Rudnick L. Lubricant Additives: Chemistry and Applications (Присадки, добавки к смазкам)
Подождите немного. Документ загружается.

424 Lubricant Additives: Chemistry and Applications
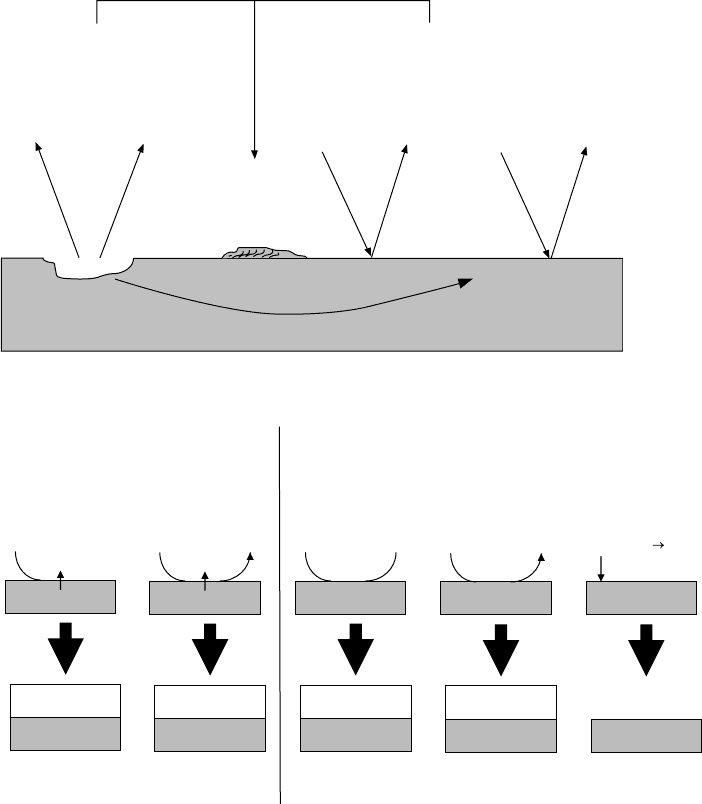
affected by the pH of the system and ultimately determines the type of protective lm that is
formed.
Anodic inhibitors (passivating inhibitors or dangerous inhibitors). Anodic inhibitors produce
a large positive shift in the corrosion potential of a metal through the production of a
protective oxide or hydroxide lm (Figure 17.3). These can be quite dangerous since they
can be corrosive at low concentration and need to be carefully monitored. There are two
main modes of action that serve to disrupt the anodic processes.
Oxidizing anions. Oxidizing anions passivate a metal in the absence of oxygen. Typical
examples include chromates and nitrites, which they function by shifting the potential into
a region where insoluble oxides or hydroxides are formed. For example, the commonly
used chromates will be reduced from Cr
6+
to Cr
3+
, which in turn oxidizes the Fe
2+
on the
surface to Fe
3+
. The Fe
3+
, which is less soluble in aqueous solutions than the Fe
2+
, then
forms a protective oxide coating and passivates the metal surface [10,11].
FIGURE 17.2 Formation of corrosion products in acidic or alkaline/neutral media.
Alkaline
Cathode
Anode
Acidic
H
2
2H
+
2OH
−
Fe
2+
Fe
3+
Fe
2
O
3
Fe
3
O
4
FeOOH
Fe(OH)
2
e
−
½
O
2
+ H
2
O
FIGURE 17.3 Schematic mechanism of cathodic and anodic corrosion inhibitors.
Fe
Fe
Fe
3+
Cr
6+
Cr
3+
Fe
2
O
3
Fe
Fe
1/2O
2
Ca(OH)
2
(Mg or Zn)
Fe
Fe
As
2
O
3
As
As (Bi or Sb)
Anode
alkaline/acid
Cathode
alkaline
Cathode
acid
Fe
Fe
Fe
3+
PO
4
3−
FePO
4
Anode
alkaline/acid
Nonoxidizing anions
Oxygen scavengerHydrogen poisons
Cathodic precipitate
Fe
Fe
1/2O
2
+ SO
2
SO
3
Cathode
acid/alkaline
X
Anodic reactions
Ca
2+
Reaction
Surface
species
Cathodic reactions
Oxidizing anions
CRC_59645_Ch017.indd 424CRC_59645_Ch017.indd 424 10/31/2008 2:31:06 PM10/31/2008 2:31:06 PM
Corrosion Inhibitors and Rust Preventatives 425
Nonoxidizing anions. Nonoxidizing anions contain species that need oxygen to passivate a
metal. Typical chemistries include silicate, carbonate, phosphate, tungstate, and molybdate.
