Rudnick L. Lubricant Additives: Chemistry and Applications (Присадки, добавки к смазкам)
Подождите немного. Документ загружается.

144 Lubricant Additives: Chemistry and Applications
is the case in imide/amide dispersants. The basicity of the imide/amide dispersants is due
to the presence of the amine functionality. Amines are weak bases and therefore possess
minimal acid-neutralizing ability. Conversely, detergents, especially basic detergents, con-
tain reserve metal bases as metal hydroxides and metal carbonates. These are strong bases,
with the ability to neutralize combustion and oxidation-derived inorganic acids, such as
sulfuric and nitric acids, and oxidation-derived organic acids.
3. Dispersants are much higher in molecular weight, approximately 4–15 times higher, than
the organic portion (soap) of the detergent. Because of this, dispersants are more effective
in ful lling the suspending and cleaning functions than detergents.
As mentioned in Chapter 4, dispersants, detergents, and oxidation inhibitors make up the general
class of additives called stabilizers and deposit control agents. The goal of oxidation inhibitors is
to minimize the formation of deposit precursors, such as hydroperoxides and radicals [3,4]. This is
because these species are reactive, and they attack the hydrocarbon base oil and additives, which
make up the lubricant, to form sludge, resin, varnish, and hard deposits. The goal of the dispersant
and the soap portion of the detergent is to keep these entities suspended in the bulk lubricant. This
not only results in deposit control but also minimizes particulate-related abrasive wear and viscosity
increase. When the lubricant in the equipment is changed, the deposit precursors and the deposit-
forming species are removed with the used oil.
The dispersants suspend deposit precursors in oil in various ways. These comprise the
following:
Including the undesirable polar species into micelles.
Associating with colloidal particles, thereby preventing them from agglomerating and fall-
ing out of solution.
Suspending aggregates in the bulk lubricant, if they are formed.
Modifying soot particles so as to prevent their aggregation. The aggregation will lead to oil
thickening, a typical problem in heavy-duty diesel engine oils [5,6].
Lowering the surface/interfacial energy of the polar species to prevent their adherence to
metal surfaces.
5.2 NATURE OF DEPOSITS AND MODE OF THEIR FORMATION
A number of undesirable materials result from the oxidative degradation of various components of
the lubricant. These are base oil, additives, and the polymeric viscosity modi er, if present. In engine
oils, the starting point for the degradation is fuel combustion, which gives rise to hydroperoxides and
free radicals [7]. The compounds in the fuel that are most likely to form peroxides, hydroperoxides,
and radicals include highly branched aliphatics, unstaurates such as ole ns, and aromatics such as
alkylbenzenes. All these are present in both gasoline and diesel fuels. American Society for Testing
and Materials (ASTM) test methods D 4420 and D 5186 are used to determine the aromatic content
of gasoline and diesel fuels, respectively [8]. The fuel degradation products (peroxides, hydroperox-
ides, and radicals) go past the piston rings into the lubricant as blowby and, because they are highly
energetic, attack largely the hydrocarbon lubricant. Again, the highly branched aliphatic, unsatu-
rated, and aromatic structures are among those that are highly susceptible. ASTM Standard D
5292 is commonly used to determine the aromatic content of the base oil [8]. The reaction between
the contents of the blowby and these compounds results in the formation of the lubricant-derived
peroxides and hydroperoxides that either oxidatively or thermally decompose to form aldehydes,
ketones, and carboxylic acids [3,4,9]. Acids can also result from the high-temperature reaction of
nitrogen and oxygen, both of which are present in the air–fuel mixture; the oxidation of the fuel
sulfur; and the oxidation, hydrolysis, or thermal decomposition of additives such as zinc dialkyl-
dithiophosphates. The reaction between nitrogen and oxygen to form NO
x
is more prevalent in diesel
•
•
•
•
•
CRC_59645_Ch005.indd 144CRC_59645_Ch005.indd 144 10/31/2008 2:00:39 PM10/31/2008 2:00:39 PM
Dispersants 145
engines and gasoline engines that are subjected to severe service, such as long-distance driving for
extended periods. The NO
x
formation initiates when the temperature reaches 137°C [10,11]. Zinc
dialkyldithiophosphates are commonly used as oxidation inhibitors in engine oils [12,13]. All these
acids are neutralized by basic detergents to form inorganic metal salts and metal carboxylates. These
compounds are of low hydrocarbon solubility and are likely to fall out of solution.
