Satas D., Tracton A.A. (ed.). Coatings Technology Handbook
Подождите немного. Документ загружается.

222
VASKELIS
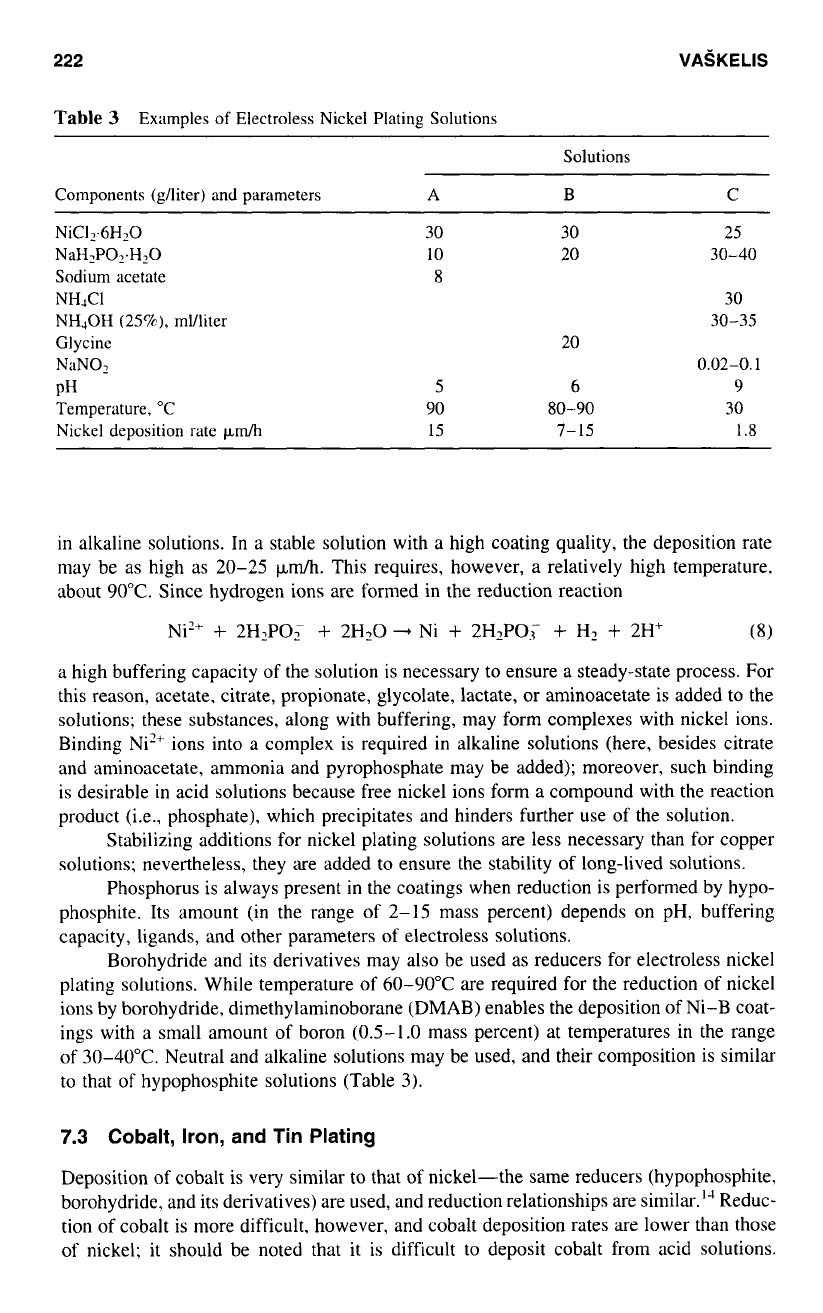
Table
3
Examples
of
Electroless Nickel Plating Solutions
Solutions
Components (&/liter) and parameters
A
B
C
NiC12.6HzO
NaH2P02.H20
Sodium acetate
NHJC1
NHJOH
(25%),
ml/liter
Glycinc
Nl1N02
PH
Temperature, "C
Nickel deposition rate
pdh
30
30 25
10 20
30-40
8
30
30-35
20
0.02-0.1
5
6 9
90
80-90
30
15
7-
15
1.8
in alkaline solutions. In a stable solution with a high coating quality, the deposition rate
may be as high as 20-25
p&.
