Rudnick L. Lubricant Additives: Chemistry and Applications (Присадки, добавки к смазкам)
Подождите немного. Документ загружается.

454 Lubricant Additives: Chemistry and Applications
These additives as well as other molybdenum, zinc, and boron compounds are excellent chemi-
cals for antiwear and friction reduction, but are typically designed for petroleum uids. They will
work well in vegetable oils and highly re ned petroleum oils, but will increase overall toxicity of
these blends.
18.11 ANTIFOAM
Vegetable oils need additional antifoam agents to prevent air entrainment. Conventional antifoam
agents have only a minor effect on vegetable oils. Silicon foam inhibitors are very effective and
widely used. Silicon is very nonpolar and is an excellent foam inhibitor. The silicon chemicals
are usually diluted in either petroleum oil base stock or vegetable oils and may need an additional
solubility agent such as an ester to maintain solubility. Silicon acts as a surfactant and prevents air
from reacting with the lubricant surface. Other foam inhibitors used with vegetable oils are dimeth-
ylsiloxane, alkylmethacrylate, and other alkylacrylates.
18.12 VISCOSITY MODIFIERS/POUR POINT DEPRESSANTS
Viscosity is commonly known as resistance to ow. Vegetable oils normally have a good natural
viscosity for industrial lubricants. Some formulators will utilize this natural characteristic and the very
high viscosity index (VI) and not use any additional viscosity modi ers.
VI is the change in viscosity compared to the change in temperature. A high VI indicates small
viscosity changes with temperature changes. Vegetable oils have very high VI usually ∼200 when
compared to petroleum oil, which has a VI of ∼100. This means that vegetable oils maintain their
design viscosity over a broader temperature range.
Finding an environmentally safe viscosity modi er is very dif cult. Typically, the long-chain
polymers do not break down in the environment and therefore reduce biodegradability. Most viscos-
ity modi ers are nonpolar and hard to solubilize in vegetable oils and will create hazy mixtures.
Ethylene propylene diene monomer (EPDM) polymers are especially dif cult to solubilize due to
their diene double bond. There are some viscosity modi ers, however, that can be dispersed in veg-
etable oils including ole n copolymer (OCP) and polymethacrylate (PMA).
The pour point is the lowest temperature at which oil will ow. Most pour point depressants
(PPDs) reduce the size and cohesiveness of the crystal structures and will thereby reduce pour point
and improve the ow at reduced temperatures. PPDs are base oil speci c. While the steric effect
of the carbon side chains of vegetable oils have more of an effect on the pour point than any other
factor, some ppds can reduce the pour suf ciently for industrial usage.
Styrene esters, methacrylates, and alkylated naphthalenes work well for vegetable oils as well as
petroleum uids. Methacrylates typically are more effective for group II petroleum oils.
REFERENCES
1. United States Department of Agriculture Biopreferred Web site, http://www.biopreferred.gov.
2. Terresolve Technologies In-house Knowledge Base.
3. Canadian Centre for Occupational Health and Safety Web site, http://www.ccohs.ca/oshanswers/
chemicals/ld50.html.
4. Bergstra, R., Emerging Opportunities for Natural Oil Based Chemicals, MTN Consulting Associates,
Plant Bio-Industrial Workshop, Saskatoon, Saskatchewan, Canada, February 27, 2007.
5. In-house Terresolve Technologies Proprietary Development Research, October 11, 2005 through
January 8, 2007.
6. McCoy, S., United Soybean Council Technical Advisory Panel, The Valvoline Company, September 20,
2005.
7. Erhan, S. Z., Oxidative Stability of Mid-Oleic Soybean Oil: Synergistic Effect of Antioxidant-Antiwear
Additives, National Center for Agricultural Utilization Research, USDA/ARS, Peoria, IL, 2006.