The aldehydes and ketones undergo aldol-type condensation in the presence of bases or acids to
form oligomeric or polymeric compounds. These can further oxidize to highly oxygenated hydrocar-
bons, commonly referred to as oxygenates. The oxygenates are usually of sticky consistency, and the
term resin is often used to describe them [14]. Resin is either the basic component in or the precur-
sor to all types of deposits. Common types of deposits include varnish, lacquer, carbon, and sludge
[15,16]. Varnish, lacquer, and carbon occur when resin separates on hot surfaces and dehydrates or
polymerizes to make tenacious lms. The quantity and the nature of deposits depend on the proxim-
ity of the engine parts to the combustion chamber. The parts closer to the combustion chamber, such
as exhaust valve head and stem that experience approximate temperatures of 630–730°C [17,18], will
develop carbon deposits. The same is true of the combustion chamber wall, piston crown, top land,
and top groove, which are exposed to approximate temperatures of 200–300°C. Carbon deposits are
more common in diesel engines than in gasoline engines and result from the burning of the liquid
lubricating oil and the high-boiling fractions of the fuel that adhere to hot surfaces [19].
As we move away from these regions to the low-temperature regions, such as the piston skirt,
the deposits are not heavy and form only a thin lm. For diesel engine pistons, this type of deposit
is referred to as lacquer; for gasoline engine pistons, this type of deposit is called varnish. The
difference between lacquer and varnish is that lacquer is lubricant-derived and varnish is largely
fuel-derived. In addition, the two differ in their solubility characteristics. That is, lacquer is water-
soluble and varnish is acetone-soluble [15]. Lacquer usually occurs on piston skirts, on cylinder
walls, and in the combustion chamber, whereas varnish occurs on valve lifters, piston rings, piston
skirts, valve covers, and positive crankcase ventilation (PCV) valves.
The coolest parts of the engine, such as rocker arm covers, oil screen, and oil pan, that are
exposed to temperatures of ≤200°C experience sludge deposits. Sludge can be watery or hard in
consistency, depending on the severity of service. If the service is extremely mild and of short
duration, as in the case of stop-and-go gasoline engine operation, the sludge is likely to be watery
or mayonnaiselike [15]. This type of sludge is called low-temperature sludge, which occurs when
the ambient temperature is <95°C. The high-temperature sludge is more common in diesel engines
and gasoline engines with long, continuous operation. This type of sludge occurs when the ambient
temperature is >120°C and is hard in consistency. In the former case, the engine does not get hot
enough to expel combustion water, which stays mixed with oil, imparting sludge, a mayonnaiselike
appearance. In the latter case, however, the ambient temperature is high enough to expel water,
thereby resulting in hard sludge. Sludge is common in areas that experience low oil ow, such as
crankcase bottoms and rocker boxes.
Another component of the combustion ef uent that must be considered is soot. Soot not only
contributes toward some types of deposits such as carbon and sludge, but it also leads to a viscosity
increase. These factors can cause poor lubricant circulation and lubricating lm formation, both of
which will result in wear and catastrophic failure. Soot is particulate in nature and results from the
incomplete combustion of the fuel and of the lubricating oil from the crankcase that might enter the
combustion chamber by traveling past the piston rings [20]. Fuel-derived soot is a chronic problem
in the case of diesel engines because diesel fuel contains high-boiling components that do not burn
easily. In addition, diesel engine combustion is largely heterogeneous, with poor air–fuel mixing,
hence poor combustion [20]. Soot is made of hydrocarbon fragments with some of the hydrogen
atoms removed. The particles are charged and hence have the tendency to form aggregates. When
aggregates occur on surfaces, such as those of the combustion chamber, soot deposits result. These
deposits are soft and aky in texture. If these occur in oil, lubricant experiences an increase in vis-
cosity. A soot-related viscosity increase usually requires the presence of polar materials in oil that
CRC_59645_Ch005.indd 145CRC_59645_Ch005.indd 145 10/31/2008 2:00:39 PM10/31/2008 2:00:39 PM
146 Lubricant Additives: Chemistry and Applications
have the ability to associate with soot. These can be additives or polar lubricant oxidation and deg-
radation products. Carbon deposits are lower in carbon content than soot and, in most cases, contain
oily material and ash. This makes knowledge of the ash-forming tendency of a lubricant important
to a formulator. This concern was addressed in Chapter 4.