The mode of action appears to promote the formation of a passivating oxide lm on the
anodic sites of the metal surface [11].
Cathodic inhibitors. Cathodic inhibitors act to retard the reduction of O
2
or H
+
or selectively
precipitate onto cathodic areas (Figure 17.3). Although the cathodic inhibitors are not as
effective at low concentration as their anodic counterparts, the cathodic inhibitors are not
corrosive at low concentrations. There are three main modes of action that serve to disrupt
the cathodic reaction.
Hydrogen poisons. Hydrogen poisons such as As (as As
2
O
3
or Na
3
AsO
4
), Bi, or Sb primarily
act in acidic media (Figure 17.3) to retard the hydrogen reduction reaction by reducing at
the cathode and precipitating a layer of the poisoning metal. Unfortunately, they also pro-
mote hydrogen absorption in steel and can cause hydrogen embrittlement if not carefully
monitored.
Cathodic precipitates. Cathodic precipitates are used in neutral or alkaline solutions
(Figure 17.3) and act to form insoluble hydroxides (such as Ca, Mg, or Zn) that will reduce
the corrosion rate where the metal is exposed. Typically when the hydroxyl ion (OH
−
)
concentration increases in the cathodic areas, cathodic precipitates such as the calcium or
magnesium carbonates will react to absorb the excess hydroxide and precipitate Ca(OH)
2
or Mg(OH)
2
on the surface of the cathode which in turn inhibits the reduction of oxygen.
Oxygen scavengers. Oxygen scavengers reduced corrosion by capturing excess oxygen in the
system. Typical aqueous oxygen scavengers used for water treatment include hydrazine,
SO
2
, NaNO
2
, and Na
2
SO
3
, but there are also many organic antioxidants based on alkylated
diphenylamine or alkylated phenols that are used in lubricating oils to scavenge oxygen,
which could also be considered in this category.
Mixed (or organic) inhibitors. Mixed inhibitors are organic materials (not inorganic ions
such as anodic and cathodic inhibitors) that absorb on a metal surface and prevent both
anodic and cathodic reactions. These materials are the typical corrosion inhibitors used in
lubricating oils and are more dif cult to remove by chemical or mechanical action than the
monolayer lms formed by the anodic and cathodic inhibitors.
17.3 MECHANISM
The basic description of the mechanism for corrosion inhibition by additives was rst proposed by
Baker et al. as the adsorption of a monolayer of the inhibitor on the metal surface to form a pro-
tective barrier. This barrier is impervious to water and prevents contact with the outside environ-
ment. They found that the amount of rust prevention depended on a complex interaction of several
variables including the lifetime of the adsorbed lm, presence of oxygen, thermal and mechanical
desorption, solubility (in water), and surface wettability [7,8]. Subsequently, Kennedy [12] added
that there is a complex equilibrium between the water on the metal surface and the micellar or
solublized water; he found that the concentration of water in the system effects the corrosion inhibi-
tion of a sulfonate. Later, Anand et al. [13] found that lowering the interfacial surface tension also
improved the corrosion resistance.
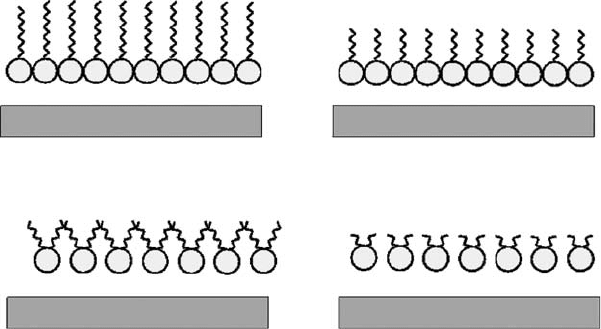
Subsequently, it was found that the monolayer of adsorbed additive was oriented with the polar
head adsorbed on the surface and the nonpolar tail closely packed and vertically aligned [14].
The matched chain lengths of the inhibitor and the basestock (e.g., C
16
-fatty acid matched with
C
16
-alkane) prevent rust due to tight surface packing, which makes the inhibitor more dif cult to
remove through chemical action (Figure 17.4) [15]. Further investigation revealed that the absorption
was accomplished by either an electrostatic (physisorption) or an electron transfer to a coordinate
type of bonding (chemisorption) [13,15]. The lm was found not only to restrict the access to the
CRC_59645_Ch017.indd 425CRC_59645_Ch017.indd 425 10/31/2008 2:31:07 PM10/31/2008 2:31:07 PM
426 Lubricant Additives: Chemistry and Applications
surface of aggressive species but also to prevent the passage of metal ions into solution, which
restricts the cathodic process to prevent the evolution of H
2
or reduction of O
2
(Figure 17.4) [14].
