Rudnick L. Lubricant Additives: Chemistry and Applications (Присадки, добавки к смазкам)
Подождите немного. Документ загружается.

154 Lubricant Additives: Chemistry and Applications
low free-amine content and better engine test performance than dispersants made from conven-
tional polyamines.
Imide and ester dispersants are made by reacting polyamines and polyhydric alcohols with
alkenylsuccinic anhydrides. The reaction typically requires a reaction temperature between 130
and 200°C to remove the resulting water and complete the reaction [44]. As mentioned earlier,
imide dispersants are made by the use of polyalkylene polyamines, such as diethylenetriamine
and triethylenetetramine. Many polyhydric alcohols can be used to make ester dispersants.
These include trimethylolpropane, tris(hydroxymethyl)aminoethane, and pentaerythritol. When
one uses tris(hydroxymethyl)aminoethane as the alcohol, one can obtain an ester dispersant
with basicity. The reactions to make succinimide and succinate dispersants are depicted in
Figure 5.11.
The alkylphenol-derived dispersants are made by reacting an alkylphenol, such as polyisobutyl-
phenol, with formaldehyde and a polyamine [58,69]. The result is the formation of 2-aminomethyl-
4-polyisobutylphenol. The reaction of ammonia or an amine, formaldehyde, and a compound with
active hydrogen(s), such as a phenol, is called the Mannich reaction [70,71]. Hence, such disper-
sants are called Mannich dispersants. For making phosphonate dispersants, the common method
is to react the free acid with an ole n epoxide, such as propylene oxide or butylene oxide, or an
amine [2,72,73]. These reactions are shown in Figure 5.12. Salts derived from the direct reaction
of amine and metal bases with ole n-phosphorus pentasul de adduct are also known [74,75]. It
is important to note that structures in gures are idealized structures. The actual structures will
depend on the substrate (alkylphenol and alkenylsuccinic anhydride)-to-reactant (formaldehyde
and polyamines) ratio.
Because of the polyfunctionality of the succinic anhydride group and of the amines and
polyhydric alcohols, various dispersants can be made by altering the anhydride-to-amine or
anhydride-to-alcohol ratios. These dispersants differ not only in their molecular weight but
also in their properties. Polyfunctionality of the two reactants leads to dispersants, which have
molecular weights that are three to seven times higher than expected if the two reactants were
monofunctional.
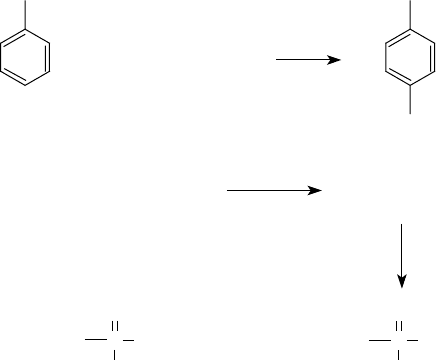
OH OH
R
+
Polyisobutylene
Polyisobutylene + P
2
S
5
Phenol
Polyisobutylphenol
Polyisobutenyl
Pol
y
isobuten
y
lthio
p
hos
p
honic and
p
ol
y
isobuten
y
l
p
hos
p
honic acids
or polyisobutenyl
Acid
Adduct
Phosphorus
pentasulfide
H
2
O
S
P
OH
OH
O
P
OH
OH
FIGURE 5.7 Synthesis of alkylphenols and alkenylphosphonic acids.
CRC_59645_Ch005.indd 154CRC_59645_Ch005.indd 154 10/31/2008 2:00:42 PM10/31/2008 2:00:42 PM
Dispersants 155
The methods to make DVMs are shown in Figures 5.13 through 5.15. These are synthesized by
Grafting or reacting of a dispersancy-imparting monomer on an already-formed polymer,
as in the case of EPRs and SDRs [76–84].
Including such a monomer during the polymerization process, as in the case of polyacry-
lates and PMAs [85].
Introducing a reactive functional group in the polymer that can be reacted with a reagent to
impart dispersancy, as in the case of styrene–maleic anhydride copolymers [40,86–93].
