Satas D., Tracton A.A. (ed.). Coatings Technology Handbook
Подождите немного. Документ загружается.

This Page Intentionally Left Blank
57
Polyimides
I
Polyimides are condensation polymers that contain the imide structure
“CO-N-CO-
as a linear or heterocyclic unit along the polymer main chain. They exhibit exceptional
thermal, thermooxidative, and chemical resistance, and good radiation resistance and di-
mensional stability, while maintaining an excellent balance of mechanical and electrical
properties.
1.0
CHEMISTRY AND PROPERTIES
Aromatic polyimides are generally produced by a two-step polycondensation reaction of
aromatic dianhydride with either aromatic diamine or aromatic diisocyanate in a suitable
reaction medium. They have the following general structure:
The direct production of high molecular weight aromatic polyimides in
a
one-step polymer-
ization could not be accomplished because the polyimides are usually insoluble and intract-
able. The polymer chains precipitate from the reaction media (whether solution or melt)
before high molecular weights are obtained. Therefore, processing of the aromatic polyim-
ides can be accomplished only with the first step intermediate amic acid varnish, which
is still soluble and fusible. Were
it
not for the processing difficulties associated with known
503

504
LEE
polymers, polyimides would be enjoying success in many more new application areas.
This processing problem was partially overcome by the development of copolymers. The
two major commercial polyimide copolymers are an amide-imide known as Torlon, a
product
of
Amoco, and an ether-imide produced by General Electric under the trade name
Ultem. Another approach to this processing problem was by incorporating a soft aromatic
segment and/or aliphatic moiety in the polymer main chain. Among the various efforts
to
overcome the processing difficulties, one commercial success was achieved by incorpora-
tion of totally asymmetric diamionophenylindane isomeric mixture into the polyimide
backbone; it is marketed as Matrimid 5218 from Ciba-Geigy. This material is soluble
in
relatively nonpolar solvents and is characterized also by exceptionally high glass transition
temperature and high thermooxidative stability.
Key properties
of
aromatic polyimides are their outstanding high temperature resis-
tance, toughness, good dielectric properties, low flammability, and high resistance to radia-
tion and structural deformation under load at elevated temperatures.
2.0
USES
Polyimides are primarily used in the aerospace, automotive, and electronic industries,
where a need exists for materials with long-term high temperature capabilities. Molded
polyimides are now widely used in loading bearing applications such as struts, chassis,
and brackets in automotive and aircraft structures because
of
their high flexural modulus
and compressive strength. Their excellent high temperature dimensional stability, chemical
resistance, and natural lubricity make them ideal bearing materials in such applications
as jet engines, appliances, and office equipment. Polyimide films are used as insulation
for electric motors, aircraft parts, missile wire cable, magnetic wire, and flat flexible cable.
The electrical properties of polyimides make them ideally suitable for applications
in
the electrical and electronic markets. They are being used in place of glass and ceramics
for high heat connectors, switches, housings, and controls. They are also used in the
fabrication of injection-molded printed circuit boards. Polyimide coatings are increasingly
being used in microelectronic applications. Major applications are
in
the following areas:
Interlayer dielectrics on silicon and gallium arsenide integrated circuits for multilevel
Passivativation, thermal-mechanical buffer, and a-particle protection coatings
on
Masking for multilayered resist processing; for negative-profile liftoff processing;
devices.
integrated circuits and other circuitry.
for harsh processing such as
ion
implantation or dry etching.
The principal features that make polyimides suitable for microelectronic applications are:
Polyamic acid varnishes and/or polyimide solutions can be easily spun
on
planarized
Polyimides have good dielectric properties.
Polyimide coatings are tough and resilient, and have good thermal and chemical
layers, exposed, and etched with existing equipment.
stability. They also have good radiation resistance.
3.0
COMMERCIAL INFORMATION
Several commercially available aromatic polyimides, such as Kapton (Du Pont), IP-2080
(Dow), Matrimid 5218 (Ciba-Geigy), Ultem (General Electric), and LARC-TPI (Mitsui-

POLYIMIDES
505
Toatsu Chemicals), are used in the form
of
films, moldings, adhesives, and composite
matrices. Numerous lacquers or varnishes based on similar chemistries are also available
from the basic resin suppliers. Several polyimide coating solutions also have been intro-
duced commercially.
REFERENCES
1.
K. L. Mittal, Ed.,
Polyitnides,
Vols.
I
and
11.
New York: Plenum Press, 1984.
2.
J.
W. Verbicky,
Jr.,
in
Encyclopedia
of
Polymer
Science and
Engineering.