When soot associates with resin, one gets either resin-coated soot particles or soot-coated resin
particles [16]. The rst type of particles results when resin is in excess, and the second type results
when soot is in excess. The amount of soot in resin determines the color of the deposits: the higher
the soot, the darker the deposits. Sludge results when resin, soot, oil, and water mix [9].
Deposit formation in gasoline engines is initiated by NO
x
and oxidation-derived hydroperoxides
that react with hydrocarbons in the fuel and the lubricant to form organic nitrates and oxygenates
[14,21]. Being thermally unstable, these species decompose and polymerize to form deposits. The
deposits typically include resin, varnish, and low-temperature sludge. In diesel engines, however,
soot is an important component of the deposits, which include lacquer, carbon deposits, and high-
temperature sludge [16]. Typically, carbon deposits are of high metal content, which is mainly due
to the presence of detergent additives in the lubricant [22,23].
Detailed mechanism of deposit formation in engines is described elsewhere [24,25]. The mecha-
nism is based on the premise that both the lubricant and the fuel contribute toward deposit forma-
tion. The role of the blowby, NO
x
, and high-temperature oxidative and thermal degradation of the
lubricant, described earlier, are substantiated [24]. The importance of oxygenated precursors—their
decomposition, condensation, and polymerization to form deposits—is also supported. The deposit
precursors consist of approximately 15–50 carbon atoms and contain multiple hydroxy and carboxy
functional groups. Because of the polyfunctionality, these molecules have the ability to thermally
polymerize to high-molecular-weight products [14,16]. As mentioned earlier, soot associates with
polar oxidation products in oil to cause a viscosity increase. Viscosity increase can also occur in
gasoline engine oils that have little or no soot. This happens when the oxygen content of the precur-
sors is low and the resulting polymer is of low molecular weight and of good oil solubility [14]. This
phenomenon is commonly referred to as oil thickening [6]. Conversely, if the oxygen content of the
precursors is high, the polymerization results in the formation of high-molecular-weight products of
low lubricant solubility. Such products constitute resin, which is of low oil solubility and separates on
surfaces. If the surfaces are hot, subsequent dehydration and polymerization lead to the formation of
varnish, lacquer, and carbon deposits. It is important to note that deposits are a consequence of lubri-
cant oxidation that accelerates once the oxidation inhibitor package in the lubricant is exhausted.
Three other internal combustion engine problems—oil consumption, ring sticking, and cor-
rosion and wear—are also related to lubricant degradation. Oil consumption is a measure of how
much lubricant travels past piston rings into the combustion chamber and burns. A certain minimum
amount of the lubricant is necessary in the vicinity of the piston rings to lubricate cylinder walls and
cylinder liners and hence facilitate piston movement and minimize scuf ng. However, if too much
lubricant ends up in the combustion chamber, serious emission problems will result. Modern piston
designs, such as articulated pistons and pistons with low crevice volume, allow just enough lubricant
to minimize scuf ng, but without adversely contributing to emissions [26,27]. Other parameters
that affect oil consumption include the integrity of pistons and cylinders and the viscosity, volatility,
and sealing characteristics of the lubricant. Pistons with stuck rings and out-of-square grooves and
cylinders with increased wear will result in a poor seal between the crankcase and the combustion
chamber [15]. As a consequence, a larger amount of blowby will enter the crankcase and increase the
rate of lubricant breakdown. This will complicate the situation further. Ring sticking occurs when
sticky deposits form in the grooves behind the piston rings. This is a serious problem because it not
only results in a poor seal but also leads to poor heat transfer from the cylinder to the wall. If not con-
trolled, this will result in nonuniform thermal expansion of the pistons, loss of compression, and ulti-
mately the failure of the engine [15]. The wear of pistons and the cylinders is undesired for the same
reasons. Wear of engine parts is either corrosive or abrasive. Corrosive wear arises from the attack
of fuel sulfur-derived products, such as sulfur oxides or sulfuric acid, or the acidic by-products of
CRC_59645_Ch005.indd 146CRC_59645_Ch005.indd 146 10/31/2008 2:00:40 PM10/31/2008 2:00:40 PM

Dispersants 147
lubricant oxidation and degradation, such as carboxylic and sulfonic acids. Fuel sulfur–derived
piston ring wear and cylinder wear are serious problems in large, slow-speed marine diesel engines
that use a high-sulfur fuel. Corrosive wear is controlled by the use of lubricants with a base reserve,
that is, those containing a large quantity of basic detergents. This was discussed in Chapter 4. Abrasive
wear results from the presence of the particulate matter, such as large soot particles, in the lubricant.