This requires, however, a relatively high temperature.
about
90°C.
Since hydrogen ions are formed in the reduction reaction
Ni'+
+
2H2P01
+
2H2O
-
Ni
+
2H2POY
+
H2
+
2H'
(8)
a high buffering capacity of the solution is necessary to ensure a steady-state process. For
this reason, acetate, citrate, propionate, glycolate, lactate,
or
aminoacetate is added to the
solutions; these substances, along with buffering, may form complexes with nickel ions.
Binding Ni" ions into a complex is required in alkaline solutions (here, besides citrate
and aminoacetate, ammonia and pyrophosphate may be added); moreover, such binding
is desirable
in
acid solutions because free nickel ions form a compound with the reaction
product (i.e., phosphate), which precipitates and hinders further use of the solution.
Stabilizing additions for nickel plating solutions are less necessary than for copper
solutions; nevertheless, they are added
to
ensure the stability of long-lived solutions.
Phosphorus is always present in the coatings when reduction is performed by hypo-
phosphite. Its amount (in the range of 2-15 mass percent) depends on pH, buffering
capacity, ligands, and other parameters of electroless solutions.
Borohydride and its derivatives may also be used as reducers for electroless nickel
plating solutions. While temperature
of
60-90°C
are required for the reduction of nickel
ions by borohydride, dimethylaminoborane (DMAB) enables the deposition
of
Ni-B coat-
ings with a small amount of boron
(0.5-1.0
mass percent) at temperatures in the range
of
30-40°C.
Neutral and alkaline solutions may be used, and their composition is similar
to that of hypophosphite solutions (Table
3).
7.3
Cobalt, Iron, and Tin Plating
Deposition of cobalt is very similar to that
of
nickel-the same reducers (hypophosphite,
borohydride. and its derivatives) are used, and reduction relationships are similar.'4 Reduc-
tion of cobalt is more difficult, however, and cobalt deposition rates are lower than those
of
nickel; it should be noted that it is difficult to deposit cobalt from acid solutions.

ELECTROLESS PLATING
223
The CO-P and CO-B coatings obtained are of particular interest due to their magnetic
properties.
Electroless iron plating is more difficult, and only one sufficiently effective iron
plating solution is known,
in
which Fe ions form a complex with tartrate and NaBHJ is
used as
a
reducer. Fe-B coatings (about
6%
B) are obtained in an alkaline solution (pH
12) at
a
temperature of 40°C and deposition rates of about 2
pd.
It is rather difficult to realize an autocatalytic tin deposition process.
A
sufficiency
effective tin deposition method is based on the tin(I1) disproportionation reaction
in
an
alkaline medium." In l-SM NaOH solutions at 80-90"C, it is possible to obtain a deposi-
tion rate of
a
few micrometers per hour.
7.4
Deposition
of
Precious Metals
Electroless silver plating is the oldest electroless metallization process: its present perfor-
mance however, lags behind nickel or copper plating.' Unstable single-use ammonia silver
plating solutions (with glucose, tartrate. formaldehyde, etc., as reducers) are usually em-
ployed. The thickness
of
coatings from such solutions is not great
(<
1
pm). Such unstable
solutions are more suitable for aerosol spray.
More effective electroless silver plating solutions have been developed using cyanide
Ag(1) complex and aminoboranes or hydrazine as reducers: at temperatures of 40-S0"C,
the deposition rate is
3-4
pndh, and in the presence of stabilizers these solutions are quite
stable. Sufficiently stable electroless silver plating solutions may be obtained using metal
ions such as Co(I1) compounds as reducers.
Gold coatings may be deposited employing various reducers: however, the solutions
are usually unstable. Solutions
of
sufficient stability have been developed with borohydride
or DMAB as reducers using a stable gold cyanide complex.'" At temperatures of 70-80°C,
the gold deposition rate reaches
S
Am/h and gold coatings of sufficient purity are obtained.
