Rudnick L. Lubricant Additives: Chemistry and Applications (Присадки, добавки к смазкам)
Подождите немного. Документ загружается.


474 Lubricant Additives: Chemistry and Applications
The acid is then neutralized with zinc oxide to yield ZDDP at ∼70 to 90°C. There are two types of
ZDDP structure, neutral and basic, both of which can be observed by
31
P NMR (nuclear magnetic
resonance). The basic salt is observed at 104 ppm with the neutral salt at 101 ppm. The neutral salt
exists as an equilibrium mixture of monomer, dimer, and oligomers. The basic salt consists of a
central oxygen atom surrounded tetrahedrally by four zinc atoms and six dithiophosphate groups
attached symmetrically to the six edges of the tetrahedron. In most industrial processes, the ZDDP
is left slightly basic for providing improved stability.
RO
RO
P
S
S
2
Zn
2(RO)
2
P(S)SH + ZnO
Neutral ZDDP
−H
2
O
RO
RO
P
S
S
6
Zn
4
O
3[(RO)
2
P(S)S]
2
Zn +
Basic ZDDP
ZnO
19.4.4 ZDDP DEGRADATION MECHANISMS
The type of alcohol used to prepare the ZDDP will determine its thermal and oxidative stabilities.
The most reactive ZDDPs are derived from secondary alcohols and especially those that are lower
in molecular weight. Solubility is a limiting factor at carbon numbers less than ve; therefore, most
ZDDPs will use alcohols with carbon numbers greater than ve. Alcohols with a lower carbon num-
ber may be used if they are combined with a higher alcohol during synthesis.
The type of alcohol used in the preparation will have a signi cant effect on the stability. In most
cases, the thermal stability of the ZDDP is as follows:
Aryl > primary alkyl > secondary alkyl
The least stable ZDDPs tend to provide improved wear at lower engine oil temperatures. Therefore,
the following applies for antiwear action:
Secondary alkyl > primary alkyl > aryl
Tables 19.4 through 19.6 indicate the performance of various ZDDPs in a range of gasoline engine
wear tests.
TABLE 19.4
Comparison of ZDDP Types in Sequence VE Wear Test
Alcohol Type Zn (%) as ZDDP
Sequence VE Wear
Average (µm) Maximum (µm)
Mixed 0.127 36 203
C
3
secondary
C
8
primary
C
8
primary 0.124 121 495
CRC_59645_Ch019.indd 474CRC_59645_Ch019.indd 474 10/31/2008 2:34:18 PM10/31/2008 2:34:18 PM

Additives for Crankcase Lubricant Applications 475
The mechanism by which secondary alcohol ZDDPs thermally degrade is shown in Figure
19.19. The degradation mechanism proceeds rapidly as the temperature rises and is made easier as a
hydrogen on the β position readily leaves to form the alkene. This mechanism may explain why the
secondary ZDDPs are much more active antiwear agents particularly at lower temperatures.
In contrast, the primary alcohol ZDDPs are more stable due to the absence a tertiary hydrogen
on the β carbon and therefore more useful for higher-temperature operation and wear such as that
found in diesel engines. The mechanism of thermal degradation is through sequential alkyl trans-
fers and relies on an intermolecular alkyl transfer (Figure 19.20).
19.4.5 SEQUENTIAL ALKYL TRANSFERS (PRIMARY ZDDP)
Other additives will affect the rate of thermal degradation of the ZDDP. For instance, it is known
that succinimide dispersants will complex with the ZDDP, making it more resistant to thermal
degradation. It is therefore important to recognize this when formulating an oil additive. Too much
dispersant may tie up the ZDDP, leaving it unable to form an effective antiwear lm. A balance has
to be found, which is dependent on the ZDDP type and the dispersant structure.