Dispersants are crucial to the control of soot-related wear.
5.3 DEPOSIT CONTROL BY DISPERSANTS
Fuel and lubricant oxidation and degradation products, such as soot, resin, varnish, lacquer, and
carbon, are of low lubricant (hydrocarbon) solubility, with a propensity to separate on surfaces.
The separation tendency of these materials is a consequence of their particle size. Small particles
are more likely to stay in oil than large particles. Therefore, resin and soot particles, which are
the two essential components of all deposit-forming species, must grow in size through agglom-
eration before separation. Growth occurs either because of dipolar interactions, as is the case in
resin molecules, or because of adsorbed polar impurities such as water and oxygen, as is the case
in soot particles. Alternatively, soot particles are caught in the sticky resin. Dispersants interfere
in agglomeration by associating with individual resin and soot particles. The particles with asso-
ciated dispersant molecules are unable to coalesce because of either steric factors or electrostatic
factors [28]. Dispersants consist of a polar group, usually oxygen- or nitrogen-based, and a large
nonpolar group. The polar group associates with the polar particles, and the nonpolar group keeps
such particles suspended in the bulk lubricant [16]. Neutral detergents, or soaps, operate by an
analogous mechanism.
5.4 DESIRABLE DISPERSANT PROPERTIES
Dispersing soot, deposit precursors, and deposits is clearly the primary function of a dispersant.
Dispersants, in addition, need other properties to perform effectively. These include thermal and
oxidative stability and good low-temperature properties. If a dispersant has poor thermal stabil-
ity, it will break down, thereby losing its ability to associate with and suspend potentially harmful
products. Poor oxidative stability translates into the dispersant molecule contributing itself toward
deposit formation. Good low-temperature properties of a lubricant are desired for many reasons:
ease of cold cranking, good lubricant circulation, and fuel economy. Base oil suppliers have devel-
oped a number of ways to achieve these properties. The methods they use include isomerization of
the base stock hydrocarbons through hydrocracking and the use of special synthetic oils as addi-
tives. Since dispersant is one of the major components of the engine oil formulations, its presence
can adversely affect these properties, which must be preserved.
5.5 DISPERSANT STRUCTURE
A dispersant molecule consists of three distinct structural features: a hydrocarbon group, a polar
group, and a connecting group or a link (see Figure 5.1). The hydrocarbon group is polymeric
FIGURE 5.1 Graphic representation of a dispersant molecule.
Nitrogen- or oxygen-
derived functionality
Polar moiety
Connecting group
Hydrocarbon group
CRC_59645_Ch005.indd 147CRC_59645_Ch005.indd 147 10/31/2008 2:00:40 PM10/31/2008 2:00:40 PM
148 Lubricant Additives: Chemistry and Applications
in nature, and depending on its molecular weight, dispersants can be classi ed into polymeric
dispersants and dispersant polymers. Polymeric dispersants are of lower molecular weight than
dispersant polymers. The molecular weight of polymeric dispersants ranges between 3,000 and
7,000 as compared to dispersant polymers, which have a molecular weight of 25,000 and higher.
Although various ole ns, such as polyisobutylene, polypropylene, polyalphaole ns, and mixtures
thereof, can be used to make polymeric dispersants, the polyisobutylene-derived dispersants are the
most common. The number average molecular weight (Mn) of polyisobutylene ranges between 500
and 3000, with an Mn of 1000–2000 being typical [29]. In addition to Mn, other polyisobutylene
parameters, such as molecular weight distribution and the length and degree of branching, are also
important in determining the overall effectiveness of a dispersant.