2nd ed., Vol. 12.
3.
J. J.
King and
B. H.
Lee,
in
High Performclme Polyners: Their Origin
and
Development,
R.
4.
Y. K. Lee and
J.
D.
Craig, in
Po1.vtner Muterictls for Electronic
App1icntion.s.
E.
D.
Feit and C.
New York: Wiley, 1988,
p.
364.
B.
Seymour and
G.
S.
Kirshenbaum. Eds. New York: Elsevier, 1986,
p.
317.
W. Wilkins,
Jr.,
Eds. Washington
D.
C.: American Chemical Society, 1982,
p.
107.
This Page Intentionally Left Blank
58
Parylene Coating
William
F. Beach
Bridgeport,
New
Jersev
1
.O
PROCESS
The parylene process’.’ is a means
of
applying a pinhole-free coating with exceptional
conformality and control
of
thickness. The coatings
so
produced have excellent dielectric
as well as barrier properties. The parylene coating, composed solely
of
poly (p-xylylene)
(PPX), a family
of
linear, high molecular weight organic polymers, is grown directly on
a substrate by vapor deposition polymerization (VDP). The gaseous p-xylylene monomer
(PX) is transformed into a solid polymer coating without passing through an intermediate
liquid stage. Since surface forces have no opportunity to alter the cross-sectional profile,
the result is a coating of extraordinary uniformity
of
thickness and continuity.
No
postdepo-
sition cure is necessary to complete the coating chemistry. The parylene process affords
exceptional control of coating thickness. While typically used in thicknesses
of
1
-
10
km,
continuous parylene films have been demonstrated at thicknesses under
500
(0.05
km).
In principle there
is
no upper limit to the thickness to which a parylene film might be
grown, but practical constraints
of
time and cost place an upper bound in the vicinity
of
100
km.
The parylene process is further distinguished by the fact that it is conducted at room
temperature. Parylene growth rates actually decrease at high temperatures. There is an
advantage in operating the process at subambient temperatures. if such operation is feasible.
Another distinguishing feature
of
the parylene process is that it proceeds without the
assistance
of
a catalyst. Thus the coating is
of
remarkable chemical purity with respect
to
catalyst residues, which in other coating systems can be ionic
or
ionogenic,
or
leachable.
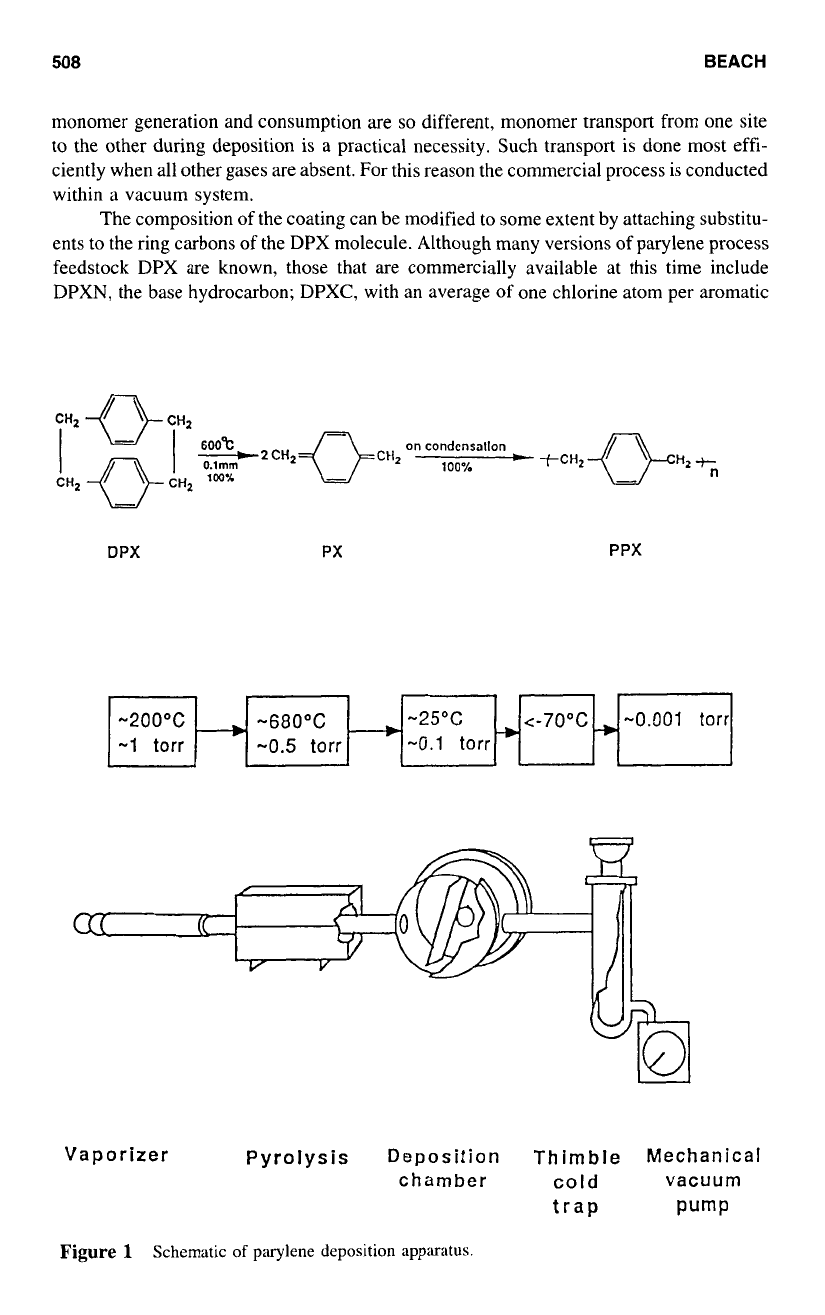
The monomer is exceptionally reactive. It cannot be stored. It can be handled only
as a rarefied, low pressure gas. It is therefore necessary to generate monomer
as
it is
required by the coating process. Monomer is conveniently generated by the pyrolytic
cleavage of its dimer, di-p-xylylene (DPX), a
[2.2]
paracyclophane. Monomer generation
from dimer proceeds in quantitative yield with no by-products. Since the temperatures for
507
508
BEACH
monomer generation and consumption are
so
different, monomer transport from one site
to
the other during deposition is
a
practical necessity. Such transport is done most effi-