TABLE 19.5
Comparison of ZDDP Types in Sequence VD Wear Test
Alcohol Type Zn (%) as ZDDP
Sequence VD Wear
Average (µm)
C
6
secondary 0.13 18
Mixed
C
6
secondary
C
8
primary 0.13 48
TABLE 19.6
Comparison of ZDDP Types in Sequence IIID Wear Test
Alcohol Type
Zn (%) as
ZDDP
Sequence IIID Wear
Average (µm)
Mixed 0.13 25
C
6
secondary
C
8
primary
C
8
primary 0.13 175
CH
3
H
2
C
CH
H
3
C
O
P=S
S
−
O
CH
CH
3
Zn
2+
H
H
CH
H
3
C
CH
2
+
Zn
2+
CH
3
CH
3
−
O
P=S
SH
O
CH
FIGURE 19.19 β-Elimination (secondary ZDDP).
CRC_59645_Ch019.indd 475CRC_59645_Ch019.indd 475 10/31/2008 2:34:18 PM10/31/2008 2:34:18 PM
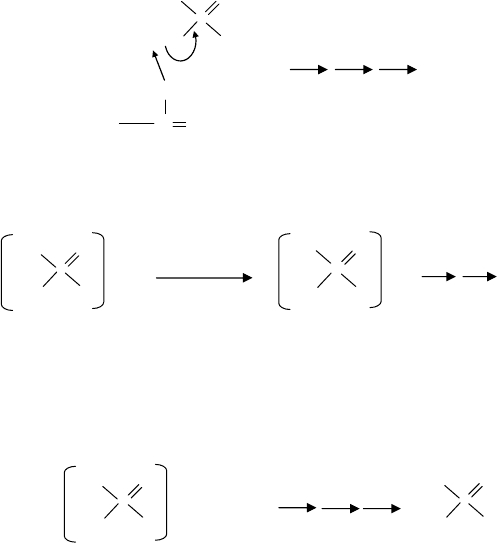
476 Lubricant Additives: Chemistry and Applications
ZDDP, also degrades at lower temperatures by oxidation (<100°C), yielding compounds that
are bene cial antiwear agents (see Figure 19.21). This leads to the mechanism of oxidative inhibi-
tion by ZDDPs, which occurs through the thiophosphoryl disul de intermediate (see Figure 19.22).
A more detailed mechanism of the antioxidant function of the thiophosphoryl disul de intermedi-
ate has been reported [18].
The effectiveness of ZDDP in decomposing hydroperoxides has been linked to wear rates in
a motored engine test [19]. Secondary ZDDPs were better at decomposing peroxides than their
primary counterparts. There is a plethora of papers in the literature with detailed analyses of the
various degradation pathways for the ZDDP molecule and its subsequent effect on the wear and
oxidation properties of the lubricant [13,14,20–22]. Although these give an excellent insight into the
ZDDP degradation mechanisms, it must be remembered that the degradation pathways are strongly
dependent on the test conditions, that is, temperature and amount and type of oxidants.
19.4.6 ANTIWEAR TESTS
The Sequence VE and Sequence IVA gasoline engine tests (for API SJ and ILSAC GF-3, respectively)
are designed to test the ability of a lubricant to prevent low-temperature sliding wear in the valve
train of a gasoline engine. Secondary ZDDPs will usually perform better than primary ZDDPs in
both tests. However, there are certain cases where it is possible to achieve a passing test result with
primary ZDDP especially with formulations in which a low level of detergent is present. High-temper-
ature gasoline wear is measured in the Sequence IIIE and Peugeot TU3 scuf ng test and responds
well to most types of ZDDP.
Wear in a diesel engine is usually accelerated by the presence of soot. There are several tests to
measure wear in diesel engines of which the four most common tests are listed in Table 19.7.
P
S
−
Zn
2+
RCH
2
−O
RCH
2
O
P
S
S
−
Zn phosphate
+
(RCH
2
S)
3
P=S
(RCH
2
O)
2
∆
S
FIGURE 19.20 Sequential alkyl transfers (primary ZDDP).
RO
RO
P
S
S
2
Thiophosphor
y
l disulfide
RO
RO
P
S
S
2
[O]
[O]/∆
Antiwea
r
species
Zn
FIGURE 19.21 Thiophosphoryl disul de formation. ([O] can be almost any oxidant such as O
2
, ROOH,
H
2
O
2
, Cu
2+
, and NO
x
.)