Although most of the examples in Figures 5.13 through 5.15 pertain to the introduction of the basic
nitrogen-containing moieties, neutral DVMs are also known in the literature. These are made by
using nonbasic reactants, such as N-vinylpyrrolidinone, alcohols, or polyether-derived methacry-
late ester [79,94,95]. Recently, dispersant viscosity–improving additives with built-in oxidation
•
•
•
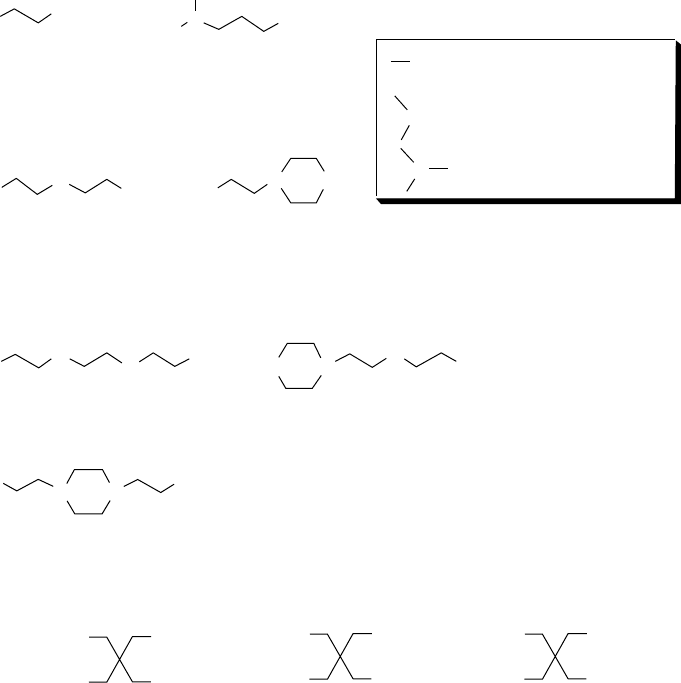
Diamines
H
2
N
H
2
N
H
2
N
H
2
N
H
2
N
H
3
C
NH
2
NH
2
NH
2
NH
2
NH
2
Ethylenediamine
Triamines
Tetramines
Triethylenetetramine
Aminoethylaminoethylpiperazine
N,N-dimethylaminopropylamine
Primary amino group (1°)
Secondary amino group (2°)
Tertiary amino group (3°)
1°
1°
1°
3° 2°
2°
2°
3°
2°
3°
2°
1°
1°
NH
2
1°
1°
3°
CH
3
N
N
N
H
N
NN
N
H
N
H
H
NH
2
NH
NH
N
Diethylenetriamine Aminoethylpiperazine
HN
N
Bis(aminoethyl) piperazine
Alcohols
HO
HO
OH
OH
HO
HO
OH
Pentaerythritol
CH
3
HO
HO
OH
NH
2
Trimethylolpropane
Tris
(
h
y
drox
y
meth
y
l
)p
ro
p
ane
Tris(hydroxymethyl)aminoethane
FIGURE 5.8 Amines and alcohols used to synthesize dispersants.
CRC_59645_Ch005.indd 155CRC_59645_Ch005.indd 155 10/31/2008 2:00:42 PM10/31/2008 2:00:42 PM
156 Lubricant Additives: Chemistry and Applications
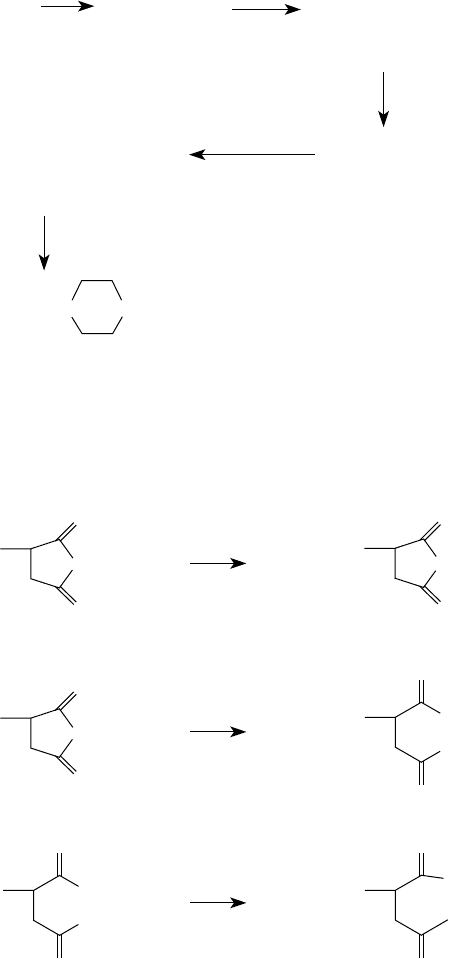
FIGURE 5.9 Manufacture of polyamines.
H
2
C=CH
2
+ CI
2
CICH
2
CH
2
CI CICH
2
CH
2
NH
2
CICH
2
CH
2
NH
2
CICH
2
CH
2
CI
NH
3
NH
3
NH
2
CH
2
CH
2
NH
2
NH
2
CH
2
CH
2
NHCH
2
CH
2
NH
2
NH
2
CH
2
CH
2
N
Ethylene
Ethylenedichloride
Chloroethylamine
Ethylenediamine
Diethylenetriamine
NH
Aminoethylpiperazine
FIGURE 5.10 Amine–anhydride reaction products. (Based on Harrison, J.J., Ruhe, R., Jr., William, R., U.S.