Thin gold coatings may be deposited on plastics by an aerosol spray method: gold
complexes with amines are employed with hydrazine as a reducer, and
a
relatively thick
coat (deposition rate as high as
0.4
pndmin) may be obtained.
Palladium coatings are easily deposited with hypophosphite as
a
reducer in alkaline
solutions, in which Pd" ions are bound in
a
complex with ammonia, EDTA, or ethylenedi-
amine. Palladium plating
is
performed at 40-S0°C, the deposition rate of the Pd-P
(4-8
P) coat being in the range of 2-S
p&.
Coatings of platinum, ruthenium, and rhodium may be deposited using borohydride
or hydrazine as
a
reducing agent. The process rate in a stable solution is low
(OS-2
pm/
h).
7.5
Deposition of Metal
Alloys
About
60
coatings of a different qualitative composition containing two or more metals
may be deposited. Such metals as copper. iron. zinc, tin, rhenium, tungsten, molybdenum,
manganese, thallium, and platinum group metals may be introduced into nickel and cobalt
coats, and nickel, cobalt, tin. zinc. cadmium, antimony, bismuth, lead. and gold into copper
coats.
In the electroless deposition of metal alloys, the same thermodynamic relationships
as
those of alloy deposition by electroplating techniques are valid;
it
is clear that it is
difficult to introduce into coatings metals that are difficult to reduce, such
as
chromium
and manganese. Besides, in the case of chemical reduction, an additional factor-catalytic
properties of metals-becomes apparent. Great amounts of additional metal may be intro-

224
VASKELIS
duced into a coat of nickel, copper. and
so
on. only when that metal is catalytic or, at
least, inert with respect to oxidation of the reducer. The amount
of
metals-catalysts in
the alloy may be as high
as
loo%, that
of
catalytically inert metals up to
5096,
and the
amount of metals-inhibitors may be only 10-20%. When
a
less catalytically active metal
is introduced, the deposition rate decreases.
8.0
PROPERTIES
OF
CHEMICALLY DEPOSITED METAL COATINGS
Only in rare cases are chemically deposited metal coatings
so
pure, and have
so
regular
a structure. that their properties are the same
as
those of the corresponding chemically pure
substance. Very different properties may be exhibited by coatings containing a nonmetallic
component-phosphorus or boron.
The density of coatings is a little lower than that of bulk metal. This is related to
a
rather irregular coatings structure: they contain more defects (pores and inclusions of
foreign matter). For example, chemically deposited copper usually has
a
great number of
microscopic voids 20-300
A
in diameter, formed by the hydrogen occluded in the coating.
NI-P and NI-B coatings usually have a layered structure, which results from the non-
uniform distribution
of
phosphorus and boron
in
the coatings.
Mechanical properties of the coatings may vary within a wide range depending on
the electroless plating conditions, plating solutions composition, and deposition rate.
For chemically deposited finish copper coatings, such
as
those on printed circuit
boards, sufficient resistance and ductility are
of
great importance. Coatings that have a
tensile strength of about 40-50 kg/mm’ may be obtained at
a
temperature of 50-70°C.
Their ultimate elongation. which characterizes ductility, may be
as
high
as
64%.
Copper
coatings obtained at room temperature are more brittle. Highly ductile coatings may be
obtained only from solutions containing special additives. Ductility increases when depos-
ited coatings are heated in an inert atmosphere at temperatures of 300-500°C.
Ni-P and Ni-B coatings are relatively hard; after deposition, their hardness. which
depends
on
the amount of P and B, is 350-600 kg/nm‘ (3400-5900 MPa) of NI-P
coatings and 500-750 kg/mm’ (4900-7400 MPa) for Ni-B coatings. while after heating
at about 400°C.
it
is 800-1000 kg/mm’ for Ni-P and 1000-12500 kg/mm’ for NI-B.
