Satas D., Tracton A.A. (ed.). Coatings Technology Handbook
Подождите немного. Документ загружается.


21
2
BREWER
5.1
2
Deionized Water
Evaporated or otherwise lost water is replaced by the use of
75.000
iLcm
of
water, which
is also used as
a
final rinse.
5.13
Bake
or Cure
Most electrocoats require a bake
of
20
minutes at
350°F.
However. lower baking materials
are available: even ambient temperature curing materials are on the market.
6.0
LABORATORY
The tank control is carried
out
in
about
3
hours per day by a technician, using the procedures
found
in
the
ASTM
Paint
T~srir~g
Mrrnurrl.’
REFERENCES
I.
SAE
J..
pp.
81-83,
August 1965.
2.
G.
E.
F.
Brewcr and
A.
D. Hamilton. “Paint for electrocoating.” ASTM Gardner-Sward
Point
Testing
Mwud.
13th
ed.
Philadelphia: American Socicty
for
Testing and Materials; 1972,
Section
8.
IO,
pp. 486-489.
BIBLIOGRAPHY
I.
2.
3.
4.
5.
6.
7.
8.
9.
Brewer.
G.
E.
F.,
Ed.
Elec.tro~le/~osi/inrr
of
Corrtirlgs,
ACS Advances
in
Chcmistry Series,
Vol.
119,
Washington, DC: American Chemical Society,
1973.
Brcwer,
G.
E.
F.,
Chairman,
“ACS
Symposium on newer developments in electrocoating.”
Org.
Cocctirlgs
Plnst.
Chem,
4.5
1-22,
92-1
13
(August 1981).
Chandler, R.
H.,
“Advances in clectrophoretic painting,”
Hi-
or
T~inrlrl~frclAh.strrcc~t,s.
Braintrcc,
Esscx,
England: R.
H.
Chandler, since 1966.
Duffy,
J.
I.,
Ed.,
Elec~rro~lrl,osi/ior1
Proccwes
ttml
Etpipr~e~~f,
Park Ridge, NJ: Noycs Data
Corp., (1982).
Kardomenos, P.
I.,
and
J.
D. Nordstrom, “Polymer compositions
for
catholic clectrodeposition
coatings,”
J.
Cotrtir~g
Techrlol.,
54(686),
33-41
(March 1982).
Machu, W.,
Hortdhook
c
Electroless Plating
1
.O
INTRODUCTION
In
electroless plating, metallic coatings are formed
as
a
result of
a
chemical reaction
between the reducing agent present
in
the solution and metal ions. The metallic phase that
appears
in
such reactions may be obtained either
in
the bulk of the solution or
as
a
precipitate in the form of
a
film
on
a
solid surface. Localization of the chemical process
on
a
particular surface requires that surface must serve
as
a
catalyst. If the catalyst is
a
reduction product (metal) itself, autocatalysis is ensured, and in this case it is possible to
deposit
a
coating, in principle, of unlimited thickness. Such autocatalytic reactions consti-
tute the essence of practical processes of electroless plating. For this reason these plating
processes are sometimes called autocatalytic.
Electroless plating may include metal plating techniques
in
which the metal is ob-
tained as
a
result of the decomposition reaction
of
a
particular compound; for example,
aluminum coatings are deposited during decomposition of complex aluminum hydrides
in organic solvents. However, such methods are rare and their practical significance is not
great.
In
a
wider sense, electroless plating
also
includes other metal deposition processes
from solutions
in
which an external electrical current is not used, such
as
immersion, and
contact plating methods in which another more negative (active) metal is used
as
a
reducing
agent. However, such methods have
a
limited application; they are not suitable for metalli-
zation of dielectric materials, and the reactions taking place are not catalytic. Therefore,
they usually are not classified
as
electroless plating.
Electroless plating now is widely used
in
modifying the surface of various materials,
such
as
nonconductors, semiconductors, and metals. Among the methods of applying
metallic coatings it is exceeded in volume only by electroplating techniques, and
it
is
almost equal
to
vacuum metallization.
Electroless plating methods have some advantages over similar electrochemical
methods. These are:
21
3
21
4
VASKELIS
1.
Coatings may be deposited on electrically nonconductive materials (on almost
2.