Patent 5,625,004, April 29, 1997.)
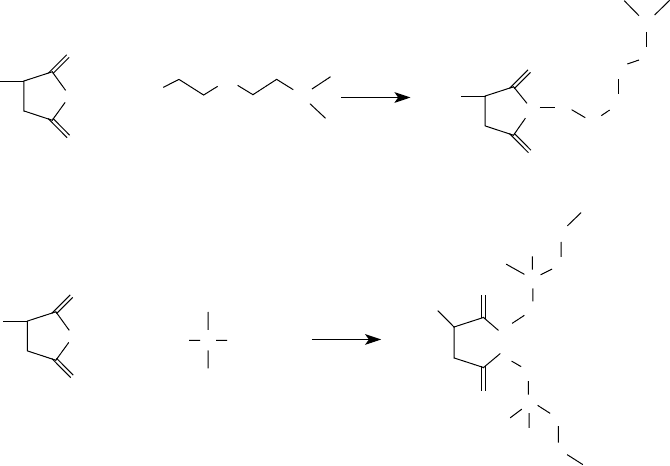
(a) Primary amine
(b) Secondary amine
(c) Tertiary amine
Polyisobutenyl
Polyisobutenyl
Polyisobutenyl
+ RNH
2
+ R
2
NH
+ R
3
N
Polyisobutenyl
Polyisobutenyl
Polyisobutenyl
Imide
Amide
Salt
O
O
O
O
O
O
NR
O
O
NR
2
NR
2
O
O
OH
NR
2
O
O
NR
2
O NHR
3
O
O
− +
inhibiting and antiwear moieties have been reported in the patent literature [77,96,97]. Dispersant
polymers containing oxidation-inhibiting moieties are commercially available from Texaco Chemi-
cal Company now part of Ethyl Petroleum Additives Company. As the examples show, grafting
usually allows the introduction of the connecting group in the dispersant polymers at the same time
as the polar moiety.
CRC_59645_Ch005.indd 156CRC_59645_Ch005.indd 156 10/31/2008 2:00:43 PM10/31/2008 2:00:43 PM
Dispersants 157
5.7 DISPERSANT PROPERTIES
A dispersant consists of a hydrocarbon chain, a connecting group, and a polar functionality. Although
each structural feature imparts unique properties to a dispersant, the dispersant’s overall perfor-
mance depends on all the three. The overall performance is assessed in terms of its dispersancy,
thermal and oxidative stability, viscosity characteristics, and seal performance. These criteria
primarily relate to engine oils, where dispersants nd major use.
5.7.1 DISPERSANCY
As mentioned, dispersancy pertains to a dispersant’s ability to suspend by-products of combustion,
such as soot, and lubricant degradation, such as resin, varnish, lacquer, and carbon deposits. The
overall performance of a dispersant depends on all the three of its structural features: the hydrocarbon
chain, the connecting group, and the polar moiety. The molecular weight of the hydrocarbon group
in a dispersant determines its ability to associate with undesirable polar species and suspend them
in the bulk lubricant. For dispersants that have the same connecting group and the polar moiety, the
lower the molecular weight, the higher the ability to associate with polar materials and the lower the
ability to suspend them. Because of the trade-off between the two properties, the hydrocarbon chain
must have the correct size and branching.
The size affects a dispersant’s af nity toward polar materials, and branching affects its solu-
bility, both before association and after association with the species, a dispersant is designed to
suspend in oil. Experience has demonstrated that hydrocarbon groups containing 70–200 carbon
atoms and extensive branching, as in the case of polyisobutylenes, are extremely suitable to design
dispersants with good dispersancy. The hydrocarbon chains of larger size, even if the branching is
similar, lead to dispersants with low af nity toward polar materials.
That is why dispersant polymers possess lower dispersancy than polymeric dispersants. How-
ever, since dispersant polymers have additional attributes, such as good thickening ef ciency and
FIGURE 5.11 Synthesis of imide and ester dispersants.