Early studies on rust inhibitors focused on the widely used dinonylnaphthalene sulfonates. It
was found that in water-saturated solutions the cation does not effect the adsorption of the sulfonate,
whereas in anhydrous solutions the cation possesses a signi cant effect, where it is proposed that
the cation coordinates directly with the oxide lm (not solublized in the aqueous phase) [16]. In the
general trends for sulfonate, it was observed that the corrosion inhibition of the cations increased in
the order: Na < Mg < Ca < Ba [13,14,16].
Overall, it can be stated that a corrosion inhibitor is a substance that forms a protective barrier
and creates a mechanical separation between the metal and environment. This barrier cannot be
easily removed and prevents transport of aggressive agents from contact with the surface, as well as
corrosion products from leaving the surface.
17.4 CHEMISTRY
17.4.1 N
ITRITES
Wachter and Smith rst described the use of sodium nitrite (NaNO
2
) as an anodic inhibitor to
prevent internal corrosion by water and air in steel pipelines for petroleum products [17,18]. It was
rst speculated that the mechanism involved the formation of a tight oxide layer of oxygen and
nitrite to prevent corrosion by forming a passivating layer [19], but subsequently it was proposed
that the nitrite accelerated the production of Fe
3+
on the metal surface and formed a less-soluble
protective barrier than the Fe
2+
species [20].
Historically, nitrites were primarily used in aqueous systems such as water treatment or
concrete emulsions as an anodic inhibitors [20], but subsequently they have also been used in
soluble oils for metalworking applications. Since the 1950s, the reaction of amines commonly
used in the soluble oils with nitrite (Figure 17.5) was found to form the carcinogenic nitrosamines
(R
2
NNO) under acidic conditions [21,22]. As a result, the use of nitrites as RPs in metalworking
uids containing ethanol amine carboxylate salts was banned by the U.S. Environmental Protection
Agency (EPA) in 1984, and the industry quickly modi ed their formulations to replace the nitrite
with other inhibitors [23]. Typical replacements used were the borate, carboxylate, or phosphate
salts of triethanolamine (TEA) [24,25]. Subsequently, it was found that of the three common
FIGURE 17.4 Schematic diagram of adsorbed lms.
High MW linear
Hi
g
h MW branched
Low MW linear
Low MW branched
M
2+
M
2+
M
2+
M
2+
M
2+
M
2+
M
2+
M
2+
M
2+
M
2+
Ba
2+
M
2+
M
2+
M
2+
M
2+
M
2+
CRC_59645_Ch017.indd 426CRC_59645_Ch017.indd 426 10/31/2008 2:31:07 PM10/31/2008 2:31:07 PM
Corrosion Inhibitors and Rust Preventatives 427
amine ethoxylates (monoethanolamine [MEA], diethanolamine [DEA], and TEA), DEA was the
most prone to the reaction with nitrite, whereas there was no evidence of nitrosamine formation
in the TEA-containing soluble oils [26]. Despite low nitrosamine formation with TEA, the use of
nitrite has been widely discontinued in metalworking applications.
17.4.2 CHROMATES
Chromates are another inhibitor that is part of the class of anodic passivators, which appear to
inhibit corrosion through the formation of an oxide coating [19,27]. Historically, chromates have
been used in steel plating and nishing, aqueous corrosion inhibition, and leather tanning, and
although there have been attempts to make oil-soluble chromate derivatives [28], they have not been
widely used in lubricants due to their instability in the presence of organics. In water treatment,
these products were applied as chromic acid, sodium chromate (Na
2
CrO
4
), or sodium dichromate
(Na
2
Cr
2
O
7
), all of which contain hexavalent chromium. Subsequently, the hexavalent chromium
(Cr
6+
) was found to be a powerful respiratory carcinogen and possessed high aquatic toxicity. As a
result, the use of chromates in aqueous water treatment was banned in 1990, and interest in using
them in lubricants has disappeared [29].
17.4.3 HYDRAZINES
Hydrazine (N
2
H
4
) has been historically used for water treatment as a corrosion inhibitor and oxygen
scavenger. The hydrazine prevents corrosion in a boiler by (1) reacting with O to form N
2
and H
2
O;
(2) reacting with Fe
2
O
3
(hematite) to form the harder (passive) Fe
3
O
4
(magnetite), which makes a
protective skin over the iron surface; and (3) forming NH
3
at high temperatures and pressures to
maintain a high alkalinity (Figure 17.6) [30]. Although it has been demonstrated that derivatives of
hydrazine can be used to inhibit copper corrosion in sulfuric acid [31], due to health concerns there
relatively few new oil-soluble derivatives have been developed, and the main focus has been to use
alternative organic treatment chemicals [32].