RO
RO
P
S
S
2
RO
RO
P
S
OH
+2ROO
·
FIGURE 19.22 Thiophosphoryl disul de decomposition of peroxy radical.
CRC_59645_Ch019.indd 476CRC_59645_Ch019.indd 476 10/31/2008 2:34:19 PM10/31/2008 2:34:19 PM
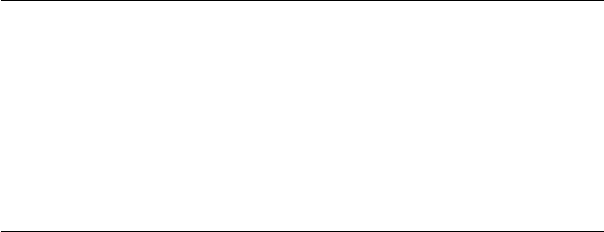
Additives for Crankcase Lubricant Applications 477
In addition, eld tests such as the Volvo VDS and Scania LDF speci cations also specify
limits for wear on critical engine components. Depending on the operating temperature of the oil,
primary or secondary ZDDPs will be used. For heavy-duty diesel engine oils, both primary and
secondary ZDDPs are widely used. In certain cases, an aryl ZDDP may be used for maximum
thermal stability.
19.4.7 OTHER ANTIWEAR AGENTS
Boron is widely used to provide antiwear properties in lubricants. A common method is to react
boric acid with the amine moiety of a succinimide dispersant. The boron works by reacting at the
metal surface to form boric acid, which has a layered structure with weak interlayer bonds [15]. On
its own, it is unlikely to give the same level of antiwear performance as ZDDP, but when used in
combination with other antiwear additives, it will reduce wear.
19.5 ANTIOXIDANTS
19.5.1 I
NTRODUCTION
Most of the lubricants, by virtue of being hydrocarbon based, are susceptible to oxidation [23,24].
If oxidation is not controlled, lubricant decomposition will lead to oil thickening; sludge formation;
and the formation of varnish, resin, and corrosive acids [25,26].
The oxidation reactions that occur in a lubricant at elevated temperatures in the presence
of atmospheric oxygen may lead to declined lubricant performance such as signi cant increase
in kinematic viscosity observed in severe service conditions or during extended drain intervals
(Figure 19.23).
In general, all types of base oils require addition of antioxidants depending on the amount of
unsaturation and natural inhibition present. The re ned mineral base oils contain natural inhibitors
in the form of sulfur and nitrogen compounds suf cient for many applications. The oxidative stabil-
ity of such oils shows a distinct, relative long induction period. In contrast, hydro ned oils do not
contain these natural inhibitors or contain them only in small quantities. Besides sulfur and nitrogen
compounds, other compounds such as aromatics or partially hydrogenated aromatics and phenolic
oxidation products can be of importance; their inhibiting effect is lost during various re ning pro-
cesses. Traditional mineral oils, such as group I and group II base stocks, show moderate oxidative
resistance. Synthetic oils such as polyol esters and hydrogenated polyalpha-ole ns (PAOs) exhibit
the highest oxidative stability due to their low unsaturated content. The most unstable oils include
highly unsaturated vegetable triglycerides such as corn, sun ower, canola, and peanut.
An overall scheme of lubricant oxidation starts with the conversion of hydrocarbon substrates to
carbonyl compounds (Figure 19.24). Coupling of these polar compounds through aldol and related
reactions builds molecular weight. Eventually, at very high molecular weight (i.e., >1000), the oil is
converted into insoluble sludge, which can precipitate on the engine hardware.
TABLE 19.7
Diesel Wear Tests
Engine Test Specifi cation Wear Measured
GM roller follower wear test API CG-4, CH-4 Roller follower wear
Cummins M11 API CH-4 Valve bridge wear
Mercedes OM602A MB Sheet 228.X, 229.X Cam nose wear
ACEA B and E sequences Cylinder wear
Mitsubishi 4D34T Global DHD-1, JASO DH-1 Cam nose wear
CRC_59645_Ch019.indd 477CRC_59645_Ch019.indd 477 10/31/2008 2:34:19 PM10/31/2008 2:34:19 PM

478 Lubricant Additives: Chemistry and Applications
19.5.2 MECHANISM OF OXIDATION OF LUBRICATING OILS
The oxidation of petroleum hydrocarbons proceeds according to a radical chain mechanism through
alkyl and peroxy radicals in three stages [27].