O
PIB
O
O
O
PIB
O
O
N
PIB
O
C
C
H
2
H
2
CH
2
H
2
C
O
+
+
H
2
N
H
N
N
NH
N
Polyisobutenylsuccinimide
Polyalkylenepolyamine
Polyisobutenylsuccinic
anhydride
Polyisobutenylsuccinic
anhydride
Polyisobutenylsuccinate ester
Polyhydric
alcohol
PIB = Polyisobutenyl
R′
R
HOH
2
C
CH
2
OH
C
O
O
O
C
O
R
O
O
PIB
CH
2
CH
2
CH
2
CH
2
C
R
R′
R′
CRC_59645_Ch005.indd 157CRC_59645_Ch005.indd 157 10/31/2008 2:00:43 PM10/31/2008 2:00:43 PM
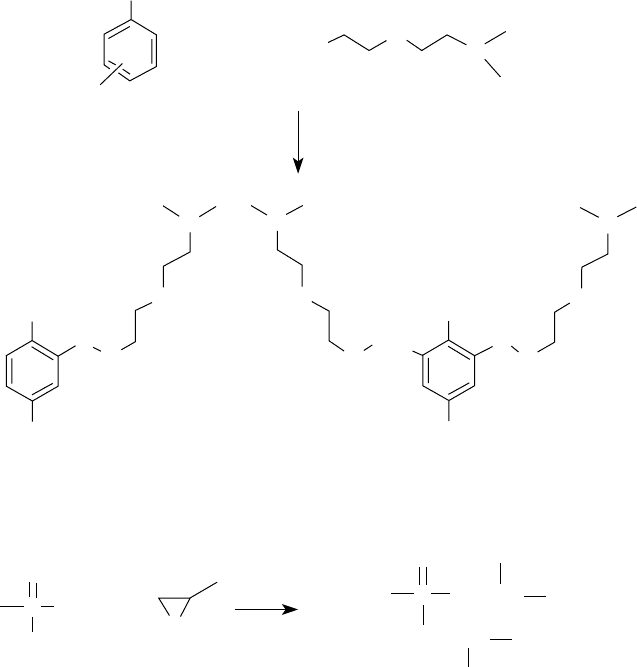
158 Lubricant Additives: Chemistry and Applications
in some cases good thermal and oxidative stability, their use is advantageous. They usually replace
additives, called viscosity modi ers, in the package. Since they impart some dispersancy because
of their structure, the amount of polymeric dispersant in engine oil formulations is somewhat
decreased [79,98].
Both the connecting group and the polar moiety are important to the dispersancy of the disper-
sant molecule. They must be considered together since both contribute toward polarity. In Mannich
dispersants, the phenol functional group, and in imide and ester dispersants, succinimide, succinate,
and phosphonate functional groups are also polar, the same as the amine and the alcohol-derived
portion of the molecule. The polarity is a consequence of the electronegativity difference between
carbon, oxygen, nitrogen, and phosphorus atoms. The greater the electronegativity difference, the
stronger the polarity. This implies that groups that contain phosphorus–oxygen bonds are more
polar than those containing carbon–oxygen bonds, carbon–nitrogen bonds, and carbon–phosphorus
bonds. The electronegativity difference for such bonds is 1.4, 1.0, 0.5, and 0.4, respectively [99].
However, since dispersants have many bonds with various combinations of atoms, the overall
polarity in a dispersant and its ability to associate with polar materials are not easy to predict.
FIGURE 5.12 Synthesis of Mannich and phosphonate dispersants.
++
OH
R
CH
2
O
H
2
N
N
N
H
Polyisobutylphenol
Polyalkylenepolyamine
OH
H
2
R
C
H
N
NH
N
NN
HN
or
H
CH
2
OH
C
N
H
H
2
R
NH
N
Polyaminomethylpolyisobutylphenols
S
P
PIB
OH
OH
+
O
Propylene
oxide
Polyisobutenylthiophosphonic acid
PIB
S
CH
3
CH
3
OCH
2
CH
OCH
2
CH
P
OH
OH
Bis-hydroxypropyl
polyisobutenylthiophosphonate
CRC_59645_Ch005.indd 158CRC_59645_Ch005.indd 158 10/31/2008 2:00:43 PM10/31/2008 2:00:43 PM
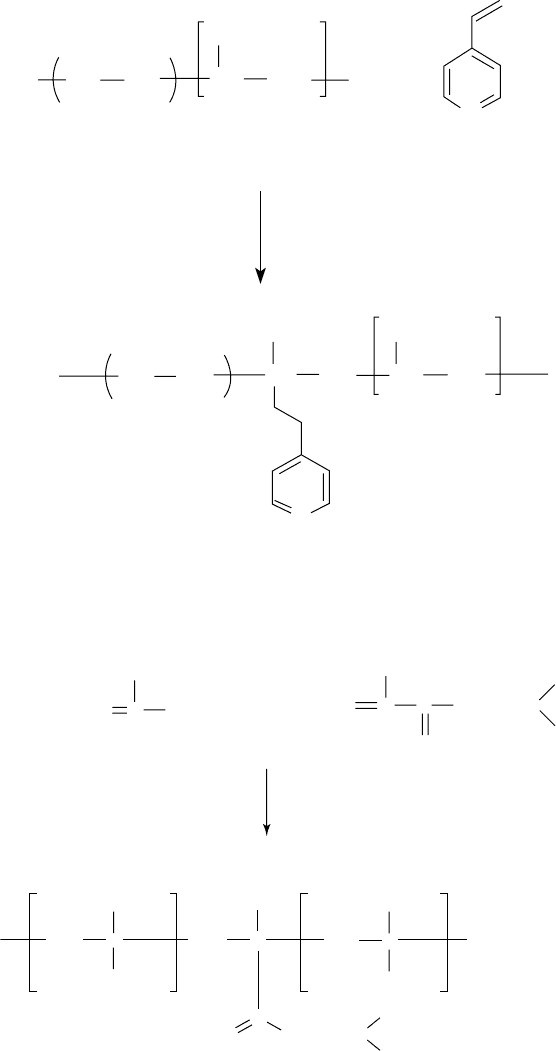
Dispersants 159
CH
3
CH
3
CH
2
CH
CH
2
m
n
+
N
4-Vinylpyridine
Ethylene − propylene copolymer
Radical
initiator
CH
2
CH
2
CH
3
CH
CH
2
CH
2
CH
2
CH
3
m
n −1
N
Dis
p
ersant olefin co
p
ol
y
mer
(
DOCP
)
FIGURE 5.13 Dispersant viscosity modi er synthesis through grafting.
CH
3
CH
3
CH
2
CH
2