Hence, such coatings have the same hardness as that of chromium coatings. The tensile
strength of NI-P coatings is
in
the range
of
40-80 kglmm’.
The ductility
of
nickel coatings, if their hardness is taken into account, is rather
high: their ultimate elongation is less than 2%. Such a combination of hardness. wear
resistance. and ductility is unique.
The electrical conductivity
of
chemically deposited coatings is usually lower than
of
the respective pure metals. Resistivity of thin copper coatings (0.5-
1
.0
km) deposited
at room temperature is 3-4
x
1.W”
iLm-twice
as
great as that
of
pure bulky copper.
The surface resistance of such coatings is 0.03-0.07
i2/0.
However, ductile copper coat-
ings that are obtained at temperatures of 50-70°C have
a
resistivity
of
2
X
IO-’
ibm,
close
to
that of pure copper.
The resistivity
of
Ni-P and NI-B coatings depends
on
the amount
of
nonmetallic
component, and it is usually in the range 3-9
x
IO”
i2.m; that
is
much higher than that
of
pure bulky nickel (0.69
X
IO”
i2.m).
Heating causes reduction
in
resistivity.
Magnetic properties of the coatings
of
such ferromagnetic materials
as
nickel and
cobalt may vary within a very wide range. With an increase
in
the amount of phosphorus

ELECTROLESS
PLATING
225
in
nickel coatings. their ferromagnetism decreases, and coatings containing more than
8
mass percent of phosphorus or
6.5
mass percent
of
boron are nonmagnetic.
Coatings of CO-P,
CO-B,
and cobalt alloys with other metals have highly different
magnetic properties. These depend on the composition of the coatings, their structure, and
thickness, and they may be controlled by changing the composition, pH, and temperature
of electroless plating solutions. Usually. cobalt coatings exhibit a high coercivity
(15-80
kA/m); however, soft magnetic coatings
(0.1
-I
.0
kA/m) may be deposited as well.
Optical properties of coatings are less varied and do not differ
so
much from those
of pure metals. Chemically deposited coatings are usually dull; when special additives are
introduced, bright coatings are obtained. Since they are not used as finish decorative
coatings, properties of appearance and brightness usually are not essential.
Silver and gold coatings are often used as mirrors, but the light-reflecting surface
is usually the inner surface, which is adjacent to the smooth glass surface. Chemically
deposited thin gold films are employed as optical filters; they pass visible light but reflect
infrared rays and radio waves.
Chemically deposited coatings are usually less porous than the respective electro-
plates; therefore. they provide better protection of the basis metal against corrosion. Corro-
sion resistance
of
the coatings themselves may be different depending on structure and
composition. NI-P and Ni-B coatings are more resistant to corrosion than nickel electro-
plates; this may be due
to
their fine crystalline structure.
REFERENCES
I.
7
-.
3.
4.
5.
6.
7.
8.
9.
IO.
1
I.
12.
13.
14.
15.
16.
A.
Brenner, and G. Riddell,
J.
Res.
Nd.
Bur..
Stmd,
37, 31 (1946).
W. Goldie,
Metnllic
Conring
of
Plmtic.s
Hatch End, Middlesex, England: Electrochcmical
Publications Ltd., Vol.
I,
1968;
Vol.
2,
1969.
F.
Pearlstein. “Electroless plating,”
Moder./l
Electropl~rtir~g.
3rd ed.,
F.
A.
Lowenheim. Ed.
New York:
1974,
p.
710.
M.
Salkauskas,
and
A.
VaSkclis,
KhirnicheskLryrr
Mercrllizcrtsiyfr
P1rrstrncr.s.s.
Leningrad: Khlm-
iya,
1984.
R. M. Lukes,
Pltrhg.
51, 969. 1066 (1964).
M. Paunovlc.
Pkrting.
55,
1161 (1968).
F.
M.
Donahue,
Ohe~~~che-Sur~fire~,
13. 301
(
1972).
A.
VaSkelis. and
J.
JaEiauskienc,
Elektrokhir~~iyrt,
17, 816 (1981).
I.
Ohno, and
S.