CRC_59645_Ch018.indd 454CRC_59645_Ch018.indd 454 10/31/2008 2:32:35 PM10/31/2008 2:32:35 PM

Part 6
Applications
CRC_59645_S006.indd 455CRC_59645_S006.indd 455 9/30/2008 4:14:19 PM9/30/2008 4:14:19 PM
CRC_59645_S006.indd 456CRC_59645_S006.indd 456 9/30/2008 4:14:19 PM9/30/2008 4:14:19 PM

457
19
Additives for Crankcase
Lubricant Applications
Ewa A. Bardasz and Gordon D. Lamb
CONTENTS
19.1 Introduction ......................................................................................................................... 458
19.2 Detergents ............................................................................................................................ 458
19.2.1 Introduction ............................................................................................................458
19.2.2 Sulfonates ...............................................................................................................460
19.2.3 Phenates, Sulfurized Phenates, and Salicylates ..................................................... 461
19.2.4 Other Detergents: Phosphates, Thiophosphates, Phosphonates, and
Th iophosphonates................................................................................... ...............462
19.2.5 Performance in Lubricants ..................................................................................... 462
19.3 Dispersants ..........................................................................................................................463
19.3.1 Introduction ............................................................................................................463
19.3.2 Dispersant Structure ...............................................................................................464
19.3.3 Polyisobutene Synthesis .........................................................................................466
19.3.4 Dispersant Basicity .................................................................................................466
19.3.5 Succinate Ester Dispersants ...................................................................................466
19.3.6 Mannich Dispersants ..............................................................................................467
19.3.7 Soot Contamination in Diesel Engine Oils ............................................................467
19.3.8 Soot-Thickening Tests ............................................................................................468
19.3.9 Seal Testing ............................................................................................................469
19.3.10 Corrosion ................................................................................................................469
19.3.11 Sludge ...................................................................................................................... 470
19.3.12 Sludge Engine Tests ................................................................................................ 471
19.4 Antiwear .............................................................................................................................. 471
19.4.1 Introduction ............................................................................................................471
19.4.2 Wear Mechanisms .................................................................................................. 471
19.4.3 ZDDP Preparation .................................................................................................. 473
19.4.4 ZDDP Degradation Mechanisms ........................................................................... 474
19.4.5 Sequential Alkyl Transfers (Primary ZDDP) ........................................................ 475
19.4.6 Antiwear Tests ........................................................................................................ 476
19.4.7 Other Antiwear Agents ........................................................................................... 477
19.5 Antioxidants ........................................................................................................................477
19.5.1 Introduction ............................................................................................................477
19.5.2 Mechanism of Oxidation of Lubricating Oils ........................................................ 478
19.5.2.1 Initiation .................................................................................................. 478
19.5.2.2 Propagation ............................................................................................. 478
19.5.2.3 Peroxide Decomposition ......................................................................... 479
19.5.2.4 Termination (Self and Chain Breaking) ................................................. 479
19.5.2.5 Radical Formation .................................................................................. 479
19.5.2.6 Decomposition and Rearrangement ....................................................... 479
CRC_59645_Ch019.indd 457CRC_59645_Ch019.indd 457 10/31/2008 2:34:12 PM10/31/2008 2:34:12 PM
458 Lubricant Additives: Chemistry and Applications
19.5.3 Oxidation Inhibitors ...............................................................................................480
19.5.4 Hindered Phenols and Arylamines.........................................................................480
19.5.5 Sulfur and Phosphorus Containing Antioxidants ..................................................482
19.5.6 Sulfur Compounds .................................................................................................483
19.5.7 Phosphorus Compounds .........................................................................................483
19.5.8 Sulfur–Phosphorus Compounds .............................................................................483
19.5.9 Antioxidant Selection, Synergism, and Testing .....................................................484
19.6 Viscosity Modi ers .............................................................................................................486
19.6.1 Introduction ............................................................................................................486
19.6.2 Viscosity Modi er Types .......................................................................................486
19.6.3 Dispersant Viscosity Modi ers ..............................................................................488
19.6.4 Shear Stability of Engine Oils ................................................................................488
19.6.5 Viscosity Grade ......................................................................................................488
19.6.6 Viscosity Modi er Requirements ...........................................................................489
19.7 Pour Point Depressants ........................................................................................................489
19.8 Foam Inhibitors/Antifoams .................................................................................................490
References ......................................................................................................................................490
19.1 INTRODUCTION
Engine oil lubricants make up nearly one-half of the lubricant market and therefore attract a lot
of interest. The principal function of the engine oil lubricant is to extend the life of moving parts
operating under different conditions of speed, temperature, and pressure. At low temperature, the
lubricant is expected to ow suf ciently in order that moving parts are not starved of oil. At higher
temperatures, they are expected to keep the moving parts apart to minimize wear. The lubricant does
this by reducing friction and removing heat from moving parts. Contaminants pose an additional
problem, as they accumulate in the engine during operation. The contaminants may be wear debris,
sludges, soot particles, acids, or peroxides. An important function of the lubricant is to prevent these
contaminants from causing any damage.