COOR
n−x
x
C
CH
3
CH
3
CH
3
CH
2
C
C C OCH
2
CH
2
N
O
n
H
2
C COOR
Dimethylaminoethyl
methacrylate
Radical
initiator
Alkyl methacrylate
CH
3
CH
3
OCH
2
CH
2
N
CH
3
CH
3
CH
2
C
C
Pol
y
acr
y
late-T
yp
e Dis
p
ersant Viscosit
y
Modifier
O
COOR
+
C
FIGURE 5.14 Dispersant viscosity modi er synthesis through copolymerization.
CRC_59645_Ch005.indd 159CRC_59645_Ch005.indd 159 10/31/2008 2:00:44 PM10/31/2008 2:00:44 PM
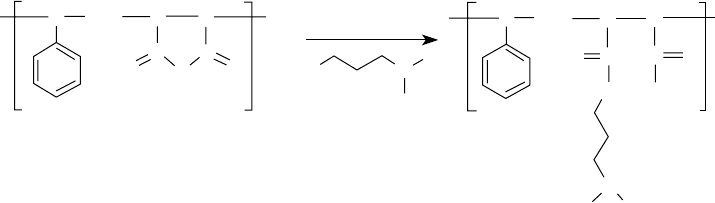
160 Lubricant Additives: Chemistry and Applications
Because some of the materials with which the dispersant associates are acidic, such as carboxylic
acids derived from lubricant oxidation, the presence of an amine nitrogen is an advantage because
of its basic character. Therefore, in certain gasoline engine tests, nitrogen dispersants are superior to
ester dispersants. Ester dispersants are usually superior in diesel engine tests because of their higher
thermo-oxidative stability. Mannich dispersants are good low-temperature dispersants; hence, they
are typically used in gasoline engine oils.
As mentioned earlier, commercial polyisobutylenes have a molecular weight distribution.
This will lead to dispersant structures of varying size, hence molecular weight. An optimum ratio
between the molecular weight of the hydrocarbon chain and that of the polar functionality (polar/
nonpolar ratio) is a prerequisite for good dispersancy. If a dispersant composition has an exces-
sive amount of components with short hydrocarbon chains, that is, of low molecular weight, its
associating ability increases, but its oil solubility suffers. This is likely to deteriorate its disper-
sancy, especially after associating with polar impurities. Such structures in dispersants are, there-
fore, undesired. Their formation can be minimized by using polyole ns of low polydispersity
index, controlling the formation of low-molecular-weight components, removing such components
through distillation [100], or postreacting with another reagent, preferably of the hydrocarbon type.
Polyole ns of low polydispersity index (≤2) are available from BP and Exxon Chemical Company.
Controlling the formation of low-molecular-weight components is exempli ed by the use of boron
tri uoride catalyst for making alkylphenols instead of aluminum chloride, which tends to fragment
polyisobutylene. Removing the lower-molecular-weight components, although not easy, is possible
at the precursor stage, which is before reacting with the alcohol or the amine. A number of reagents
can be used for the postreaction [101]. Hydrocarbon posttreatment agents include polyepoxides
[102], polycarboxylic acid [103], alkylbenzenesulfonic acids [104], and alkenylnitriles [105].