Coatings have more uniform thickness irrespective of the shape
of
the product
3.
Deposition is simple-it is enough to immerse the (pretreated) product
in
the
4.
It is possible
to
obtain coatings having unique mechanical, magnetic, and chemi-
any surfaces that are stable in electroless plating solutions).
to
be plated.
electroless plating solution.
cal properties.
Application
of
electroless plating,
in
comparison with electroplating techniques, is
limited by the two factors: (a) it is more expensive, since the reducing agent costs more
than an equivalent amount of electricity, and (b)
it
is less intensive, since the metal deposi-
tion rate is limited by metal ion reduction in the bulk of the solution.
2.0 PLATING
SYSTEMS
To
ensure chemical reduction of metal ions in a solution. The solution must contain
a
sufficiently strong and active reducing agent; that is it must have a sufficiently negative
redox potential. The more easily the metal ions are reduced, the greater is the number
of available reducing agents. Since only autocatalytic reduction reactions may be used
successfully for deposition of coatings, the number of electroless plating Me-Red (metal-
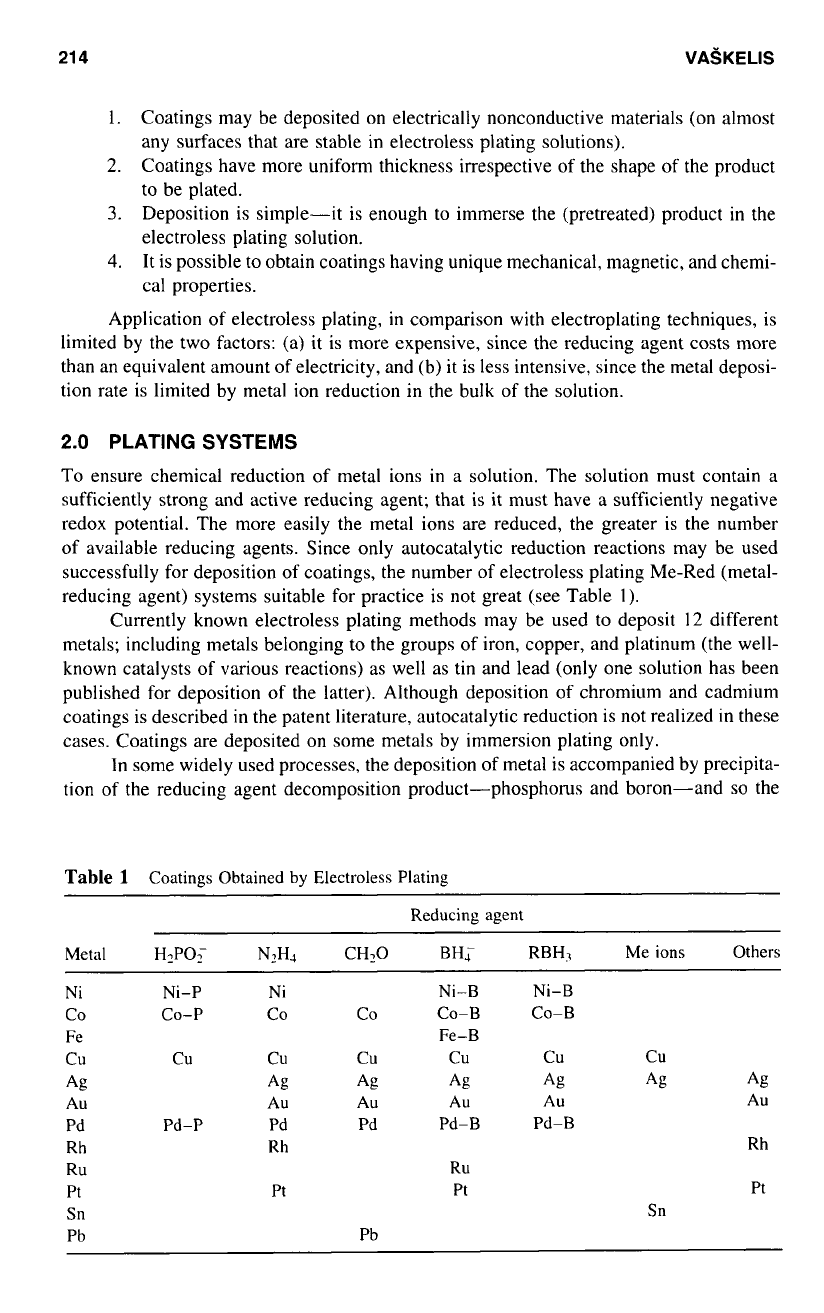
reducing agent) systems suitable for practice is not great (see Table
1).