To function effectively, the lubricant needs chemical additives as well as base oils. Depending
on the application, various combinations of additives are used to meet the required performance
level; the most important ones are listed as follows:
Detergents
Dispersants
Antiwear
Antioxidants
Viscosity modi ers
Pour point depressants
Foam inhibitors
In addition to these additives, there are several other additives for anticorrosion, antirust, seal swelling,
biocide, and demulsability.
19.2 DETERGENTS
19.2.1 I
NTRODUCTION
Detergents play an essential role in protecting various metallic components of internal combustion
engines by neutralizing acidic compounds formed during combustion processes [1–3]. Gasoline and
diesel engine oils account for more than 75% of the total detergent consumption. Detergent treat-
ment in engine lubricant can reach 6–10 wt%, with marine diesel engine lubricants containing the
•
•
•
•
•
•
•
CRC_59645_Ch019.indd 458CRC_59645_Ch019.indd 458 10/31/2008 2:34:13 PM10/31/2008 2:34:13 PM
Additives for Crankcase Lubricant Applications 459
highest concentration levels due to combustion of high-sulfur fuel, which leads to the formation of
inorganic acidic combustion products such as sulfuric acid.
The purpose of detergents in crankcase oils is
1. To suspend/disperse oil-insoluble combustion products such as sludge or soot and oxidation
products
2. To neutralize combustion products (inorganic acids)
3. To neutralize organic acid products of oil degradation processes
4. To control rust, corrosion, and deposit-forming resinous species [4]
Why are these speci c functions critical to engine durability? Coke and varnish-like deposits can
restrict the free movement of the piston rings, allowing a portion of the combustion gases to pass
into the crankcase or combustion chamber, leading to heavy contamination of the oil, impacting
engine out emissions and even causing piston seizure if the engine operates at high loads [5]. Heavy
sludge can plug oil lters, leading to oil starvation and thus to catastrophic wear especially during
cold temperature start-ups [6]. Acidic fuel combustion products can cause corrosion.
Detergents can react with hydroxyacids, deposit precursors, formed during the oxidation of the
oil. Deposit precursors are attracted to detergent micelles and trapped within them and, thus, cannot
settle onto metal surfaces and form resinous deposits. The cleaning action of detergent additives is
attributed to chemisorption processes and formation of metal salts.
To satisfy the abovementioned requirements, practically all detergent additives contain
Polar head. Hydrophilic, acidic groups (e.g., sulfonate, hydroxyl, mercapton, carboxylic, or
carbonamide groups) that react with metal oxides or hydroxides
Hydrocarbon tail. Oleophilic aliphatic, cycloaliphatic, or alkyaromatic hydrocarbon
radicals that provide oil solubility
One or several metal ions
Idealized representation of the detergent structures is shown in Figure 19.1.
Although several metals have been incorporated into detergents, only three metal cations are now
commonly used—calcium, magnesium, and sodium. Heavy metals such as barium are no longer used.
Detergents are described chemically in terms of their metal ratio, soap content, percent sulfate
ash, degree of overbasing or conversion, and total base number (TBN) [2]. The metal ratio is de ned
as total equivalents of metal per equivalent of sulfonate acid. Soap content refers to the amount of
neutral salt and re ects the detergent’s cleansing ability or detergency. The percent sulfate ash is the
ash obtained after treating the detergent with sulfuric acid and complete combustion. The degree
of overbasing (conversion) describes the ratio of equivalents of the metal base to equivalents of
the acid substrate and is usually expressed as conversion. Conversion provides the amount of inor-
ganic material relative to that of the organic material and is expressed as number of equivalents of
base per equivalent of acid times 100. The overbased part of detergent is needed to neutralize acid
•
•
•
CaCO
3
CaCO
3
Metal
Oil
FIGURE 19.1 Idealized representations of neutral and overbased detergents.