Haruyama,
Surfirce
Techrlol.,
13,
1
(198
I
).
A.
VaSkelis,
Elekr,ok/~irl~iytr,
14, 1970
(
1978).
E.
B. Saubestre,
Pltrtirrg
59. S63 (1972).
K.
M. Gorbunova, and
A.
A.
Nikiforova,
Physit~oc~her~lictrl
Prirlc,iple.s ofNickel
Plating,
trans-
lated from Russian,
1963,
TT
63-1
1003.
G.
Gnwrilov, C/~rr~~isc~/~elStr-orlllnse/vc.r7lickr/r~r~~~.
Saulg~~,
Wurt.: Eugen Lcuze Verlag,
1974.
K.
M. Gorbunova
et
al.,
Fi~iko-K/lirllic/~e.sk/ye
Osr~ovy
Processcr
Khirr~ic/tr.sko,~o
Ko/xr/rir.owl-
iyrr.
Moscow: Nauka,
1974.
A.
Molenaar. and
J.
J.
C.
Coumans,
Storfirc~
Techrlol..
16, 265 (1982).
Y.
Okinaka,
Gold
Plcrtir~g
Techr~ology,
H. Reid and W. Goldie, Eds. Hatch End. Middlesex,
England: Electrochemlcal Publications, Ltd.,
1974,
p.
82.
This Page Intentionally Left Blank
25
The Electrolizing Thin, Dense,
Chromium Process
Michael O’Mary
The
Annoloy
Corporution,
DeKdb.
Illirzois
1
.O
GENERAL DEFINITION
The electrolizing process uniformly deposits
a
dense, high chromium, nonmagnetic
alloy
on the surface of the basic metal being treated. The alloy used in Electrolizing provides
an unusual combination of bearing properties: remarkable wear resistance, an extremely
low coefficient
of
friction, smooth sliding properties, excellent antiseizure characteristics,
and beneficial corrosion resistance. Electrolized parts perform better and last up
to
IO
times longer than untreated ones.
The solution and application processes are carefully monitored at
all
Electrolizing
facilities. The result is a fine-grained chromium coating that is very hard, thin, and dense
and has absolute adhesive qualities. The Electrolizing process deposits
a
99%
chromium
coating on the basis metallic surfaces, whereas normal conventional chromium plating
processes tend to deposit
8248%
chromium in most applications.
Electrolizing calls for the cleaning and removal
of
the matrix on the basis metal’s
surface by multicleaning process, using
a
modified electrocoating process that causes the
chromium metallic elements of the solution to bond
to
the surface porosity
of
the basis
metal. It is during this process that the absolute adhesive characteristics and qualities of
Electrolizing are generated. The Electrolizing coating will not flake, chip, or peel off the
basis metal substrate when conventional ASTM bend tests and impact tests are performed.
Three basic factors are always present after applying Electrolizing to metal surfaces:
Increased wear (Rockwell surface hardness of
70-72
R,)
Added lubricity characteristics
Excellent corrosion resistance
227

228
O’MARY
2.0
APPLICATIONS
2.1
General
Electrolizing can meet a variety
of
engineering needs: chrome plating according
to
specifi-
cations, flash chrome plating, repair or salvage work, and heavy chrome application before
grind. Electrolizing increases wear resistance, reduces friction. prevents galling and seiz-
ing. minimizes fretting corrosion, offers resistance
to
erosion, and provides corrosion
resistance.
It
can be applied
to
all commonly machined ferrous and nonferrous metals,
including aluminum, titanium, stainless steels, coppers, brass, and bronze. The coating
is
not recommended for magnesium, beryllium, columbium, lead, and their respective alloys.
On
aluminum, Electrolizing increases wear life and surface strength. reduces oxida-
tion and corrosion, enhances appearance, and prevents galling, seizing, and erosion. Elec-
trolizing is also conductive, whereas other treatments to aluminum are nonconductive.
The result with Electrolizing is no static buildup.