Currently known electroless plating methods may be used
to
deposit
12
different
metals; including metals belonging to the groups
of
iron, copper, and platinum (the well-
known catalysts
of
various reactions)
as
well
as
tin and lead (only one solution has been
published for deposition
of
the latter). Although deposition of chromium and cadmium
coatings is described
in
the patent literature, autocatalytic reduction is not realized in these
cases. Coatings are deposited on some metals by immersion plating only.
In some widely used processes, the deposition of metal is accompanied by precipita-
tion of the reducing agent decomposition product-phosphorus and boron-and
so
the
Table
1
Coatings Obtained by Electroless Plating
Reducing agent
Metal HIPOF NZHI CH20 BH; RBH7 Me ions Others
Ni Ni-P Ni Ni-B Ni-B
CO CO-P CO CO CO-B
CO-B
Fe Fe-B
cu
cu
cu
cu
cu
cu
cu
AU
Au
AU
Au
Au
Au
Pd Pd-P
Pd Pd Pd-B Pd-B
Rh
Rh
Rh
RU
RU
Pt
Pt Pt Pt
Sn
Sn
Pb
Pb
A&
Ag
Ag
Ag
Ag Ag
A&

ELECTROLESS PLATING
21
5
respective alloys are obtained. It is not difficult to deposit two or more metals at
a
time;
electroless plating methods are known for deposition
of
more than
50
alloys of different
qualitative composition, mostly based on nickel, cobalt, and copper.
The majority of reducing agents used in electroless plating are hydrogen compounds,
in which H is linked to phosphorus, nitrogen, and carbon. It is
in
the reactions of these
compounds that significant catalytic effects are possible, since
in
the absence of catalysts
these reactions proceed slowly.
The most effective autocatalysis is obtained when the strongest reducer-hypophos-
phite-is used. In the absence of catalysts the reducer is inert and does not react even
with the strong oxidants; only
a
few catalysts are suitable for it (e.g., Ni, CO, and Pd),
but they provide for
a
catalytic process of the highest rate without reduction in the bulk
of
a
solution. Other reducers are more versatile, for example, by using borohydride we may
deposit coatings of almost all the metals mentioned. The reducing capacity of hydrogen
compounds increases with an increase in pH of a solution. For this reason, the majority
of electroless plating solutions are alkaline.
Such simple reducing agents
as
metal ions of variable valence (Fe’+, Cr2+ and Ti’+)
usually are not suitable for deposition of coatings because noncatalytic reduction occurs
rather easily. Recently, conditions have been established for autocatalytic deposition of
tin and silver coatings using
as
reducing agents such metal complexes
as
Sn(0H);- and
Depositions
of
some metals (Ag, Au, Cu) by chemical reduction techniques was
known
as
long
ago
as
the nineteenth century, but it became popular after Brenner found
(in 1945)
a
very efficient electroless nickel plating process using hypophosphite.’ It was
then that the term “electroless plating” was coined.
CO(NH.&
.
3.0
ELECTROLESS PLATING SOLUTIONS
The electroless plating solutions used in practice, in addition to the basic components (the
salt of the metal to be deposited and
a
reducing agent), contain other substances
as
well.
Usually. these are
as
follows.
1.
Ligands. which form soluble complexes with metal ions, are necessary for alka-
line solutions. Also. the use of stable complexes sometimes enhances the autocat-
alytic effect.
2.
Substances controlling and maintaining a certain pH value of the solution: buffer
additions are especially important, since in the course of metal reduction. Hydro-
gen ions are formed.
3.
Stabilizers that decelerate reduction reaction in the bulk of
a
solution, hence
enhance autocatalysis.
Sometimes, agents such
as
brighteners are also added to the solution.
The basic technological parameters of electroless plating solutions are discussed in
Sections
3.1-3.4.