CRC_59645_Ch019.indd 459CRC_59645_Ch019.indd 459 10/31/2008 2:34:13 PM10/31/2008 2:34:13 PM
460 Lubricant Additives: Chemistry and Applications
by-products. The TBN indicates its acid-neutralizing ability and is expressed as milligrams of KOH
per gram of additive. It is measured using a potentiometric method (e.g., ASTM D-2897).
The alkaline reserve of all modern detergents may vary considerably. Neutral detergents
contain the stoichiometric amounts of metals, corresponding to the basicity of the acids. Basic
(or overbased) detergents contain a signi cant excess of metal oxides, hydroxides, carbonates, etc.,
in colloidally dispersed form. The structure of detergents can be envisioned as a reverse micelle,
with an amorphous carbonate molecule encapsulated by metal soap molecules with their nonpolar
ends extended into the oil (Figure 19.1).
In practice, virtually all commercial detergents are overbased to some extent. For example,
commercial neutral sulfonates have a TBN of 30 or less. Basic detergents have a TBN in the range
of 200–500.
Some detergents can act as oxidation inhibitors, depending on the nature of their functional
group. Most modern motor oils contain combinations of several detergent types, which are selected
to give optimum performance.
Preparation of calcium detergents be represented schematically as follows:
2RSO
3
H
+
CaO (RSO
3
)
2
Ca + H
2
O
(RSO
3
)
2
Ca
+
Ca(OH)
2
xCa(OH)
2
xCO
2
Promoters
(RSO
3
)
2
Ca(CaCO
3
)
x
+
H
2
O
Promoter
+
19.2.2 SULFONATES
The salts of long-chain alkylarylsulfonic acids are being widely used as detergents. Basic calcium
sulfonates make up 65% of the total detergent market.
As the demand for sulfonic acids has rapidly increased, synthetic products with the general
structure (RSO
3
)
v
Me
w
(CO
3
)
x
(OH)
y
are also used besides the sulfonated alkylaromatics from
petroleum re ning known as natural sulfonates. Synthetic products are produced by the sulfonation
of suitable alkylaromatics, for example, the di- and polyalkylation products from the dodecylben-
zene production; their alkyl radicals should contain together at least 20 C atoms. Other starting
materials are alpha-ole n polymers with mean molecular mass of ∼1000.
The neutral sulfonates (schematically shown in Figure 19.2) contain the stoichiometric amounts of
metal ion and acid. Besides Na, Ca, and Mg, patents have been issued describing detergents containing
S
O
O
O
Ca
O
S
O
O
CaCO
3
Hydrocarbon
chain
Sulphonate
head group
Basic sulphonate inverse
micelle structure
Size: 100−150 Å
Neutral calcium sulphonate
FIGURE 19.2 Neutral and basic sulfonates structures.
CRC_59645_Ch019.indd 460CRC_59645_Ch019.indd 460 10/31/2008 2:34:13 PM10/31/2008 2:34:13 PM
Additives for Crankcase Lubricant Applications 461
tin, chromium, zinc, nickel, and aluminum; however, the importance of these metals is inferior to that
of the alkaline earth metals.
Neutral oil–soluble metal petroleum sulfonates can be converted into basic sulfonates by mixing
and heating with metal oxides or hydroxides, followed by ltration. In these products, the metal
oxides and hydroxides are present in colloidally dispersed form (Figure 19.2). Such basic sulfonates
have a considerably increased alkaline reserve and thus a higher neutralizing power.
Treatment with carbon dioxide converts basic sulfonates into metal sulfonate–carbonate
complexes that have the same alkaline reserve, yet a lower basicity. Efforts to produce additives with
even higher neutralizing power have led to the development of the overbased sulfonates. Besides
high neutralizing power, these additives also possess a high dispersing capacity due to the large
amount of polar inorganic bases present.
Overbased sulfonates are produced, for instance, by heating an oil-soluble sulfonate with metal oxides
in the presence of substances that act as catalysts such as phenols and phosphoric acid derivatives.