17.4.4 SILICATES
These materials are mainly used as inhibitors in water treatment for potable water due to their low cost.
Owing to their poor oil solubility, silicates have limited use as inhibitors in lubricant formulations.
17.4.5 OXIDATES
Oxidates are one of the oldest classes of RP additives and were historically made from oxidized
oils, waxes, and petrolatums obtained from the re ning process [3–5]. The production of oxidates
can be accomplished by either heating the petroleum product air in a closed vessel in the presence
of a catalyst or chemical treatment using nitric acid, sulfuric acid, or KMnO
4
. Both these methods
typically make a combination of polar compounds (including carboxylic acids, esters, alcohols,
FIGURE 17.5 Nitrosamine formation.
2HNO
2
→ N
2
O
3
+ H
2
O
R
2
NH + N
2
O
3
→ R
2
N−N=O + HNO
2
FIGURE 17.6 Hydrazine decomposition.
N
2
H
4
→ N
2
+ 4H
+
+ 4e
−
4H
+
+ O
2
+ 4e
−
→ 2H
2
O
CRC_59645_Ch017.indd 427CRC_59645_Ch017.indd 427 10/31/2008 2:31:07 PM10/31/2008 2:31:07 PM
428 Lubricant Additives: Chemistry and Applications
aldehydes, and ketones), where the total acid number (TAN) of the resulting mixture is between
10 and 200 mgKOH/g and the saponi cation number (SAP#) is between 10 and 200 mgKOH/g.
Typically, commercial grade products possess a TAN of 50 and 100 mgKOH/g and a SAP# of
10 and 50 mgKoh/g. Depending on the application, oxidates can be applied to a surface in various
methods as follows:
1. Aqueous dispersions. The oxidate can be dissolved or suspended in a water-based formula-
tion that is then applied to the surface. Either the water is removed with washing or the lm
is allowed to dry to make an RP coating.
2. Solvent based. The oxidate is dissolved in a low-boiling solvent (like naphtha), and the
solvent is allowed to quickly evaporate to form the RP coating.
3. Solid lm (typically wax). Either the oxidate is heated to apply the coating as a liquid and
then allowed to cool to solidify or the solid oxidate is applied through mechanical methods
as a solid at ambient temperatures.
Although these materials can be used as effective RP additives in their acid form, they are typically
neutralized to form more effective coatings. It has been found that oxidized wax neutralized with
Ca(OH)
2
or Ba(OH)
2
are effective RPs [33], and that the use of Ca(OH)
2
as a neutralizing agent is
effective at preventing gelation [34]. Basic materials that contain CaCO
3
have also been found to be
effective when combined with petroleum oxidate. The combination of petroleum oxidates and overbased
calcium sulfonates has demonstrated both improved lubricity and corrosion protection in forming and
engine oils [35,36]. Additionally, the petroleum oxidates can be neutralized with amines to form the
carboxylate amine salts. Although there are various amines listed in the patent literature (piperadines
[37] and polyamines [38]), the most common amines used are the simple alkanolamines [3–5].
17.4.6 SULFONATES
The sulfonates comprise a class of compounds that can be derived from petroleum (natural) or
synthetic feedstocks. The sulfonic acids are formed in the reaction of SO
3
with a feedstock and can
be neutralized with various bases to form the Na, Ca, Mg, or Ba salts, all of which have demonstrated
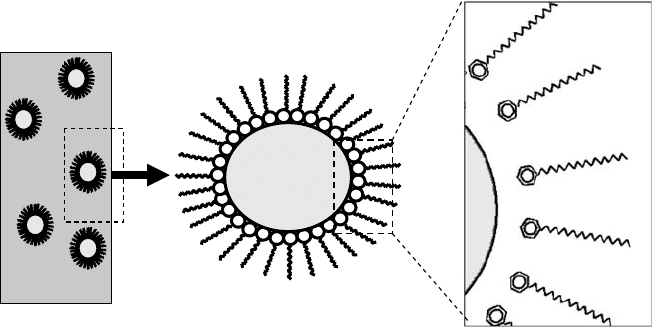
activity as RPs in various applications. Additionally, the neutral salts can be overbased by the addi-
tion of excess base and carbon dioxide. For example, in the case of a calcium petroleum sulfonate,
an excess of Ca(OH)
2
and CO
2
can be added to the sulfonic acid to form a colloidal suspension of
CaCO
3
in oil, where calcium sulfonate serves to disperse the CaCO
3
in the oil carrier (Figure 17.7).