Because it is a thin, dense coating, Electrolizing exhibits its best wear and lubricity
properties on hardened surfaces. It is most effective when the basis metal is
40
R,
or
harder. In severe wear applications. a basis metal should be hardened to the
50-62
R,
range before Electrolizing is performed. Electrolizing will improve performance on any
basis metal, but
it
is not a substitute for heat treating.
Table
I
lists appropriate uses for Electrolizing at various basis metal Rockwell
hardness ranges.
2.2
Specific
Electrolizing is used across a variety of industries for a multitude
of
purposes. With
Electrolizing, dies and molds for both rubber and plastics have better release characteristics
and reduced wear (especially when abrasive materials are involved); cutting tolls experi-
ence longer wear life; nuclear components exhibit better antigalling and corrosion resis-
tance properties; dies used in stamping. drawing, forming, and blanking have sharper cuts
Table
1
Uses
for Electrolizing
Basis metal
hardness
range
(R,)
Application
Thickness of
Electrolizing
rccommeldxl (in.)
~ ~~~~
18-30 Electrolizing will handle low-loaded or strcss conditions
0.0001
and provide basic corrosion resistance.
will basically support the wear factor. Corrosion
resistance is good in this range.
harder.
Wear
resistance propertics begin to improve
greatly.
an
Electrolizing application. Corrosion reststance is
superior.
and
the maximum wear resistance benefits
exhibited.
30-40 Electrolizing increases wear resistance where the metal
0.0007-0.0009
40-50 Corrosion resistance increases
21s
basis mctal gets
0.0003-0.0005
250
This is the most common and recommended rangc for
0.00005-0.0003

THE ELECTROLIZING THIN, DENSE, CHROMIUM PROCESS
229
and increases in work life; engine and transmission parts (such a valves, valve guides,
pistons, gears, and splines) are protected without detrimental tolerance changes or varia-
tions; standard pumps and meters handle corrosive liquids and materials much better; and
bearing and bearing surfaces can run longer and cooler and are superior to
a
400 series
stainless steel for corrosion protection. In fact, Electrolizing makes it possible to use
standard ferrous steels in place of stainless steel in many applications, including food
processing, medical environments. and ball or roller bearing applications.
Other applications include drive transmissions. power transmissions, gears, molds,
screws. sleeves, threaded parts, and valves. In the automotive industry, applications include
not only engine parts, but also large metal form dies for auto body parts. Electrolizing
has been utilized on applications in these and other industries:
aerospace
aircraft and missiles
armament
automotive
business machines
cameras and projectors
computers
cryogenics
data processing
electronics
food processing
gauges and measuring equipment
pharmaceutical
photography (motion and still)
medical instruments
metalworking
molds (plastic and rubber)
motor industry
nuclear energy
refrigeration
textile industry
transportation
Specifically, Electrolizing is approved and meets the following aerospace, nuclear,
and commercial specifications:
Air Research Company, Garrett, CO
American Can Company
AMS 2406
AMS 2438
AVCO Lycoming-AMS
2406
Bell Helicopter
Bendix Company
Utica,
NY,
division
Teterboro,
NJ,
division
Kansas City, MO. division
South Bend.
IN,
division
BAC
5709
Class
11,
Class
IV
QQC 320
Cleveland Pneumatic Tool-CPC Specs (Chromium), QQC320
Colt Industries
Boeing
Menasco,
TX.
division
DuPont
Fairchild Camera
Fairchild Republic
General Dynamics

230
O’MARY
General Electric
Lynn, MA
Cincinnati, OH (aircraft)
Wilmington, MA
Wilmington, NC (nuclear)
Fitchburg, MA
Gillette Company, Boston
Grumman Aircraft
IBM. 40 to 45
Johnson
&
Johnson, New Jersey
Kaman Aircraft
QQ-C-320
AMS 2438
McDonnell/Douglas
PS 13102
QQC320
MIL-C-23422
Nabisco Company
Ozone Industries
Perkin Elmer Company, most divisions
Pratt
&
Whitney
QQC320
PWA
48
AMS 2406
Procter
&
Gamble, Cincinnati, OH
QQ-C-320 B Class
I1
Raytheon
U.S.