19.5.2.1 Initiation
RH + O
2
→ R
•
+ HOO
•
The initiation step starts by the slow abstraction of a hydrocarbon proton by molecular oxygen
to form alkyl and hydroperoxy free radicals. This process is also referred to as autooxidation
and is favored by time, higher temperature, and transition metal (i.e., iron, nickel, and copper)
catalysis.
19.5.2.2 Propagation
Propagation starts by the rapid reaction of more oxygen with an alkyl-free radical to form an
alkyl peroxy radical, which is also capable of hydrocarbon abstraction to form hydroperoxide
FIGURE 19.23 Antioxidants can prevent premature oil thickening.
Hydrocarbon
Air (with or without metal)
Primary oxidation products
(e.g., aldehydes, ketones, and carboxylic acids)
Aldol condensation
High-molecular-weight products
Polycondensation
polymerization
Slud
g
e
FIGURE 19.24 Degradation of mineral oils.
CRC_59645_Ch019.indd 478CRC_59645_Ch019.indd 478 10/31/2008 2:34:19 PM10/31/2008 2:34:19 PM
Additives for Crankcase Lubricant Applications 479
and another alkyl radical. The alkyl radical then may react with more oxygen starting the chain
over [25].
R
•
+ O
2
→ ROO
•
ROO
•
+ RH → ROOH + R
•
19.5.2.3 Peroxide Decomposition
Alkyl hydroperoxides are quite reactive and may decompose, especially at higher temperature, to
form additional radical species. These can undergo further abstraction and chain propagation reac-
tions that increase the overall oxidation process. Alkyl peroxides and alkyl peroxy radicals also
decompose to neutral oxidation products such as alcohols, aldehydes, ketones, and carboxylic acids.
Hydroperoxide decomposition to neutral oxidation products can be viewed as a chain termination
step, since free additional radicals are not formed.
ROOH RO
.
+
.
OH
2ROOH RO
.
+ ROO
.
+ H
2
O
RO
.
+ ROOH Various products
Engine surface
.
Free radicals
or lubricant + ROOH
.
Alcohols
.
Inactive products
19.5.2.4 Termination (Self and Chain Breaking)
During termination stage, the radicals either self-terminate or terminate by reacting with oxidation
inhibitors [23].
ROO
•
+ ROO
•
→ Inactive products
ROO
•
+ IH → ROOH + I
•
Alternative oxidation pathways are listed later.
19.5.2.5 Radical Formation
RH O ROOH
ROOH RO OH
ROH NO R HNO
R
hydroperoxides
1
⫹
⫹
⫹⫹
⫹
2
2
→
→
→
••
•
xx
••
→⫹⫹ ⫹O NO RONO RONO
2
nitrite and nitrite esters
2
2x
Nitrogen oxides can react with alcohols (an oxidation product) to form alkyl free radicals, which
react with NO
x
and oxygen to form nitrate and nitrate ester oxidation products.
19.5.2.6 Decomposition and Rearrangement
ROOH + SO
2
→ ROH + SO
3
ROOH + H
2
SO
4
→ Carbonyl compounds
RO
•
and ROO
•
→ Oxidation products
CRC_59645_Ch019.indd 479CRC_59645_Ch019.indd 479 10/31/2008 2:34:20 PM10/31/2008 2:34:20 PM
480 Lubricant Additives: Chemistry and Applications
The action of sulfur dioxide and H
2
SO
4
(from SO
3
+ water) on alkyl hydroperoxides also leads to
neutral oxidation products such as alcohols and carbonyl compounds. Aldehydes and ketones can
react further and form polymers. Carboxylic acids can attack metallic hardware: rings, valve train,
and bearings leading to extensive wear. Furthermore, they can form metal carboxylates, which
further increase the oxidation rate. Wear metals can also enhance the rate of oxidation [26].