The overbased sulfonates serve a dual role in rust prevention, where the sulfonate acts to form a
protective layer, and the calcium carbonate acts to absorb any acidic by-products of corrosion. As a
result, a combination of neutral and overbased sulfonates can be a quite effective RP.
The two types of sulfonates that are commonly manufactured are as follows:
1. Petroleum (natural) sulfonates. The petroleum sulfonates were originally made from the
by-products of the acid treating of petroleum oil but have also been intentionally made as
coproducts from the acid-treating process to manufacture technical and medicinal white
oil [6,39]. In this process, the oil-soluble petroleum sulfonic acids formed are typically
isolated from the oil layer as the sodium salts by treatment with sodium carbonate followed
by extraction with alcohol, where the typical activity of the extracted product is 60%,
which signi cantly reduces the viscosity and facilitates easier storage and handling.
2. Synthetic sulfonates. The synthetic sulfonates are made from the sulfonation of long-
chained alkyl aromatics. Depending on the aromatic structure used, the alkyl chain length
varies, but it is important for the overall alkylation to be suf cient to render the com-
pound oil-soluble. The alkylbenzene sulfonate derivatives are typically monoalkylated
with a long-chained (C16–40) moiety, whereas alkylnaphthalene derivatives are typically
CRC_59645_Ch017.indd 428CRC_59645_Ch017.indd 428 10/31/2008 2:31:08 PM10/31/2008 2:31:08 PM
Corrosion Inhibitors and Rust Preventatives 429
dialkylated with short-chain (C9–10) lengths, where barium dinonyl naphthalene sulfonate
(BaDNNS) is a common structure [6,40,41].
A natural or synthetic sulfonate can be neutralized and overbased with various cations depending
on the application. For example,
Sodium sulfonate. In general, the high equivalent weight (500–550 EW) petroleum or
(390–700 EW) synthetic alkylbenzene sulfonic acids are neutralized with NaOH to form
sodium sulfonates, which are commonly used in soluble oils for metalworking applica-
tions, where the divalent cations (Mg, Ca, and Ba) are detrimental to the stability of the
soluble oil. The sodium salts of the synthetic dialkyl naphthalene sulfonates have been
used, but their high cost has limited their use in this application.
Calcium sulfonate. Both the synthetic and natural sulfonic acids have been neutralized with
CaO or Ca(OH)
2
to form the neutral calcium sulfonates, but generally these products are
more effective when they have been overbased. The overbased calcium sulfonates can
contain either amorphous or crystalline form of calcium carbonate. The amorphous calcium
carbonates are easily soluble in oil and are commonly used as a detergent in engine oils
[42,43], whereas the crystalline form of calcium carbonate, called calcite, typically contains
colloidal particles that are too large to be held in suspension in the uid and precipitate to
form a gel or gelled solid and are commonly used in RP coatings and greases [44–47].
Magnesium sulfonate. Both the natural and synthetic sulfonic acids can be neutralized with
MgO or Mg(OH)
2
to form the magnesium sulfonates. Although magnesium sulfonates have
not been generally used in RP oils, the overbased magnesium sulfonates are extensively used
as fuel oil additives [48,49]. In particular, the undesirable contaminants in fuel oil such as
V and Na can form low-melting corrosive slags on the reside of a commercial boiler used
for power generation. The molten V
2
O
5
can act as an oxygen carrier and can accelerate cor-
rosion. The addition of the overbased magnesium sulfonate to the fuel oil serves to react with
low-melting sodium vanadates to form high-melting magnesium vanadates that increase the
viscosity, reduce the oxygen uptake, and counteract the destruction of the protective oxide
lm. Additionally, magnesium sulfonate reacts with sulfur oxides (SO
3
/SO
2
) forming high-
melting friable MgSO
4
, which can be easily removed by washing, whereas the carbonate
neutralizes any free acids to reduce the pH and lower the acid deposition rate (ADR) [50,51].
FIGURE 17.7 Overbased sulfonate structure.
Carrier Carbonate Sulfonate
CaCO
3
• Mineral oil • Amorphous • Natural
• Fuel oil • Cr
y
stalline • S
y
nthetic
Ca
2+
Ca
2+
Ca
2+
O
3
S
O
3
S
O
2
S
O
3
S
O
S
CRC_59645_Ch017.indd 429CRC_59645_Ch017.indd 429 10/31/2008 2:31:08 PM10/31/2008 2:31:08 PM
430 Lubricant Additives: Chemistry and Applications
Barium sulfonate. Both the natural and synthetic sulfonic acids can be neutralized with BaO or
Ba(OH)
2
to form the barium sulfonates. The barium sulfonates have been found to be effective
when both neutral and overbased barium sulfonates are combined in a formulation and applied
to a metal surface, where these products are generally used as RPs in mill and slushing oils
[8,13,39–41,52,53]. The BaDNNS was found to be very effective at low concentrations [6,39–
41] compared to Ca and Na derivatives, and, in general, the RP effectiveness of the sulfonate
increases with ionic radius (Table 17.2) where Ba > Ca > Mg > Na.