19.2.3 PHENATES, SULFURIZED PHENATES, AND SALICYLATES
Basic phenates make up 31% of the total detergent market. Schematic structures of phenates and
sulfurized phenates are shown in Figure 19.3.
Phenate detergents are available as calcium and magnesium salts. Metal salts of alkylphenols
and alkylphenol sul des, (R) (OH) C
6
H
3
-S
x
-C
6
H
3
(OH) (R), where x = 1 or 2 and R is ∼12 C, can
be prepared at elevated temperatures by the reaction of alcoholates such as magnesium ethylate
with alkylphenols or by the reaction of phenols or phenol sul des with an excess of metal oxide or
hydroxide (particularly of Ca) sul des with an excess of metal oxides or hydroxides in neutral phen-
ates. Besides their neutralization power, phenates also possess good dispersant properties.
As in the case of the sulfonates, the phenates can be overbased. Overbased phenates are often
used as components of marine diesel cylinder lubricants.
In many commercial lubricant applications, sulfonate and phenate detergents are used in com-
bination and often contain various metals to obtain an optimum detergent action and neutralizing
power. Besides better neutralizing power, the main incentive for the use of basic phenates is lower
manufacturing cost compared with the normal phenates.
Salicylates are less commonly used as detergents in crankcase lubrication. The typical structure
of salicylate detergent is given in Figure 19.4.
Besides their detergent properties, the metal alkylsalicylates also possess oxidation-inhibiting
and anticorrosion properties. Their solubility in mineral oils can be improved in the case of esters
by extending the chain length of the alcohol radicals or generally by alkylation of the aromatic ring.
OH
R
S
OH
R
OH
R
OH
R
OH
R
OH
R
FIGURE 19.3 Phenates and sulfurized
phenates structures.
C
C
C
C
C
C
OH
R
C
O
O
H
HH
C
C
C
C
C
C
OH
R
C
O
O
H
HH
Ca
FIGURE 19.4 Calcium salicylate structure.
CRC_59645_Ch019.indd 461CRC_59645_Ch019.indd 461 10/31/2008 2:34:14 PM10/31/2008 2:34:14 PM
462 Lubricant Additives: Chemistry and Applications
The alkaline earth salicylates are usually overbased by the incorporation of alkaline earth carbon-
ates, stabilized in the form of micelles.
19.2.4 OTHER DETERGENTS: PHOSPHATES, THIOPHOSPHATES,
P
HOSPHONATES, AND THIOPHOSPHONATES
Besides their use as oxidation inhibitors, phosphates and thiophosphates also serve in various
variations and combinations as detergents. Thiophosphonates are obtained by the reaction of
phosphorus pentasul de with polyisobutenes (PIBs), ole ns, fatty alcohols, and esters are neutralized
after hydrolysis with metal hydroxide.
19.2.5 PERFORMANCE IN LUBRICANTS
For crankcase engine oils, which include passenger car, heavy-duty diesel, marine diesel, and
stationary gas applications, detergents provide several key performance functions. One of the
primary functions of overbased detergents is to neutralize acidic combustion by-products [2–4]. In
all reciprocating piston internal combustion engines, gases from the combustion chamber are forced
around and through the piston rings and into the crankcase where they interact with the lubricant.
These combustion gases and by-products contain such components as oxides of sulfur, derived from
the sulfur content of fuels. Particularly in diesel engines, these sulfur oxide compounds interact with
oxidized components from the fuel and base oil to produce sulfuric acid and organic acids [6].
Another form of combustion by-products comes from oxides of nitrogen, derived from the
high-temperature combination of nitrogen and oxygen from the intake air. These by-products
are predominant in gasoline engines in which the oxides of nitrogen materials can further react
with water (from the combustion process), oxidized oil and fuel, and soot (if present) to produce
engine sludge and piston varnish [5]. Obviously, these acidic combustion gases and by-products are
detrimental to the extended life of both the engine components and the lubricant itself. They can
give rise to increased rusting of steel parts and corrosion of bearings.
The use of high TBN, overbased detergents can combat these problems. However, one must be care-
ful in formulating with an appropriate mix of detergents for acid control and corrosion performance.
The use of several appropriate detergents in a lubricant for excellent engine rust and bearing corrosion
performance may not necessarily be favorable to maintain good valve train wear performance, such as
in the well-known gasoline engine speci cation tests, the Sequence VE and Sequence IVA.