Substances obtained through a polymerization reaction, especially those made by using an acid
catalyst or a free-radical initiator, often contain molecules of different sizes. Molecular weight
distribution, or polydispersity index, is commonly used to assess the heterogeneity in molecular
size. Polydispersity index is the ratio of weight average molecular weight (Mw) and Mn, or Mw/Mn
[30–32]. These molecular weights are determined by subjecting the polymer to gel permeation
chromatography (GPC). The method separates molecules based on size [33]. The larger molecules
come out rst, followed by the next size. When the molecules are of the same size, Mw/Mn equals
1 and the polymer is called a monodisperse polymer. The polymers with an index >1 are called
polydisperse polymers. For most applications, monodispersity is desired. Polyisobutylene, derived
from acid-catalyzed polymerization reaction, typically has a polydispersity index between 2 and 3.
This will impact many of the dispersant properties described below.
Dispersant polymers, also called dispersant viscosity modi ers (DVMs) and dispersant viscos-
ity index improvers (DVIIs), are derived from hydrocarbon polymers of molecular weights between
25,000 and 500,000. Polymer substrates used to make DVMs include high-molecular-weight ole n
copolymers (OCPs), such as ethylene–propylene copolymers (EPRs), ethylene–propylene–diene
copolymers (EPDMs), polymethacrylates (PMAs), styrene–diene rubbers (SDRs) of both linear and
star con gurations, and styrene–ester polymers (SEs).
The polar group is usually nitrogen- or oxygen-derived. Nitrogen-based groups are derived
from amines and are usually basic in character. Oxygen-based groups are alcohol-derived and are
neutral. The amines commonly used to synthesize dispersants are polyalkylene polyamines such
as diethylenetriamine and triethylenetetramine. In the case of DVMs or dispersant polymers, the
polar group is introduced by direct grafting, copolymerization, or by introducing a reactable func-
tionality. The compounds used for this purpose include monomers such as 2- or 4-vinylpyridine,
N-vinylpyrrolidinone, and N,N-dialkylaminoalkyl acrylate and unsaturated anhydrides and acids
such as maleic anhydride, acrylic acid, and glyoxylic acid. The details of these reactions are
described in Section 5.6, which deals with the dispersant synthesis. Amine-derived dispersants are
called nitrogen or amine dispersants, and those that are alcohol-derived are called oxygen or ester
dispersants [28]. Oxygen-derived phosphonate ester dispersants were popular at one time, but their
use in engine oils is now restrained because of the phosphorus limit. Phosphorus limit pertains
to its tendency to poison noble metal catalysts used in catalytic converters. Formulators prefer to
take advantage of the phosphorus limit by using zinc dialkyldithiophosphates, which are excellent
oxidation inhibitors and antiwear agents. In the case of amine dispersants, it is customary to leave
some of the amino groups unreacted to impart basicity to the dispersant. The reasons for this are
described in Section 5.7.
5.6 DISPERSANT SYNTHESIS
Since it is not easy to attach the polar group directly to the hydrocarbon group, except in the case
of ole ns that are used to make DVMs, the need for a connecting group or a link arises. Although
many such groups can be used, the two common ones are phenol and succinic anhydride. Ole n, such
as polyisobutylene, is reacted either with phenol to form an alkylphenol or with maleic anhydride
CRC_59645_Ch005.indd 148CRC_59645_Ch005.indd 148 10/31/2008 2:00:40 PM10/31/2008 2:00:40 PM
Dispersants 149
to form an alkenylsuccinic anhydride. The polar functionality is then introduced by reacting these
substrates with appropriate reagents.