ciently when all other gases are absent. For this reason the commercial process is conducted
within a vacuum system.
The composition
of
the coating can be modified
to
some extent by attaching substitu-
ents to the ring carbons
of
the DPX molecule. Although many versions
of
parylene process
feedstock DPX are known, those that are commercially available at this time include
DPXN, the base hydrocarbon; DPXC, with an average of one chlorine atom per aromatic
n
DPX PX PPX
-200°C
-25°C
-
-680°C
-
-1
torr
-w
-0.1
torr
-0.5
torr
Vaporizer Pyrolysis
Deposition
chamber
Thimble
Mechanical
cold
vacuum
trap
Pump
Figure
1
Schematic
of
parylene deposition apparatus.

PARYLENE COATING
509
ring, and DPXD, averaging two clorine atoms per aromatic ring. The coatings prepared
starting with these dimers are called Parylene
N.
Parylene
C,
and Parylene D, respectively.
Substituents reduce the volatility
of
the monomer, causing the processing characteristics
of the modified versions to be somewhat different.
The discovery of the base member of the family, poly(p-xylylene), was first reported
in 1947. However,
it
was not until
1965,
when the Gorham process starting with DPX was
announced,3 that the polymer family became industrially viable. Figure
1
is a schematic
of
the parylene equipment, with typical operating conditions indicated.
2.0
PROPERTIES
The glass transition temperatures of the parylenes are
in
the vicinity of room tenlperature.
However, the parylenes are crystalline polymers, and as such retain substantial physical
strength and solvent resistance at temperatures approaching their crystalline melting points
(for Parylenes
N.
C,
and D:
420, 290,
and
380°C.
respectively). Long-term contact with
solvents results in a mere few percent swelling. Equilibrium moisture absorption is very
low. Significantly, no mode of moisture-induced degradation is chemically feasible. The
dielectric constants and
loss
factors of the parylenes are low and invariant over a wide
range
of
frequencies. Although permeable, the parylenes at a given thickness are superior
as barriers to other organic polymers that can conveniently be prepared as coatings.
The parylenes are vulnerable to attack by oxygen, particularly at elevated tempera-
tures and in the presence
of
ultraviolet radiation. In air, 10-year use temperatures for
Parylenes
N,
C,
and D are projected to be
60, 80,
and
100"C,
respectively.
In
oxygen-
free environments, the thermal endurance of each is substantially better.
While it is tempting to categorize the parylene vapor deposition polymerization
process with the superficially similar processes of evaporation, sputtering. or chemical
vapor deposition of metals or inorganics, there are important distinctions to be made.
In
the latter processes, the growth action is confined to the outer substrate surfaces, while
the parylene polymerization chemistry actually occurs under the surface
of
the growing
coating.