3.1
Deposition Rate
Deposition rate usually is expressed in micrometers per hour
(@h;
or milk, kin.& mg/
cm’h). In the course of deposition, if the concentrations of reacting substances are
not
maintained at
a
constant level, this rate decreases. The values given in the literature are

21
6
VASKELIS
often averages, reflecting only the initial period. Such average rates depend on the ratio
of the surface to be plated to the solution volume (dm'/liter).
The dependence of the deposition rate
(v)
on the concentration
of
reacting substances
for a general case is rather complicated. It is often described by empirical equations, for
example:
11
=
k
[Me"+]" [Red]"
[H']"
[L]"
(1)
where
k
is the rate constant (a constant value for a system of the given type) and [L] is
the concentration of a free ligand (not bound with metal ions
in
a complex). The exponents
n
and
b
are usually smaller than unity, while
c
is a negative value (in alkaline solutions
OH-ion concentration is used, and in such
a
case the exponent is often positive.
0
<
c
<
1).
Exponent
d
is usually close to zero: when the ligand is substituted, however, the
deposition rate may change substantially. With constant concentrations of other solution
components, the deposition rate decreases when the stability
of
a metal complex increases
(when the concentration of free metal ions is lowered); however, this relationship for a
general case is not rigorous.
The electroless deposition rate of most metals under suitable conditions is about
2-5 pnl/h, and only electroless nickel plating rate may be as high as 20 pdh (this
corresponds to an electroplating process at current densities of 200 A/m').
3.2 Solution Life
Solution life represents the maximum duration of solution usefulness. The beginning of
metal ion reduction in the bulk of a solution may terminate its exploitation.
In
most modern
electroless plating solutions, however, the reduction
in
the bulk usually does not occur
under normal operating conditions, and the solution life is limited by the accumulation
of
reaction products or impurities. Thus,
it
is better
to
characterize the life of
a
solution
not by time. which depends on the intensity of exploitation. but rather by the maximum
amount of metal deposited from a volume unit of the solution &/liter or pndliter) or by
turnover number showing how many times the initial amount of metal
in
the solution may
be deposited in the form of a coating. This number may be as big as 10-20. After removal
of undesirable substances accumulated in the solution,
it
may be used longer, just like
electrolytes for electroplating.
After protracted exploitation of solutions, a certain amount
of
sediment may appear,
since the bulk reaction may proceed
on
a limited scale even in fully stable solutions.
3.3
Reducing Agent Efficiency Factor
The amount of reducing agent (in moles or grams) that is consumed for deposition of a
mole or gram of coating is indicated by the reducing agent efficiency factors. The required
amount (according to the reduction reaction) of a reducing agent, which is equal. for
example, to 2 moles for
1
mole of metal (nickel ion reduction by hyposphosphite or copper
ion
reduction by formaldehyde) is exceeded in real electroless plating processes as a result
of the side reactions taking place.
3.4
Solution Sensitivity to Activation
The minimum amount of catalyst that must be present
on
the dielectric surface to initiate
a reduction reaction is shown by the solution sensitivity
to
activation. This parameter is

ELECTROLESS PLATING
21
7
related to solution stability. The lower the stability of a solution. the easier is the initiation
of a reaction, even on surfaces with low catalytic activity.
A
high sensitivity of a solution
to activation is not always desirable. since metal from such solutions may be deposited
even on surfaces that had not been activated;
in
such cases selective plating becomes
impossible. When palladium compounds are used for activation, there should be
no
less
than
0.01
and 0.03-0.05 Kg of lead per square centimeter of a dielectric surface for nickel
and copper plating, respectively. When silver is used as an activator, which is suitable
only
for some electroless copper plating solutions.
it
is necessary to have about
0.4
pg
of silver per square centimeter.
4.0
PRACTICAL
The applications of chemically deposited coatings may be divided into two groups. For
decorative metallization of plastics, a thin
(0.3-1
.O
km) layer of metal is chemically
deposited
on
a
dielectric surface, and its thickness is then increased by electroplating
techniques.
In
this case. the properties of chemically deposited coatings and the nature of
the metal are not of great significance;
it
is important
only
to ensure compactness and
sufficient electrical conductivity
of
such coatings
(a)
for subsequent electroplating and
(A)
for providing the required adhesion of the metal layer. The metal for the chemically
deposited underlayer is selected for process convenience and cost. For this purpose. nickel
and copper coatings are used. Nickel is more convenient. since electroless nickel plating
solutions are more stable and their compositions simpler than those
of
similar electroless
copper plating solutions.