19.5.3 OXIDATION INHIBITORS
Oxidation inhibitors can be classi ed into the following manner:
1. Radical scavengers
a. Nitrogen-containing inhibitors—aryl amines
b. Oxygen-containing inhibitors—phenols
c. ZDDPs
2. Hydroperoxide decomposers
a. Sulfur-containing inhibitors—sul des, dithiocarbamates, and sulfurized ole ns
b. Phosphorus-containing inhibitors—phosphites and ZDDPs
Note that ZDDPs function by both antioxidant mechanisms in addition to providing antiwear
protection (covered in Section 19.4.2). This dual- (or tri-) functional ability of ZDDPs to provide
antiwear performance and antioxidancy by two different pathways explains why these additives are
by far the most effective inhibitors, especially on a cost/performance basis.
Phenols or amines of speci c structures function as radical acceptors by transfer of a hydrogen
atom from the oxygen or nitrogen atom to the hydrocarbon or peroxy radical. The inhibitor radicals
thus formed react through radical combination or electron transfer to give ionic compounds or by
addition reactions or formation of complexes that do not maintain the radical chain mechanism of
the autoxidation reaction. In further reactions, very often ethers, betones, polyaromatic systems,
etc. are formed.
19.5.4 HINDERED PHENOLS AND ARYLAMINES
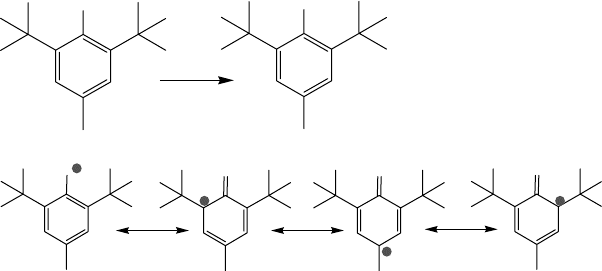
Hindered phenols and arylamines are two prominent examples of inhibitors that act as radical
scavengers through hydrogen transfer. Figure 19.25 illustrates the mechanism of phenol
performance.
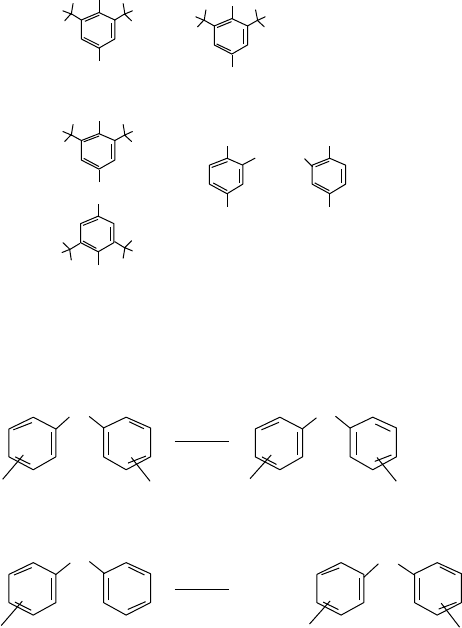
Among various types of phenols, polyalkylphenols have signi cant performance advantage
over nonalkylated compounds (Figure 19.26). The effect of substituents demonstrates the role of
OH
O
.
RO
.
+
+ ROH
O
OO
O
Resonance
stability
FIGURE 19.25 Mechanism of inhibition by hindered phenols.
CRC_59645_Ch019.indd 480CRC_59645_Ch019.indd 480 10/31/2008 2:34:20 PM10/31/2008 2:34:20 PM
Additives for Crankcase Lubricant Applications 481
electron density and steric hindrance at the phenolic oxygen; an increase in the number of alkyl
radicals and the introduction of electron donors in the o- and p-positions increase the ef ciency,
whereas the introduction of electron acceptors reduces it. α-branching of alkyl groups in o- position
or increasing the alkyl group to C4 in p-position has a bene cial effect [27].
A new type of antioxidant involves the base-catalyzed addition of hindered phenol to Michael
acceptors such as acrylate esters. Although more expensive than simple alkylphenols, the originators
of this chemistry claim improved upper piston deposit control in certain diesel engine tests and
better seal compatibility than arylamines.
S-coupled (X = 1,2) alkylphenols combine the antioxidant bene ts of the phenol and sul de
groups.