As
a result, the parylenes deposit in a condition
of
compressive stress and adhere
to organic substrates, which are permeable to monomer by an interpenetration mechanism.
Adhesion
to
impermeable metallic
or
inorganic substrates such as aluminum or silica can
be achieved by pretreatment with an organosilane primer such as y-methacryloxypropyl
trimethoxysilane (A-174).
3.0
APPLICATIONS
The parylenes first found use
in
electronics construction. Because of its exceptionally low
and frequency-independent dielectric constant, Parylene
N
continues to be used as the
functional dielectric in high quality miniature film capacitors. Very early on. the parylenes
were qualified under
MIL-1-46058,
the specification for coating printed circuit assemblies.
Parylene
C
continues to enjoy a reputation as a high performance coating for military
circuitry, particularly in avionics. Parylene
C
is
also
an Underwriters Laboratories (UL)
approved conformal coating. The use of the parylenes as circuit coatings has extended
to
hybrids. The exceptional conformality
of
the parylenes has served them well in these
applications, but
so
far has impeded their use in multilevel interconnection schemes, where
a planarizing dielectric is sought. In the Inanufacture of miniature electric motors, such
as those used
in
wristwatches, palylene is used as an insulating coating on the armature.

51
0
BEACH
Parylene’s exceptional thickness control permits the winding
of
a maximum number
of
turns, and therefore superior motor performance. Furthermore,
a
parylene process variation
in which the parts are tumbled during deposition permits economies through the coating
of
thousands
of
parts at
a
time.
Parylene’s use today has broadened to such diverse missions
as
the immobilization
of
loose particles that otherwise would result in early device failure (Winchester drives
and hybrids), the modification
of
surface abrasiveness
on
ferrite toroids, and the reinforce-
ment and preservation
of
embrittled paper in old books and museum artifacts.
REFERENCES
1. W.
F.
Beach, C. Lee,
D.
R.
Bassett,
T.
M.
Austin, and
R.
Olson,
“Xylylene polymers,” in
Blcyclopedirl
of
Polyrner Science and Engiueerirzg,
2nd ed., New York: Wiley-Interscience,
1988.
2.
(a)
S.
M.
Lee. in
Kirk-Otlmer Elqdoperlia
of
Chernicul
Technology.
3rd ed.,
Vol.
24. New
York: Wiley-Interscience, 1983, pp. 744-771.
(b)
M.
Szwarc,
Polw.
Eng.
SCE.,
16(7), 473-479
(1976). (c) W.
F.
Gorham and
W.
D.
Niegisch, in
Enc~clopedia
oj‘folymer
Sci~vzce
and
Technol-
ogy.
Vol,
15.
1971, pp. 98-124. (d) L. A. Errede and
M.
Szwarc
Q.
Rev.
12.
301-320 (1958)
3.
W.
F.
Gorham U.S. Patent 3,342,754 (Sept. 19, 1967); Union Carbide Corp.

59
Nitrocellulose
Daniel
M.
Zavisza
Hercules
Incorporrrted,
Wilrnington.
Delmcnre
When most people think of nitrocellulose, they think
of
guncotton, a material that was
developed for explosives or gun propellant. But they are only partially correct. Nitrocellu-
lose is one
of
the oldest and most widely used film formers adaptable to a number
of
uses. It is derived from cellulose, a material from plants, and therefore a renewable source.
Soluble nitrocellulose possesses a unique combination of properties such as toughness,
durability, solubility, gloss, and rapid solvent release.
As
the film former
in
lacquer sys-
tems, it affords protective and decorative coatings for wood and metal. In addition. it finds
use in flexible coatings for paper, foil and plastic film, printing inks, and adhesives. This
chapter briefly covers the properties, uses, and handling procedures for nitrocellulose and
the formulations made from it.
1
.O
PREPARATION
Nitrocellulose is the common name for the nitration product of cellulose. Other names
include cellulose (tri)nitrate and guncotton. The commercial product is made by reacting
cellulose with nitric acid. Cellulose is composed
of
a large number of P-anhydroglucose
units, which are joined together into a chain. The anhydroglucose units are six-membered
rings having three hydroxyl
("OH)
groups attached to them. The number of anhydroglu-
cose units in the typical cellulose chain ranges from
500
to
2500
in chemically purified
cellulose.
1
.l
Degree
of
Substitution
Nitric acid can react with these three hydroxyl groups of the anhydroglucose units to form
the nitrate ester. Fully nitrated cellulose would then be a trinitrate-that is a nitrate having
a
degree of substitution of
3.
The calculated nitrogen content of such a fully nitrated
cellulose is
14.14%.
51
1