The adhesion
of
a
coating to the nonconducting surface is essentially determined
by the state of the surface. while the nature of the metal (at least for nickel and copper)
usually has only a slight effect on adhesion. Copper coatings might be preferred because
of
their higher electrical conductivity.
A
copper underlayer is almost always used in the
production of printed circuit boards.
Chemically deposited finished coatings, on the other hand, are thicker and their use
is determined by their mechanical. electrical. and magnetic properties. The most popular
are nickel (NI-P and Ni-B) coatings deposited on metal products. Copper coatings
20-30
pm
thick, deposited on plastics. exhibit good electrical conductivity and ductility
and, therefore. are used
in
the production
of
printed circuit boards by additive processes.
The entire circuit pattern is obtained by electroless techniques.
Coatings of cobalt and its alloys may be used
to
take advantage of their specific
magnetic properties; silver and gold coatings are used because
of
their
good
electrical
conductivity, optical properties, and inertness.
Electroless plating may be performed by using the plating solution once
(until
a
greater part of any component in the solution is consumed and the reaction rate has sharply
decreased) or by replenishing the substances that have been consumed in the course of
plating. Long-term exploitation
of
solutions reduces the amount of plating wastes and
ensures a higher labor productivity, but at the same time.
it
imposes more stringent require-
ments
on
plating solutions: they must be stable, and their parameters should
not
vary
significantly with time. Besides, special equipment is required for monitoring and control-
ling the composition of such solutions. For this reason, long-term exploitation of solutions
is applied
only
in
large-scale production processes.
Single-use solutions are more versatile. but they are less economic and less efficient.
A
single-use method may be applied rather efficiently. however, when the solution has a

21 8
simple composition and the basic components (first of all, metal ions) are
fully
consumed
in the plating process, while the remaining components (such as ligands) are inexpensive
and do not pollute the environment. In this case, single-use processes may be practically
acceptable even in mass production.
An extreme case of single use
of
plating solutions is aerosol spray plating,'
in
which
droplets of two solutions being sprayed by a special gun collide
on,
or close to, the
surface being plated. One solution usually contains metal ions, while the other contains
the reducing agent. Metal
ion
reduction in this case should be rapid enough to permit a
greater part of the metal to precipitate on the surface before the solution film runs off it.
This method is practical for deposition of such easily reducible metals as silver and gold,
though such aerosol solutions are known for deposition
of
copper and nickel as well. The
aerosol spray method is highly suitable for deposition of thin coatings on large, flat sur-
faces: this process is similar to spray painting.
Since the components of electroless plating solutions, first
of
all metal ions, may
be toxic and pollute the environment, techniques have been developed for recovery of
metals from spent plating solutions and rinse water. Other valuable solution components,
such as ligands (EDTA, tartrate). may also be recovered.
Electroless plating usually does not require sophisticated equipment. The tank for
keeping plating solutions must exhibit sufficient chemical inertness, and its lining should
not catalyze deposition of metals. Such tanks are usually made
of
chemically stable plastics;
metal tanks may be used as well-they can be made
of
stainless steel or titanium.
To
prevent possible deposition
of
metals
on
the walls, a sufficiently positive potential is
applied to them using
a
special current source (anodic protection). Parts for plating may
be mounted on racks; small parts may be placed in barrels immersed in the plating bath.
Heating and filtration
of
solutions are carried out in the same way as
in
electroplating
processes. Special automatic devices have been developed for monitoring and controlling
the composition of plating solutions.
5.0
MECHANISMS
OF
AUTOCATALYTIC METAL ION REDUCTION
Autocatalytic metal ion reduction processes are highly complex: they contain many stages.
and their mechanism is not understood in detail. At present, it is possible to give an
accurate description only of the basic stages
of
the catalytic process. Localization of the
reduction reaction on the metal-catalyst surface (the cause of catalysis) is usually attrib-
uted
to
the requirement for a catalytic surface for one or more stages of the process to
proceed. In accordance with one of the earlier explanations, only on a catalytic surface is
an active intermediate product obtained, which then reduces metal ions. First, atomic
hydrogen and, later,
a
negative hydrogen ionhydride-were considered to be such prod-
ucts.