Whenever postreacted dispersants are used in engine oils, improved dispersancy, viscosity index
credit, improved uorocarbon elastomer compatibility, hydrolytic stability, and shear stability are
often claimed.
5.7.2 THERMAL AND OXIDATIVE STABILITY
All the three components of the dispersant structure determine its thermal and oxidative stability,
the same as dispersancy. The hydrocarbon group can oxidize in the same manner as the lubricant
hydrocarbons to form oxidation products that can contribute toward deposit-forming species [4,9].
(This is described in Section 5.2.) Although the rate of oxidation is quite slow for largely paraf nic
hydrocarbon groups, such as polyisobutyl group, it is quite high for those that contain multiple bonds,
such as polyisobutenyl, and the benzylic groups. The benzylic functional group is present in styrene
FIGURE 5.15 Dispersant viscosity modi er synthesis through chemical reaction.
CC
HH
H
C
C
C
O
O
O
CH
2
Styrene−maleic
anhydride polymer
n
n
H
2
N
ROH
R
H
C
C
C
H
H
CC
O
O
CH
2
R′
R′
R
N
N
NH
OR
Styrene ester−based
dispersant viscosity
modifier
CRC_59645_Ch005.indd 160CRC_59645_Ch005.indd 160 10/31/2008 2:00:44 PM10/31/2008 2:00:44 PM
Dispersants 161
butadiene and styrene ester–derived dispersant polymers. Purely paraf nic hydrocarbon groups that
contain tertiary hydrogen atoms, such as EPRs, oxidize at a faster rate than those that contain only
primary and secondary hydrogen atoms. Styrene isoprene–derived materials contain both benzylic
and tertiary hydrogen atoms. This implies that highly branched alkyl groups, such as polyisobutyl
and polyisobutenyl, have a higher susceptibility toward oxidation than linear or unbranched alkyl
groups. Dispersant polymers with built-in oxidation-inhibiting moieties are known in the literature
[77,78,96]. The polar moiety in an amine-derived dispersant is also likely to oxidize at a faster rate
than the oxygen-derived moiety because of the facile formation of the amine oxide functional group on
oxidation. Such groups are known to thermally undergo β-elimination [40], called the cope reaction, to
form an ole n. This can oxidize at a faster rate as well as lead to deposit-forming polymeric products.
From a thermal stability perspective, the hydrocarbon group in the case of high-molecular-
weight dispersant polymers, such as those derived from OCPs, is more likely to break down (unzip)
than that derived from the low-molecular-weight polymers. Dispersants based on 1000–2000
molecular weight polyisobutylenes are relatively stable, except at very high temperatures that are
experienced in some engine parts, such as near the top of the piston [17,18]. Thermal breakdown of
the oxidized amine polar group is mentioned in the previous paragraph.
The chemical reactivity of certain dispersants toward water and other reactive chemicals pres-
ent in the lubricant formulation is an additional concern. The most likely reaction site is the con-
necting group. The common connecting groups are amide and imide in amine-derived dispersants
and ester in alcohol-derived dispersants. All three can hydrolyze in the presence of water [106], but
at different rates. Esters are easier to hydrolyze than amides and imides. The hydrolysis is facilitated
by the presence of bases and acids. Basic detergents are the source of the metal carbonate and metal
hydroxide bases, which at high temperatures catalyze the hydrolysis reaction. Additives, such as
zinc dialkyldithiophosphates, are a source of strong acids that result when these additives hydro-
lyze, thermally decompose, or oxidize. The fate of the ester-, amide-, and imide-type dispersant
polymers, such as those derived from polyacrylates, PMAs, and styrene ester substrates, is the same.
Some OCP-derived dispersant polymers, such as those obtained by grafting of monomers 2- or
4-vinylpyridine and 1-vinyl-2-pyrrolidinone [76,80], do not suffer from this problem since they do
not contain easily hydrolyzable groups. Reactivity toward other chemicals present in the formula-
tion is again prevalent in the case of ester-derived dispersants. Reaction with metal-containing addi-
tives, such as detergents and zinc dialkyldithiophosphates, can occur after hydrolysis to form metal
salts. This can destroy the polymeric structure of a dispersant and hence its effectiveness. Some
formulations contain amines or their salts as corrosion inhibitors or friction modi ers. Depending
on the molecular weight and the ambient temperature, these can displace the polyol or sometimes
the polyamine, thereby altering the dispersant structure, hence its properties.