The effectiveness of alkaline earth metals can be correlated to their ionic radius, which is inversely
proportional to the solubility of the metal carbonate (and metal sulfonate) of the additive. For
example, the large ionic radius of Ba results in a small release of energy (enthalpy of solution) due
to the small amount of solvation necessary for this large polarizable cation. As a result, the large
cations require more energy to solubilize and are more dif cult to remove from a metal surface by
a humid atmosphere or aqueous washing.
17.4.7 CARBOXYLATES
Carboxylates are some of the oldest known corrosion inhibitors and can be made from animal
(lanolin, lard, or tallow), vegetable (tall oil fatty acids [TOFAs]), or mineral (naphthenic or aromatic
acids) oils and were commonly used in early slushing oil formulations [3–5]. The carboxylic acids
can be neutralized with many exotic cations (such as Bi [54]), but they are commonly reacted with
NaOH to form the sodium carboxylate salts.
Owing to their corrosivity in aqueous systems [55], carboxylates are more typically combined
with alkanolamines to form the alkanolamine salts in situ in soluble oil applications for metal-
working. In metalworking, the use of the alkanolamine carboxylates provides good coupling with
other lubricity additives, enhanced lubricity, and the formation of soft (noncalcium containing)
residues, but the alkanolamine carboxylates do suffer from hard water sensitivity, and many short-
chained derivatives can cause odor and excessive foaming. The dicarboxylates, which possess good
corrosion protection and low foam, can remedy this problem, but they are not popular due to their
high cost and poor coupling with other lubricity additives.
In other applications, carboxylates have been used with varying amounts of success, and it had been
found that a small amount of the C
6
–C
18
carboxylic acids prevented corrosion in turbines, whereas acids
with chain length <C
6
promoted corrosion [56]. Additionally, in stamping applications, it was found
that a simple fatty acid ester provides good lubricity as well as possesses inherent RP properties [57].
17.4.8 ALKYL AMINES
The alkyl amines represent the largest and most diverse chemistry of all the RP types and are used
in various metalworking and industrial oil applications. The most common alkylated amines that are
used include MEA, DEA, and TEA, fatty amines, diamines, phenylene diamine, cyclohexylamine,
morpholine, and ethylenediamine, triethylene tetramine (TETA), and tetraethylene pentamine
TABLE 17.2
Ionic Radii of Cations
Type
Solubility
Product (Ksp)
Ionic
Radius (pm)
∆H
soln
(kJ/mol)
∆H
lattice
(kJ/mol)
MgCO
3
6.82 × 10
–6
65 −25.3 3180
CaCO
3
4.96 × 10
–9
99 −12.3 2804
SrCO
3
1.1 × 10
–10
113 −3.4 2720
BaCO
3
2.58 × 10
–9
135 4.2 2615
CRC_59645_Ch017.indd 430CRC_59645_Ch017.indd 430 10/31/2008 2:31:08 PM10/31/2008 2:31:08 PM
Corrosion Inhibitors and Rust Preventatives 431
(TEPA). It had initially been recognized that the amine was preferentially adsorbed onto the metal
surface and inhibited the reaction of the corrosive species and the metal [58], but now it is believed
that the alkyl amines rst displace the water on the metal surface to form a bond between the lone
pair on the nitrogen and the unoccupied orbitals on the metal through defects present in the oxide
coatings. As a result, the amines provide cathodic protection by creating a barrier and inhibiting H
2
formation in acidic environments [59–62]. In general, it has been found that longer-chained amines
are more effective than the shorter-chained amines [60] and that the nucleophilicity of the nitrogen
strongly correlates with rust inhibition effectiveness where the tertiary is more effective than the
secondary or primary amine [61].
Owing to their high volatility and low ash formation, products such as cyclohexyl amine,
morpholine, and piperadine can be used as VCIs. In systems that are unsuitable for oil, grease, or
hard coatings, they expand in the vapor phase to ll the void space in a metal enclosure to form an
extremely thin lm over the entire metal surface (including intricate interior parts in a red engine)
[59,63]. Owing to the absence of inorganic salts (such as the phosphates, borates, or carboxylates),
they do not have the tendency to leave dry residues on the surface [64]. They are also commonly
found in sweet (CO
2
) and sour (H
2
S) gas applications where they volatilize to ll the entire void
space of the pipeline [65].