The mechanism of oxidation inhibition (based on generation of nitrogen radicals) by arylamines
is presented in Figure 19.27.
In general, the alkylated arylamines are more effective antioxidants than alkylphenols because
they are
Able to trap more equivalents of radicals: four versus two
Able to better stabilize nitrogen or nitroxyl radicals (by two aryl rings instead of one)
Able to operate at both low and high-temperature mechanisms (the latter of which
regenerates the alkylated arylamine)
•
•
•
OH
R
OH
CH
2
OH
OH
CH
2
−CH
2
−CO
2
R
OH
R
(S)
X
OH
R
FIGURE 19.26 Major types of phenolic antioxidants.
ROH+
RO
.
+
ROO
.
+
Diarylamine
Diarylamine
+ ROOH
N
N
H
N
H
N
R
R
R
R
R
R
R
R
.
.
FIGURE 19.27 Mechanism of inhibition by arylamines.
CRC_59645_Ch019.indd 481CRC_59645_Ch019.indd 481 10/31/2008 2:34:21 PM10/31/2008 2:34:21 PM
482 Lubricant Additives: Chemistry and Applications
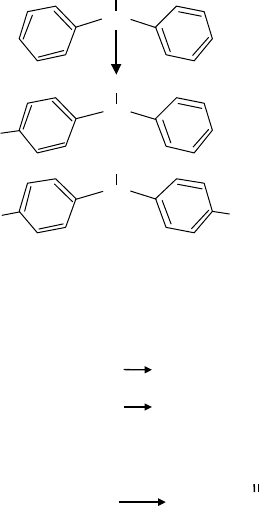
Oil-soluble amines such as diphenylamine, phenyl-α-naphthylamine are the most common type of
amine antioxidants used in lubricants. Diphenylamine can be alkylated with aluminum chloride
catalyst and a mixture of branched nonene ole ns. A mixture of mono- and dialkylate (Figure 19.28)
is intentionally targeted by the reactant charge ratio to produce a liquid product. Diphenylamine is
particularly suited for applications at elevated temperatures. Therefore, it is often used to lubricate
supersonic aircraft engines and bearings. In these applications, arylamines prevent sludge forma-
tion in synthetic ester oils.
19.5.5 SULFUR AND PHOSPHORUS CONTAINING ANTIOXIDANTS
Sulfur, phosphorus, and compounds containing both the elements decompose peroxides by reducing
the hydroperoxide in the radical chain to alcohols; the sulfur or phosphorus atoms are correspond-
ingly oxidized (Figure 19.29). Bivalent sulfur compounds (sul des, etc.) yield sulfoxides and sulfones;
trivalent phosphorus compounds (phosphates) are transformed into pentavalent ones (phosphates).
Organic compounds of tetravalent sulfur act as peroxide decomposers, but the corresponding hexava-
lent, in line with theory, do not. Destruction of hydroperoxides is important so that these intermedi-
ates do not decompose into radicals, which can continue the chain oxidation process.
Phosphates are somewhat hydrolytically unstable, and due to limits on phosphorus level in
crankcase oils, these additives are generally limited to application in gear lubricants. ZDDPs are
more ef cient phosphorus-containing antioxidants for crankcase oils, with the added bene t of
antiwear performance.
ZDDPs convert hydroperoxides to one equivalent of an alcohol and a carbonyl compound
(Figure 19.30). The ZDDP is regenerated intact and is available to convert another hydroperoxide
molecule. Many cycles of hydroperoxide decomposition are carried out by the ZDDP, before its
eventual breakdown.
N
H
N
H
N
+
+
DPA
(DPA)
22%
76%
2%
C
9
H
19
C
9
H
19
C
9
H
19
H
FIGURE 19.28 Alkyl aromatic amine as antioxidant.
ROOH + R′
3
P
ROOH + (R′O)
3
P
ROH + R′
3
P=O
ROH + (R′O)
3
P=O
Phosphite Phosphate
ROOH + R′ S R′′
ROH + R′ S R′′
SulfoxideSulfide
O
FIGURE 19.29 Mechanism of S- and P-containing antioxidants.