5.7.3 VISCOSITY CHARACTERISTICS
The amount of dispersant in automotive engine oils typically ranges between 3 and 7% by weight
[79], making it the highest among additives. In addition, dispersant is the highest molecular-weight
component except the viscosity improver [107]. Both of these factors can alter some physical prop-
erties, such as viscosity, of the lubricant. A boost in the viscosity of a lubricant at high temperatures
is desired, but at low temperatures it is a disadvantage. At high temperatures, the lubricant loses
some of its viscosity [108], hence its lm-forming ability, resulting in poor lubrication. Maintaining
good high-temperature viscosity of a lubricant is therefore imperative to minimize wear damage.
This is usually achieved by the use of polymeric viscosity modi ers [3,109]. Some dispersants,
especially those that are based on high-molecular-weight polyole ns and have been oversuccinated
partly ful ll this need [44]. Therefore, the amount of polymeric viscosity modi er necessary to
achieve speci c high-temperature viscosity is reduced. Unfortunately, dispersants that provide a
viscosity advantage lead to a viscosity increase at low temperatures as well. The low-temperature
viscosity requirements for engine oils have two components: cranking viscosity and pumping
CRC_59645_Ch005.indd 161CRC_59645_Ch005.indd 161 10/31/2008 2:00:44 PM10/31/2008 2:00:44 PM
162 Lubricant Additives: Chemistry and Applications
viscosity [110]. Cranking viscosity is an indication of how easily the engine will turn over in
extremely cold weather conditions. Pumping viscosity is the ability of the lubricant to be pumped
to reach various parts of the engine. For cold weather operation, low to moderate cranking and
pumping viscosities are highly desirable. Although pumping viscosity and the pour point can be
lowered by the use of additives, called pour point depressants [3,13], lowering cranking viscosity
is not easy. In the case of base oils, this is usually achieved by blending carefully selected base
stocks. An ideal polymeric dispersant must provide high-temperature viscosity advantage with-
out adversely affecting the cold-cranking viscosity of the lubricant. Dispersant polymers have
the same requirement. Good high-temperature viscosity to cranking viscosity ratio in polymeric
dispersants can be achieved by
Carefully balancing the type and the molecular weight of the hydrocarbon chain [111]
Choosing the optimum ole n to maleic anhydride molar ratio [112]
Selecting the type and the amount of the polyamine used
In dispersant polymers this can be achieved by selecting (1) a polymer of correct molecular weight
and branching and (2) a suitable pendant group. Dispersant polymers derived from medium-
molecular-weight, highly branched structures, and ester-type pendant groups are best suited for use
as additives. Examples include polyacrylate, PMA, and styrene ester–derived dispersants. These
additives not only act as viscosity modi ers and dispersants but also act as pour point depressants,
thereby improving the low-temperature properties of the lubricant.
A number of patents pertaining to dispersants with balanced high-temperature viscos-
ity and low-temperature properties are reported in the patent literature [113–117]. A Mannich
(alkylphenol) dispersant, derived from ethylene/1-butene polymers of Mn 1500–7500, has been
claimed to possess improved dispersancy and pour point [113]. Another patent claiming the syn-
thesis of a dispersant with superior dispersancy and pour point depressing properties has also
been issued [114]. The dispersant is based on the reaction of maleic anhydride/lauryl methacry-
late/stearyl methacrylate terpolymer with dimethylaminopropylamine, and a Mannich base was
obtained by reacting N- aminoethylpiperazine, paraformaldehyde, and 2,6-di-t-butyl phenol. A
number of patents describe the use of ethylene/α-ole n/diene interpolymers to make dispersants
[115–117]. These dispersants are claimed to possess excellent high- and low-temperature viscosi-
ties, as de ned by VR´/VR. Here VR´ pertains to the dispersant and VR pertains to the precursor,
such as alkylphenol or alkenylsuccinic anhydride. VR´ is the ratio of the –20°C cold-cranking
simulator (CCS) viscosity (cP) of a 2% solution of dispersant in a reference oil to the 100°C kine-
matic viscosity (cSt) of the dispersant. VR is the ratio of the –20°C CCS viscosity (cP) of a 2%
solution of precursor in the reference oil to the 100°C kinematic viscosity (cSt) of the precursor.
The values of 2.0–3.9 for VR and VR´ and of <1.11 for VR´/VR are considered suitable for bal-
anced low- and high-temperature viscosities.
5.7.4 SEAL PERFORMANCE
Seals in automotive equipment are used for many purposes, the most prominent of which are to
have easy access to malfunctioning parts to perform repair and to minimize contamination and loss
of lubricant. Various polymeric materials are used to make seals. These include uoroelastomers,
nitrile rubber, polyacrylates, and polysiloxanes (silicones). Maintaining the integrity of seals is criti-
cal; otherwise, the lubricant will be lost, and wear damage and equipment failure will occur. The
seals fail in a number of ways. They can shrink, elongate, or become brittle and thus deteriorate.
