methyl pentynol
N-Methyl-2-pyrrolidone
Nitropropane
Pentoxol
Propyl alcohols
Phenol
Propylene dichloride
Tetrachlorethane
Tetrahydrofuran
Toluene/cthyl alcohol (95%)
(6040
by weight)
Toluene
VMgLP Naphtha
Xylene-rz-butyl alcohol (60:40 by weight)
Xylcnc
S
1
S
I
I
I
1
S
I
I
I
S
S
S
I
l
I
S
I
I
I
I
S
S
I
1
I
S
S
I
I
I
S
I
S
S
S
I
I
I
I
"
Key:
S,
Completely
soluble;
I.
Insoluble
or
not
complctely
soluble
Source;
Ref.
3.

POLYVINYL ACETAL RESINS
499
Table
7
Properties
of
Butvar Dispcrsion BR
Property DescriptionNaluc
Form
Milk-white aqueous dispersion
of
plasticized
polyvinyl butyral
Total
solids 50.0-52%
Viscosity 500-
I500
mPa"
Particle sue
Most particles between 0.25 and
1.5
p
Particle charge
Anionic
Plasticizer content
40
parts per
100
parts
of
resin (28.6%
of
solids)
Pounds per gallon at 25°C
8.4
Grams pcr liter
1008
''
Determmed
on
n
Brookfield
vlscometer,
LVF,
no.
3
Spndle.
30
qm.
25°C.
PH
8.0-
10.5
adipate diesters are useful for polyvinyl formal. By proper choice of plasticizer type and
level, the physical-mechanical, chemical. and adhesion properties
of
these resins can be
tailored for
a
wide variety
of
applications. Fitzhugh and Crozier5 have studied the effect
of a number of plasticizers
on
the mechanical properties of polyvinyl acetal resins.
3.5 Toxicology
Butvar resins are regulated by the
U.S.
Food and Drug Administration under the Code of
Federal Regulations,
as
indirect food additives. Butvar resins have also been subjected
to
acute toxicity studies
on
laboratory animals. Subject to the appropriate regulations, they
are useful
in
a
number of packaging applications for both fatty and aqueous foods.
Both Butvar and Formvar resins have flash points
in
excess of 370°C. The lower
explosive limit
(LEL)
for Butvar dust in air is
20
g/m3. Although these materials are
considered to be nontoxic
in
ordinary everyday handling, good industrial hygienic practices
should be observed when using them.
4.0
SURFACE COATING APPLICATIONS
4.1
Polyvinyl
The inherent properties of adhesion
to
a
wide variety
of
surfaces, film toughness and
chemical/solvent resistance, and film clarity of the polyvinyl butyral resins makes them
the vehicle of choice in
a
wide variety of specialty coating applications. They adhere
tenaciously to most polar surfaces: wood, glass, metals, ceramics, pigments, etc. Their
high binding efficiency allows them
to
be used at very high pigment loadings. Ceramic
films are typically cast
in
thicknesses from fractions of
a
mil to several millimeters, using
4-5%
Butvar B-76
as
the sole binder"; most permanent coatings applications will require
considerably higher binder levels than this for adequate film strength. Polyvinyl butyral
resins have been used
as
the binder of choice in thermophotographic and photographic
coatings,' in photoconductive coatings, in electrophotographic coatings, and
as
a
coating
on optical recording disks,
all
of
which depend
on
film toughness, binder efficiency, and
optical clarity. Their toughness, chemical and heat resistance, and binding efficiency render
them useful in powder coatings and photothermographic coating applications.

500
BLOMSTROM
Table
8
Wcstern Pine Association Knot Sealer, WP578: Brush Application
Material
p/r
by weight
Butvar B-90 (Monsanto Chemical Co.)
Durite P-97 (Borden Chemical Co.)
SDA 3SA.
95%
ethanol
3.3
40.0
56.7
100.0
-
Polyvinyl butyrals are used extensively as wood coatings, where their resistance
to
natural wood oils makes them a primary choice for sealers and wash coats. An example
of an application
on
the Western Pine Association Knot Sealer number WP578 is given
in Table
8.
A well-known application of polyvinyl butyrals is
in
the manufacture of wash
primers for the priming of metal surfaces to be used
in
hostile environments (e.g., the
hulls
of
naval vessels). There are a number of formulations available, both single- and
two-package systems. Another example of a metal coating based on polyvinyl butyral is
Metal Coating
2009,
described
in
Table
9.
Other coating applications of the polyvinyl butyrals include solder masks for printed
circuit board manufacture, heat-fusible wire coatings, and zinc oxide-based photosensitive
paper coatings; they also serve as the bindedvehicle for iron oxide
in
the production of
magnetic recording tapes. They are widely used as
toughness/flexibility/adhesion
pro-
moters
in
the production
of
inks for letterpress, flexographic, and gravure printing, and
as
a component in toners for reprography.
4.2
Polyvinyl Butyral Dispersions
The dispersion
of
plasticized polyvinyl butyral
in
water, marketed by Monsanto as Butvar
Dispersion
BR.'
is widely used
as
a permanent surface size in critical textile applications
Table
9
Metal Coating 2009: Spray or Roller Application
Material
76
by weight
Diacetone alcohol 17.4
SDA-3SA.
95%
ethanol 7.1
Xylene 34.7
Mcthylon 75-
IO8
(Bakelite Thermosets, Ltd.)
S.
I
Epon 1007 (Shell Chemical Corp.)
13.0
Butvar B-90 (Monsanto Chemical Co.) 2.0
10%
Phosphoric acid (diluted with a portion
of
the given
-
2.7
solvent mixture) 100.0
Cure
cycle:
15
minute air dry
+
30 minutes at 190°F and
20
minutes at 400°F
S(JI~WC:
Ref.
3.
/l-Butanol 17.4