Unfortunately, it has also been found that the amines tend to cause occupational skin diseases
(including irritant and allergic contact dermatitis) in workers exposed to metalworking uids
[66–68]. In particular, the commonly used mono- and diethanol amines were found to elicit a
signi cant amount of positive reactions [69], and as a result, they have been largely phased out of
this application in favor of the tertiary amines such as TEA. The tertiary amines possess the added
bene t of low ecotoxicity and are used environmentally sensitive applications. For example, they
are particularly effective in oil eld acidizing operations as corrosion inhibitors where they are
directly introduced to the outdoor environment [70].
17.4.9 ALKYL AMINE SALTS
Although the alkylamines are commonly used in the gas phase, the amine salts of the carboxylates,
borates, and phosphates are most commonly used in metalworking uids where they are formed in
situ by reaction with the corresponding acid. For example, the combination TOFA neutralized with
triethanol amine to form the amine carboxylate salt is commonly used as a RP. Each amine salt
formed for metalworking and RP applications possesses its own unique chemistry and performance
issues, each of which will be discussed.
17.4.10 AMINE CARBOXYLATE SALTS
In metalworking formulations, typically tertiary amines such as TEA are neutralized with organic
fatty acids to form the amine carboxylates in situ to not only provide corrosion inhibition but also
improve lubricity and emulsi cation. Although there have been many combinations of carboxylic
acid and amines proposed, the most effective combinations include the long-chained carboxylic
acids from C
18
to C
22
[71–73].
17.4.11 AMINE BORATE SALTS
The borates represent the most chemically diverse and least understood of the RP classes. Although
the structure of the borates and borate esters can be written empirically as B(OR)
3
, where R is a H
or alkyl group, the actual three-dimensional structure of the borates are complex chains and rings
that contain both sp
2
(3-coordinate)- and sp
3
(4-coordinate)-hybridized boron species. For example,
the species Na
2
(B
4
O
5
(OH)
4
) contains both sp
3
and sp
2
-hybridized species, where two of the
sp
2
-hybridized B have empty p-orbitals that can be used to bond to an amine or any other lone pair
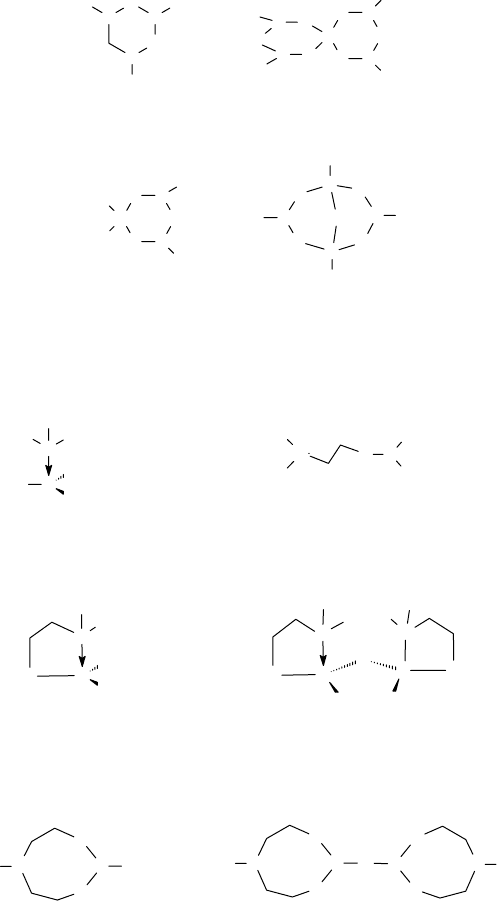
of electrons as well as use the terminal oxygen to form borate ester linkages (Figure 17.8).
CRC_59645_Ch017.indd 431CRC_59645_Ch017.indd 431 10/31/2008 2:31:09 PM10/31/2008 2:31:09 PM
432 Lubricant Additives: Chemistry and Applications
As a result, common ethoxylated amines, such as the triethanol amine, can bond to the borate
using four different modes of connectivity (Figure 17.9), which can be described as follows:
1. The TEA can datively bond to the unoccupied sp
2
-hybridized orbital of the boron to make
the borate salt that is nominatively of the form R
3
N:B(OR)
3
.
2. The TEA can bond through one oxygen on an sp
2
-hybridized boron to form the mono-
dentate −(N−CH
2
−CH
2
−O−B)− bond.
FIGURE 17.8 Proposed structures of borates.