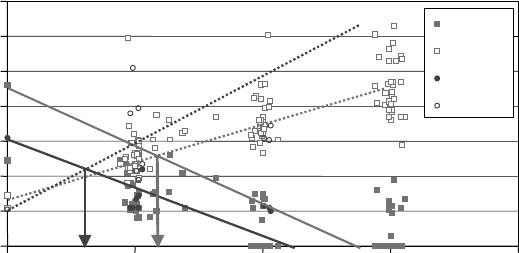
Figure 19.5 plots data from a recent passenger car eld test showing the decrease in TBN and the
increase in total acid number (TAN) with use. Original equipment manufacturer (OEM) oil drain
recommendations are often determined by this type of testing. Typically, it is considered desirable
0
2
4
6
8
10
12
14
0 5 10 15 20
Miles (1000 s)
Total acid−base number
TBN-H
TAN-H
TBN-L
TAN-L
FIGURE 19.5 Passenger car eld testing: TBN/TAN relation.
CRC_59645_Ch019.indd 462CRC_59645_Ch019.indd 462 10/31/2008 2:34:14 PM10/31/2008 2:34:14 PM

Additives for Crankcase Lubricant Applications 463
to change the oil before the TBN and TAN cross. In this “severe service” example, the oil that starts
at a TBN of 6 would need to be changed twice as often as the oil that has an initial TBN of ∼9.
Evidence such as this results in oils with higher TBN being recommended for longer drain
intervals.
A second function of a detergent is to retard deposit formation on engine parts, especially parts
that are operating at high temperatures, such as pistons and piston rings (Figure 19.6). Detergency of
North American diesel engine oils is evaluated using both single-cylinder engine tests (Caterpillar 1N,
1K, 1P, and 1Q) and multicylinder engine tests (Cummins M11, Mack T-9, and T-10). The selection of
detergents to give the best piston and ring cleanliness is highly dependent on the temperature of the
piston ring area, the metallurgy of the piston, the ring pack design, and the base stock of the lubricant
being tested. Metallurgy variances in engine designs such as aluminum versus articulated steel diesel
pistons complicate proper detergent selection. A particular mixture of detergents may be excellent
with aluminum hardware but may only perform marginally with steel hardware.
Some types of detergents perform additional functions in an engine oil formulation. For
example, coupled-coupled alkyl phenols enhance high-temperature oxidation inhibition. Owing to
their speci c structure and thermal stability, these detergents help prevent oxidation of the lubricant
under high speed and high-load engine conditions; the result is a lower viscosity increase of the oil.
Of course, high-temperature oxidation inhibition synergizes with the detergent’s ability to enhance
cleanliness.
In summary, the best overall cost and performance by using a selected combination of deter-
gents in a lubricant depends on many factors. Several of these factors entail complete engine per-
formance, customer desires, and regulations including the maximum total amount of metal or metal
ash allowed in a lubricant as set by speci cation requirements.
19.3 DISPERSANTS
19.3.1 I
NTRODUCTION
Dispersants are typically the highest treat additive in an engine oil formulation. They are similar
to detergents in that they have a polar head group with an oil-soluble hydrocarbon tail. Although
detergents are used to clean engine surfaces and neutralize acidic by-products, their effectiveness
is limited when it comes to dispersing oil-insoluble products resulting from the by-products of
combustion. The principal function of a dispersant is to minimize the deleterious effects of these
contaminants. The most obvious contaminants related to engine lubricants are black sludge and soot
particles. Sludges range from thick oil-like deposits to a harder deposit; soot is composed primarily
of carbon particles and is typically found in diesel engines. Dispersants are used to disperse these
contaminants within the engine thereby ensuring that the oil ows freely. The dispersing ability of
the dispersants helps keep the engine clean and, in some cases, will maintain piston cleanliness.
Some formulators will actually refer to certain dispersants as ashless detergents.
Acceptable
Unacce
p
table
FIGURE 19.6 Detergents keep upper pistons clean.
CRC_59645_Ch019.indd 463CRC_59645_Ch019.indd 463 10/31/2008 2:34:14 PM10/31/2008 2:34:14 PM