A
reaction scheme with an intermediate hydride gives a good explanation of the
relationships observed
in
nickel and cooper plating processes.5 However. there is
no
direct
proof that hydride ions are really formed during these processes. Moreover, the hydride
theory explains only the reactions with strong hydrogen-containing reducers, which really
may be
H-
donors.
A more versatile explanation of the causes of catalysis in these processes is based
on electrochemical reactions. It is suggested that reducing agents are anodically oxidized
on the catalyst surface and the electrons obtained are transferred
to
metal ions. which are
cathodically reduced. The catalytic process comprises two simultaneous and mutually
compensating electrochemical reactions. In this explanation of the catalytic process, elec-

ELECTROLESS PLATING
21
9
trons are the active intermediate product. However, electrons are fundamentally different
from the conversational intermediate products
of
reactions. They may be easily transferred
along the catalyst without transfer of the mass, and for this reason, the catalyst reaction,
contrary
to
all other possible mechanisms (which are conventionally called “chemical
mechanisms”), occurs not as a result of a direct contact between the reactants, or the
reactants or the reactant and an intermediate substance, but because of the exchange of
“anonymous” electrons via metal.
On the metal surface, when anodic oxidation of the reducer
Red
-
Ox
+
ne
(2)
and cathodic reduction of metal ions
Mell
~t
+
ne
(3)
proceed simultaneously. A steady state in the catalytic system of electroless plating is
obtained, in which the rates
of
both electrochemical reactions are equal, while the metal
catalyst acquires a mixed potential
E,,,.
The magnitude of this potential is between the
equilibrium potentials
E,
of the reducer and of the metal. The specific value
E,,,
depends
on the kinetic parameters of these two electrochemical reactions.
Electrochemical studies of catalytic metal deposition reactions have shown that the
electrochemical mechanism is realized practically in all the systems of electroless
plating.‘.‘.’
At the same time, it has become clear that the process is often not
so
simple. It
appears that anodic and cathodic reactions occurring simultaneously often do not remain
kinetically independent but affect each other. For example, copper ion reduction increases
along with anodic oxidation of formaldehyde.x The cathodic reduction
of
nickel ions and
the anodic oxidation of hypophosphite in electroless nickel plating solutions are faster
than in the case in which these electrochemical reactions occur separately. This interaction
of electrochemical reactions probably is related to the changes in the state of the
metal-catalyst surface.
Electrochemical reactions may also hinder each other: for example, in reducing silver
ions by hydrazine form cyanide solutions, their rate is lower than is separate Ag-Ag(
1)
and
redox systems.
The electrochemical nature of most
of
the autocatalytic processes discussed enables
us to apply electrochemical methods
to
their investigation. Only they must be applied to the
entire system of electroless plating, without separating the anodic and cathodic processes in
space. One suitable method is based
on
the measurement
of
polarization resistance. It can
provide information
on
the mechanism of the process and may be used for measuring the
metal deposition rate (both in laboratory and in industry).’ The polarization resistance
R,
is inversely proportional to the process rate
i:
R,
=
($)
1-0
where
b;,
and
b,
are Tafel equation coefficients
(b
=
l/anj),
a
is the transfer coefficient,
tz
is the number of electrons taking part in the reaction for one molecule of reactant, and
f
=
F/RT
(F
=
Faraday number).

220
VASKELIS
Autocatalytic metal reduction reactions also may
not
proceed in an electrochemical
manner. Two courses
of
such reactions have been shown: (a) an intermediate metal hydride
is formed, which decomposes
to
metal and hydrogen (reduction of copper ions by borohy-
dride and, (b) the metal complex is hydrolyzed, resulting in precipitation
of
metal oxide
on the surface, which then is reduced to metal by the reducer present in the solution
(reduction of silver ions by tartrate).
6.0
STABILITY OF PLATING SOLUTIONS
Electroless plating solutions containing metal ions and reducing agents thermodynamically
unstable systems. Metal ion reduction must proceed in the bulk of the solution.