B
B
O
B
O
O
−
−
O
B
OB
O
BO
O
O
B
B
O
O
−
O
−
O
−
O
−
O
−
−
O
−
O
−
O
B
OB
O
BO
−
O
−
O
O
−
O
−
B
O
O
B
O
B
O
B
O
Triborate
Boroxyl ring Pentaborate
Diborate
O
−
FIGURE 17.9 Proposed structures of borated amines.
N
B
OR
OR
RO
R
R
R
Nitrogen dative bond
N
B
OR
OR
O
R
R
Oxygen single bridge
Nitrogen dative bond
Oxygen single bridge
N
O
O
BOR
R
N
O
O
B
R
O
N
O
O
B
R
Oxygen double bridge
N
R
R
OB
OR
OR
N
B
O
R
R
O
BO
N
R
R
OR
OR
CRC_59645_Ch017.indd 432CRC_59645_Ch017.indd 432 10/31/2008 2:31:09 PM10/31/2008 2:31:09 PM
Corrosion Inhibitors and Rust Preventatives 433
3. The TEA can datively bond to the unoccupied sp
2
-hybridized orbital of the boron to make
the borate salt and can bond through one oxygen on an sp
2
-hybridized boron to form the
bidentate −(N−CH
2
−CH
2
−O−B)− bond.
4. The TEA can bond through two oxygen on an sp
2
-hybridized boron to form the bidentate
−(N−(CH
2
−CH
2
−O)
2
−B)− bond.
The advantages of the borate salts are their low cost, low toxicity, hard water stability, excellent
antiwear, and reserve alkalinity. The large disadvantage is the possible formation of a tacky residue
that remains after the part is machined due to the partially dehydrated products such as meta-boric
acid and its esters depositing on the surface [74].
The borates not only serve to inhibit corrosion but have also been found to be bacteriostatic
(biostatic) agents, and a large synergistic inhibitory effect has been found when combined with
amines. Although the mechanism of the effect is still unclear, it is believed that it may be due to the
release of boric acid at low pH that may react to form cis-diols with the ribonucleotides to promote
the antimicrobial activity [75–77].
Although the borated amines have been cited for use in engine oils [78,79], hydraulic uids [80],
and slushing oils [81,82], they are most commonly used in metalworking uids [83] for both their
rust inhibition and biostatic properties.
17.4.12 PHOSPHATES
The phosphating of metals is a well-known technique to improve both wear and corrosion resistance
and is primarily used as a surface preparation before painting. The process of phosphate coating is
the treatment of iron, steel, or a steel-based substrate with a solution of phosphoric acid or as K, Na,
or Ca phosphate salts, in the presence of heavy metal accelerators (Zn or Mg) whereby the surface
of the metal is converted to a mildly protective layer of insoluble crystalline phosphate. Phosphating
is considered the heart of pretreatment operations in a steel mill and where the top surface of the
metal is converted into a highly insoluble, corrosion-resistant coating [84]. The mechanism of this
cathodic inhibitor is believed to be the precipitation of a phosphate lm on the surface of the steel
that prevents the corrosive action of acids and water. Additionally, phosphates have been extensively
used in water treatment where the phosphate combines with calcium to form calcium phosphate
precipitates [10].
Although the inorganic phosphates (mono-, ortho-, or polyphosphates) are not oil soluble, the
phosphate esters can be synthesized to provide oil-soluble derivatives for lubricants. In particular,
the tertiary and secondary phosphate esters of the form (RO)
3
P=O or (RO)
2
OHP=O, where R is
typically an alkylaryl group have been used in slushing oils alone or in combination with other
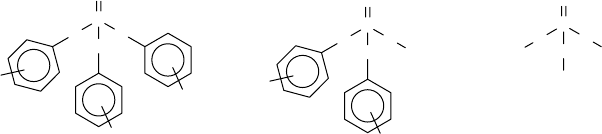
additives (Figure 17.10) [84–90]. The most common alkylaryl derivatives include the tricresyl
phosphate (TCP) and trixylyl phosphate (TXP). Although the trialkyl phosphates (such as tributyl
or trioctyl) could be used for this application, the instability of these species due to facile thermal,
oxidative, and hydrolytic degradation makes them less desirable as RPs [91,92].
FIGURE 17.10 Phosphate ester structures.
P
O
O
O
O
R
R
R
P
O
O
O
O
R
R
R
P
O
O
O
O
R
R
Triaryl phosphate Aryl-alkyl phosphate
Trialkyl phosphate
R
CRC_59645_Ch017.indd 433CRC_59645_Ch017.indd 433 10/31/2008 2:31:09 PM10/31/2008 2:31:09 PM