The damage to elastomer seals is assessed by examining volume, hardness, tensile strength change,
and the tendency to elongate and rupture [118]. Two primary mechanisms by which seal damage can
occur include abrasion due to particulate matter in the lubricant and the attack of various lubricant
components on the seals. The lubricant-related damage can occur when some of its components
•
•
•
CRC_59645_Ch005.indd 162CRC_59645_Ch005.indd 162 10/31/2008 2:00:45 PM10/31/2008 2:00:45 PM
Dispersants 163
diffuse into the seals. This will either cause a change in the seal’s hardness, thereby leading to
swelling and or elongation, or extract the plasticizer, an agent used to impart exibility and strength
to polymeric materials.
Abrasive damage is not common since most equipment has an installed lubricant ltration sys-
tem. The lubricant-related damage, however, is of primary interest to us. The lubricant is a blend of
base stocks and an additive package. Certain base stocks, such as those of high aromatics content
or those that are of the ester type, have the tendency to extract the plasticizer because of their high
polarity. Additives, however, have the ability to diffuse into the seal material and alter its properties
as well as remove the plasticizer. Among additives, dispersants are the most implicated in causing
seal damage, especially to uoroelastomer (Viton
®
) seals. Although in many cases seal failure can
be corrected by the use of additives, called the seal-swell agents, it is wise to eliminate such damage
by prevention. Elastomer compatibility requirements are a part of the current United States, Asso-
ciation des Contsructeurs Européens de l’Automobile (ACEA), and Japanese standards for engine
oils and worldwide automotive transmission and tractor hydraulic uid speci cations [119]. Damage
to seals is prevalent in the case of nitrogen dispersants. In general, the higher the nitrogen content,
the higher the seal problems [118]. Rationally, these problems occur due to the presence of low-
molecular-weight molecules in the dispersant. These include free amine either as such or in a labile
form, such as an alkylammonium salt, or low-molecular-weight succinimides and succinamides.
Because of their high polarity and smaller size, these molecules are more likely to diffuse into the
seal material and alter its physical and mechanical properties [120]. It is believed that in the case of
Viton seals, the loss of uoride ions is responsible for seal deterioration. Removal of the free amine
and of low-molecular-weight succinimides will improve seal performance. Alternatively, one can
posttreat dispersants with reagents, such as boric acid and epoxides, which will either make
such species innocuous or hinder their diffusion into the seal material. Many chemical treat-
ments of dispersants, covered in Section 5.7.1, claim to improve seal performance of dispersants
and crankcase lubricants that use them. These reagents react with seal-damaging amines and low-
molecular-weight succinimides to make them harmless. Strategies other than those listed earlier are
also reported in the patent literature [121–125].
5.8 PERFORMANCE TESTING
Engine oils account for almost 80% of the automatic transmission dispersant use. Other applica-
tions that use these additives include automatic transmission uids, gear lubricants, hydraulic uids,
and re nery processes as antifoulants. Dispersants of relatively lower molecular weight are also
used in fuels to control injector and combustion chamber deposits [126,127]. Such dispersants usu-
ally contain a polyether functionality [128].
Succinimide and succinate ester–type polymeric dispersants are used in gasoline and die-
sel engine oils, but the use of alkylphenol-derived dispersants, that is, of the Mannich type, is
limited to gasoline engine oils. Dispersant polymers derived from ethylene–propylene rubbers,
styrene–diene copolymers, and PMAs are also used in both gasoline and diesel engine oils. As
mentioned earlier, dispersant polymers lack suf cient dispersancy to be used alone and hence are
used in combination with polymeric dispersants. The PMA and styrene ester–derived dispersant
polymers are used in automatic transmission uids, in power-steering uids, and, to a limited
extent, in gear oils.
Additive manufacturers use various laboratory screen tests and engine tests to evaluate a
dispersant’s effectiveness. Many of the screen tests are proprietary, but all are developed around
evaluating performance in terms of a dispersant’s ability to disperse lamp black or used engine oil
sludge. The laboratory engine tests are industry-required tests and include both gasoline engine
and diesel engine tests. These are listed in International Lubricant Standardization and Approval
Committee (ILSAC), American Petroleum Institute (API), ACEA 2002, Japanese Automobile
Standards Organization (JASO), and Bureau of Indian Standards (BIS) standards. It is important
CRC_59645_Ch005.indd 163CRC_59645_Ch005.indd 163 10/31/2008 2:00:45 PM10/31/2008 2:00:45 PM