POLYVINYL ACETAL RESINS
501
e.g.. seat belt and parachute webbing). where the toughness of the butyral film lends
outstanding abrasion resistance to the fabric. The relatively high surfactant levels used
in
the manufacture of this dispersion reduce the inherent adhesion
of
the resin to most highly
polar surfaces such as metals and glass. This property is used to advantage
in
the prepara-
tion
of
removable coating for temporary protection of sensitive surfaces. This dispersion
is typically formulated with other dispersions/latex as extenders to reduce cost. Pigments
and plasticizers can be incorporated. as can small amounts of solvents to enhance adhesion
properties.
4.3
Polyvinyl
Formal’,8
Polyvinyl formal, available in several viscosity grades from Monsanto as Formvar, is
widely used as an oil-resistant insulating coating for magnetic wire. For this application
it
is normally cross-linked by formulating with phenolic, epoxy, or urethane resins to
enhance properties. These coatings are tough, strongly adhering, abrasion resistant. and
totally impervious
to
hydrocarbon oils and lubricants. Polyvinyl formal serves as the base
vehicle for coatings for printed circuit boards. both permanent and fugitive (e.g., solder
masking). for the surface treatment
of
glass fibers and boron filaments (abrasion resis-
tance), and
as
an antifog coating
on
glass.
It
finds application in photothermographic and
electrographic coatings. It is used as a binder
in
magnetic tape coatings. particularly for
the high performance chromium oxide coated tapes.
It
finds use as an
ink
binder and as
a lacquer to protect the surface of lithographic plates.
In
general, polyvinyl formal is higher
in
modulus and less susceptible to solvent
attack than is polyvinyl butyral. Conversely, solvent choices are more limited for the
formal. Films are somewhat yellow, reducing their utility
in
applications calling for a
colorless film.
REFERENCES
1.
2.
3.
4.
5.
6.
7.
8.
K. Toyoshima,
in
Po/yirlyl
Alcohol
C.
A.
Finch, Ed. New York: Wiley. 1973,
pp.
381-41
I.
T. P. Blomstrom, in
Blcyc/o/mfirr
of
Polyrrw
Scier~ce
trrld
D~~gir~eerir~,g.
2nd ed.,
Vol.
16.
J.
I.
Kroschwitz, Ed. New York: Wiley-Intcrscience, 1989.
EurwdFornlvor Brnchtre.
publication No 6070E, Montanto Chemical Company. Plastics and
Resins Division, 800 North Lindbergh Avenue, St. Louis, MO 63167.
Butvrrr
Dis/~er:~ior~s
ER
Drrfa
Sheet.
Monsanto Chemical Company. Plastics and Resins Division.
800 North Lindbcrgh Avenue, St. Louis,
MO
63 167.
A.
F.
Fitzhugh and R. N. Crozler,
J.
Polwl.
Sci..
K.
225 (1952);
9.
96
(1952).
K.
Hoshi,
S.
Tasaka, and
T.
Yoshimi,
Jpn.
Kokat. 62/46, 953 (1987): Taiyo Yudcn Co. Ltd.,
[
Cherrl.
ADstr..
/116(22), 18
I,
48
I
h.]
T.
T.
Boersma.
J.
hr~tr,girrg
Techr~ol..
13(2), 75,
(
1987).
E. Lavin and
J.
A. Snelgrove, “Polyvinyl acetals,”
in
Erzc:\.c/o~pedit~
of
Cherrlicd
Tech~ology,
3rd ed.,
Vol.
23.
New
York: Wiley-lnterscicnce, 1983.
pp.
798-816.