5.6.1 THE HYDROCARBON GROUP
Polyisobutylene is the most common source of the hydrocarbon group in polymeric dispersants. It is
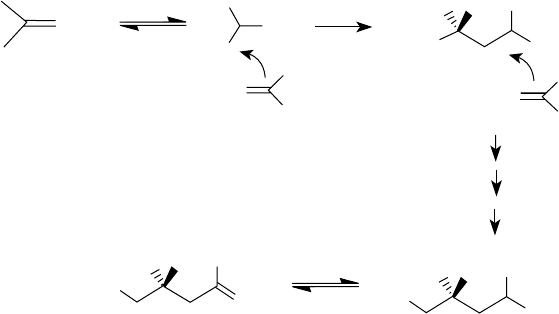
manufactured through acid-catalyzed polymerization of isobutylene [34,35]. Figure 5.2 depicts the
mechanism of its formation. In Figure 5.2, polyisobutylene is shown as a terminal ole n, whereas
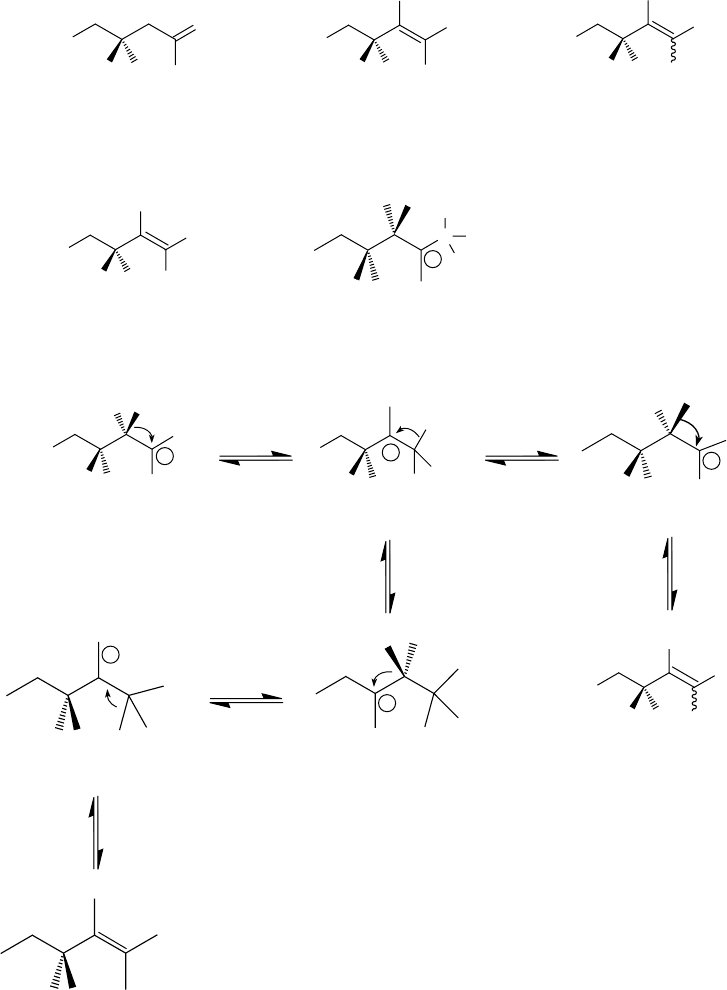
in reality it is a mixture of various isomers. Those that predominate include geminally disubstituted
(vinylidene), trisubstituted, and tetrasubstituted ole ns. Figure 5.3 shows their structure and the pos-
sible mechanism of their formation. Polyisobutylenes of structures I and II result from the loss of a
proton from carbon 1 and carbon 3 of the intermediate of structure V. Polyisobutylenes of structures
III and IV result from the rearrangement of the initially formed carbocation, as shown in Figure 5.3.
The reactivity of these ole ns toward phenol and maleic anhydride varies. In general, the more sub-
stituted the ole n, the lower the reactivity, which is a consequence of the steric factors. Similarly,
the larger the size of the polyisobutyl pendant group, that is, the higher the molecular weight, the
lower the reactivity. This is due to the dilution effect, which results from low ole n-to-hydrocarbon
ratio. As mentioned earlier, polyisobutylene is the most commonly used ole n. One of the reasons
for its preference is its extensive branching. This makes the derived dispersants to possess excellent
oil solubility, in both nonassociated and associated forms. However, if the hydrocarbon chain in the
dispersant is too small, its lubricant solubility greatly suffers. Because of this, the low-molecular-
weight components in polyisobutylene are not desired. This is despite their higher reactivity. These
must be removed, which is carried out through distillation. Alternatively, one can minimize the
formation of these components by decreasing the amount of the catalyst during polymerization and
by lowering the polymerization reaction temperature.
A new class of dispersants derived from ethylene/α-OCP with an Mn of 300–20,000 has also
been reported, primarily by the Exxon scientists [36,37]. Such dispersants are claimed to have supe-
rior low- and high-temperature viscometrics than those of the polyisobutylene-derived materials.