The difference in the rate of metal ion reduction on the required surface (controlled
catalytic reaction) and that of a reduction reaction in the bulk of
a
solution shows the
effect of catalysis. and it determines, to a substantial degree, the practical usefulness of
plating solutions. In an ideal case. the reaction in the bulk
of
a solution should not occur
at all.
Formation of metal in the bulk
of
a solution is hindered by energy barriers: the
activation barrier of homogenous reactions between metal ions and reducer and the barrier
of
the formation
of
a new phase (metal). The magnitude
of
the second barrier may be
evaluated on the basis
of
thermodynamic principles.
")
It was established empirically that the stability
of
plating solutions decreases with
an increase in the concentration of reactants and temperature. with a decrease
in
the stability
of metal ion complexes, and with the presence of solid foreign particles in the solution.
Besides. it was found that stability decreases as the catalytic process rate and load increase.
This may be attributed
to
the transfer of intermediate catalytic reaction products from the
catalytic surface to the solution, where they may initiate a reduction reaction.
To
enhance
the stability of solutions, it is recommended that lower concentration solutions and more
stable metal complexes be used and that solid particles
in
the solution be removed by
filtration. The most effective solution stabilization method is the introduction of special
addition agents-that is, stabilizers.'." Stabilizers. the number of which is very great,
may be divided into two large groups: (a) catalytic poisons, such as
S(II),
Se(I1) com-
pounds, cyanides. heterocyclic compounds with nitrogen and sulfur, and some metal ions,
and (b) oxidizers.
It
is assumed that stabilizers hinder the growth of fine metal particles,
close to critical ones, by absorbing on them (catalytic poison) or passivating them (oxi-
dizers).
Modern electroless plating solutions always contain stabilizers. Their concentration
may be within the range of
1-100
mg/liter. Stabilizers. by hindering deposition
of
metal
on
fine particles, usually slow down the rate of the catalytic process on the surface being
plated. This process Inay stop completely at a sufficiently high concentration of the stabiliz-
ers.
In
some cases. however, small amounts
of
stabilizers increase the deposition rate.
7.0
ELECTROLESS PLATING
7.1
Copper
Deposition
Though copper coatings may be deposited using various reducers, only formaldehyde