Navy
Newport News, Electric Boat, Portsmouth Naval Shipyard
Hamilton Standard
HS 332, HS246
QQ-C-320B
Western Gear Company
Western Electric Company
Westinghouse
3.0
SURFACE PREPARATION
Substrate surfaces must be free
of
oil, grease, oxides, and sulfides. Parts should be surface
finished before shipment to an Electrolizing facility. where they will undergo further
cleaning and surface preparation through a multicleaning process. Surfaces will
not
be
changed significantly in configuration by the Electrolizing process.
A clean surface is very important. When conventional chrome is utilized, component
failure is often erroneously attributed
to
“spalling.” The effect is actually due
to
a residual
contaminant, which was covered or surrounded by electrodeposited chrome and subse-
quently dislodged as
a
result of sliding or other mechanical action. This leaves
a
void in
the chrome plating which,
in
turn, causes further deterioration of the surface.

THE ELECTROLIZING THIN, DENSE, CHROMIUM PROCESS
231
The surface must be free from scale and soils to secure the best corrosion resistance.
Any scale left on the surface will gradually acquire a rusty appearance and act
as
a nucleus
for additional rust
to
form. On articles that are to have a “stainless” appearance, every
trace of scale must be removed by grinding, pickling, or polishing.
The solution of Electrolizing, Inc., is to use a multicleaning procedure. Multicleaning
is not
a
trade name, nor is it
a
novel, or supercleaning, technique. It is merely
a
designation
for a carefully planned cleaning program prescribed for each individual part, utilizing
every possible cleaning method available-such as vapor degreasing; solvent, detergent,
diphase, and alkaline cleaning; dry vapor honing; electrolytic and ultrasonic cleaning;
vibratory cleaning; and hydroblasting. Maintained throughout are high level quality control
standards, consisting of microscopic before-and-after inspection
of
each part, intermittent
inspection between processing phases, and disciplined handling procedures.
The significant surfaces to be coated should be completely finished prior to process-
ing. For best results, the surface finish should be
32
RMS or better. The finer the surface,
the better it
is
to Electrolize. This is true to a finish of 2-4 RMS or better. Surface finishes
that are received by Electrolizing with
4
RMS
or less may show some slight roughing of
the surface after application. In most cases these finishes can be lapped or polished back
to their original condition. All surfaces should be free from plated coatings. Parts that
have been nitrided should be machined or vapor-blasted before processing. The Electroliz-
ing coating will not adhere to brazed or welded areas unless these are machined or vapor-
blasted to remove
all
impurities before processing. Unless otherwise specified, the coating
should be applied after
all
basis metal processing has been completed. This includes heat
treatment, stress relieving (when required), machining, brazing, welding, and forming.
The coating should be applied directly
to
the basis metal without any intermediate coating.
Electrolizing is
a
low temperature process. The temperature of parts during the
cleaning and coating process does not exceed
180°F.
There is no distortion or annealing
of the basis metal.
Generally, parts should be less than
20
ft. long,
30
in. in diameter, and 4000 Ib
in
weight. Parts exceeding these limits should be discussed with Electrolizing, Inc.
4.0
SOLUTION
Electrolizing is
a
blend of some of the best chrome salts and selected proprietary catalysts
and additives that create its unique proprietary features. The solution is carefully monitored
and its quality maintained through the use
of
exacting, sophisticated equipment and labora-
tory controls. The materials are distributed only
of
accredited licenses within the Electroliz-
ing licensee program.
5.0
PROPERTIES
5.1
Thickness
Electrolizing is applied in
a
very thin, dense layer on the basis metal. The Electrolizing
process does not create
a
buildup
on
comers or sharp edges; it conforms exactly to the
surface. There is no change in either the conductivity or magnetic properties of the basis
metal.
Electrolizing deposits range from
0.000010
to
0.003
in. per side. The practical coat-
ing range
is
from 0.000025 to
0.001
in. The average deposit is
0.0004-0.0008
in. per