CRC_59645_Ch019.indd 482CRC_59645_Ch019.indd 482 10/31/2008 2:34:21 PM10/31/2008 2:34:21 PM

Additives for Crankcase Lubricant Applications 483
19.5.6 SULFUR COMPOUNDS
Elemental sulfur is an ef cient oxidation inhibitor; however, it shows a strong corrosion tendency.
From the practical approach, numerous dialkyl sul des and polysul des, diaryl sul des, modi ed
thiols, mercaptobenzimidazoles, thiophene derivatives, xanthogenates, zinc dialkyldithiocarba-
mates, thioglycols, thioaldehydes, and others have been examined as inhibitors. Dibenzyl disul de
must be mentioned among the alkyaromatic sulfur compounds.
Alkylphenol sul des that are formed in the reaction of alkylphenols such as butyl-, amyl-, or
octylphenol with sulfur chloride are more active than the compounds of the dibenzyl disul de type,
due to the position of sulfur next to the OH group.
Modern compounds of this type contain mostly tert-butyl radicals besides the methyl groups, as
for instance, 4,4′-thio-bis-(2-tert-butyl)-5-methylphenol. Sulfur–nitrogen compounds are also suited
as oxidation inhibitors for lubricating oils (2-mercaptobenzimidazole, mercaptotriazines, reaction
products of benzotriazole alkylvinyl ethers or esters, phenothiazine, and its alkyl derivatives).
Among the sulfur-containing carboxylic acid esters, 3,3′-thio-bis-(propionic acid dodecyl ester)
and bis-(3,5-di-tert-butyl-4-hydroxybenzyl)-malonic acid-bis-(3-thio-pentadecyl) ester have been
applied with success. These compounds have been replaced by the dialkyldithiophosphates due
to their broad application spectrum. Sulfoxides, sometimes in combination with aromatic amines,
have also been utilized.
19.5.7 PHOSPHORUS COMPOUNDS
Red phosphorus possesses oxidation-inhibiting properties but cannot be used because of its
corrosivity toward nonferrous metals and alloys. Triaryl and trialkyl phosphates have been proposed
as thermally stable inhibitors; however, their applications are limited. Combined phosphoric acid–
phenol derivatives such as 3,5-di-tert-butyl-4-hydroxybenzyl-phosphonic acid dialkyl esters or
phosphonic acid piperazides have a better effect.
19.5.8 SULFUR–PHOSPHORUS COMPOUNDS
Today, metal salts of thiophosphoric acids are used predominantly as oxidation inhibitors for
crankcase oils. In principle, compounds that contain sulfur and phosphorus are signi cantly more
ef cient than inhibitors that contain only sulfur or phosphorus. Most widely used are the ZDDPs
that are prepared by the reaction of P
2
S
5
with the respective higher alcohols (e.g., hexyl, 2-ethyhexyl,
and octyl alcohols), followed by the reaction with zinc oxide. The temperature of the exothermic salt
formation is kept at 20°C by successive addition of zinc oxide and cooling and is limited even at the
end of the reaction to 80°C because of the thermal ability of the free dialkyl dithiophosphoric acids.
They react very corrosively with metals and are toxic. Metal dialkyldithiophosphates are prepared
in mineral oil solution. Their solubility in hydrocarbon oils increases with the increasing number
of carbon atoms of the alkyl residues and is satisfactory with the diamyl compounds and higher.
Longer-chain derivatives act as solubilizers for short-chain products. Metal dialkyldithiophosphates
act not only as antioxidants but also as corrosion inhibitors and EP additives.
The ef ciency of Zn dialkyldithiophosphates (with octyl or cetyl and, respectively, propyl,
butyl, or octyl radicals in various combinations) decreases with increasing molecular mass of the
alcohol substituents. The best results have been obtained with isopropyl and isoamyl radicals.
ZDDP+HOOCR
3
ZDDP + ROH+
R
R
O
FIGURE 19.30 ZDDPs hydroperoxide destruction.
CRC_59645_Ch019.indd 483CRC_59645_Ch019.indd 483 10/31/2008 2:34:21 PM10/31/2008 2:34:21 PM