copper plating solutions are of practical importance. Autocatalytic reduction
of
copper
ions by formaldehyde proceeds at room temperature in alkaline solutions (pH
=
1
1
-
14);
ELECTROLESS
PLATING
221
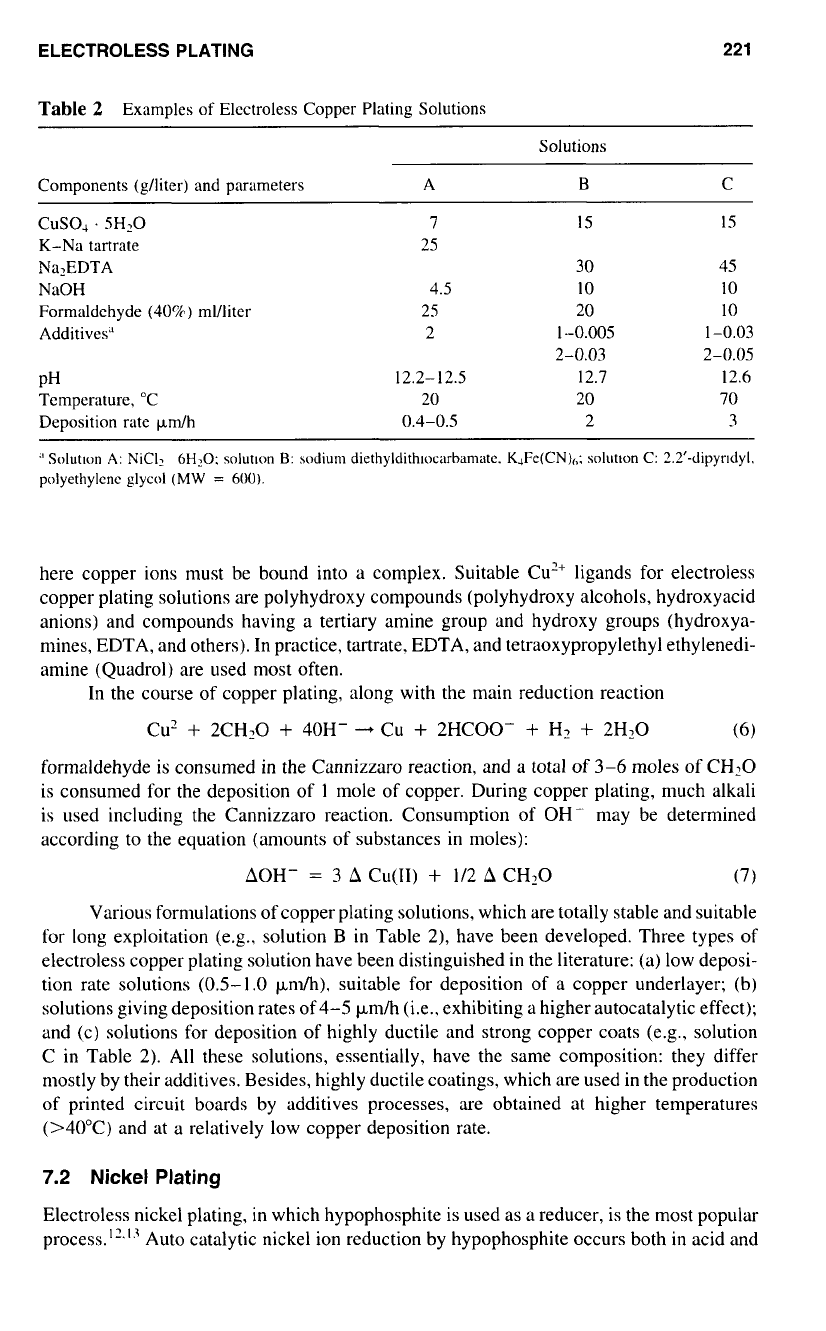
Table
2
Examples
of
Electroless Copper Plating Solutions
Solutions
Components (glliter) and parameters A
B
C
CUSOA
. 5H2O
K-Na tartrate
NaOH
Formaldehyde
(40%)
ml/liter
Additives"
NaZEDTA
PH
Temperature,
"C
Deposition rate Fdh
7
15
30
4.5 10
25 20
2 1-0.005
25
2-0.03
12.2-12.5 12.7
20 20
0.4-0.5 2
15
45
IO
10
1-0.03
2-0.05
12.6
70
3
here copper ions must be bound into a complex. Suitable Cu2+ ligands for electroless
copper plating solutions are polyhydroxy compounds (polyhydroxy alcohols, hydroxyacid
anions) and compounds having a tertiary amine group and hydroxy groups (hydroxya-
mines, EDTA, and others).
In
practice, tartrate.
EDTA,
and tetraoxypropylethyl ethylenedi-
amine (Quadrol) are used most often.
In the course
of
copper plating, along with the main reduction reaction
CU'
+
2CH20
+
40H-
-+
CU
+
2HC00-
+
Hz
+
2H70
(6)
formaldehyde is consumed
in
the Cannizzaro reaction, and a total of
3-6
moles of CH20
is consumed for the deposition
of
1
mole of copper. During copper plating, much alkali
is used including the Cannizzaro reaction. Consumption
of
OH-. may be determined
according
to
the equation (amounts of substances in moles):
AOH-
=
3
A
Cu(I1)
+
1/2
A
CH20
(7)
Various formulations of copper plating solutions, which are totally stable and suitable
for long exploitation (e.g.. solution B
in
Table 2), have been developed. Three types
of
electroless copper plating solution have been distinguished in the literature: (a) low deposi-
tion rate solutions (0.5-1
.O
p.ndh). suitable for deposition of a copper underlayer; (b)
solutions giving deposition rates of 4-5 pm/h (i.e.. exhibiting a higher autocatalytic effect);
and (c) solutions for deposition
of
highly ductile and strong copper coats (e.g., solution
C in Table 2).
All
these solutions, essentially, have the same composition: they differ
mostly by their additives. Besides, highly ductile coatings, which are used in the production
of printed circuit boards by additives processes, are obtained at higher temperatures
(>40"C) and at a relatively low copper deposition rate.
7.2
Nickel Plating
Electroless nickel plating,
in
which hypophosphite is used as a reducer, is the most popular
process.".'.3 Auto catalytic nickel ion reduction by hypophosphite occurs both in acid and