As mentioned earlier, dispersant polymers are derived from EPRs, styrene–butadiene copoly-
mers, polyacrylates, PMAs, and styrene esters. The ethylene–propylene rubbers are synthesized
by Ziegler–Natta catalysis [38]. The styrene–butadiene rubbers are synthesized through anionic
polymerization [38]. Polyacrylates and PMAs are synthesized through polymerization of the
monomers using free-radical initiators [38]. Styrene esters are made by reacting styrene–maleic
H
3
C
H
3
C
H
2
C
H
2
C
H
3
C
H
3
C
H
3
C
H
3
C
H
3
C
H
3
C
CH
2
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
2
CH
3
Isobutylene
R = polyisobutyl
− H
+
H
+
+
+
+
R
R
FIGURE 5.2 Acid-catalyzed polymerization of isobutylene.
CRC_59645_Ch005.indd 149CRC_59645_Ch005.indd 149 10/31/2008 2:00:40 PM10/31/2008 2:00:40 PM
150 Lubricant Additives: Chemistry and Applications
anhydride copolymer or styrene–maleic anhydride–alkyl acrylate terpolymer with alcohols, usually
in the presence of a protic acid, such as sulfuric or methanesulfonic acid, catalyst. Since complete
esteri cation of the anhydride is hard to achieve, the neutralization of the residual carboxylic acid
anhydride is carried out by alternative means [38–40].
FIGURE 5.3 Polyisobutylene structures and the mode of their formation.
R
CH
3
H
3
C
CH
3
CH
2
Terminal olefin (vinylidene)
I
IV
V
IX VIII III
Trisubstituted olefin
Tetrasubstituted olefin
IV
V
VI VII
Trisubstituted olefin
Tetrasubstituted olefin
Carbocation intermediate
Trisubstituted olefin
R = polyisobutyl
II III
R
H
H
3
C
CH
3 CH
3
CH
3
R
H
3
C
CH
3
CH
3
CH
3
H
R
H
R
H
H
H
H
H
1
1
2
3
4
H
3
C
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
C
+
+
+
+
R
CH
3
CH
3
H
H
3
C
CH
3
H
4
3
2
1
1
+
+
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
2
2
2
3
4
4
3
1
CH
3
1
1
1
3
4
R
1
1
1
2
4
3
R
H
R
H
H
H
H
1
1
3
2
2
3
1
H
4
4
C
3
to C
2
hydride
transfer
C
2
to C
3
methide
transfer
C
4
to C
3
methide
transfer
C
3
to C
4
hydride
transfer
C
3
proton
loss
R
H
CH
3
CH
3
CH
3
CH
3
CH
3
H
3
C
H
H
3
C
H
3
C
R
H
Not shown
by arrow
C
2
proton
loss
R
H
3
C
CH
3
CH
3
CH
3
H
CRC_59645_Ch005.indd 150CRC_59645_Ch005.indd 150 10/31/2008 2:00:41 PM10/31/2008 2:00:41 PM
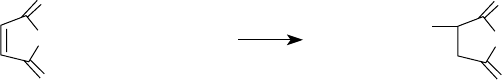
Dispersants 151
5.6.2 THE CONNECTING GROUP
As mentioned in Section 5.5, succinimide, phenol, and phosphonate are the common connecting
groups used to make dispersants. Of these, succinimide and phenol are the most prevalent [2].
Succinimide group results when a cyclic carboxylic acid anhydride is reacted with a primary amino
group. Alkenylsuccinic anhydride is the precursor for introducing the succinimide connecting
group in dispersants. Alkenylsuccinic anhydride is synthesized by reacting an ole n, such as
polyisobutylene, with maleic anhydride [2]. This is shown in Figure 5.4.
The reaction is carried out either thermally [29,41,42] or in the presence of chlorine [43]. The
thermal process involves heating the two reactants together usually >200°C [29,41,42], whereas the
chlorine-mediated reaction with a mixture is carried out by introducing chlorine to react contain-
ing polyisobutylene and maleic anhydride [43–48]. Depending on the manner in which chlorine is
added, the procedure is either one-step or two-step [44]. If chlorine is rst reacted with polyisobu-
tylene before adding maleic anhydride, the procedure is considered two-step. If chlorine is added
to a mixture of polyisobutylene and maleic anhydride, it is a one-step procedure. The one-step
procedure is generally preferred.
The chlorine-mediated process has several advantages, which include having a low reaction
temperature, having a faster reaction rate, and working well with internalized or highly substi-
tuted ole ns. The low reaction temperature minimizes the chances of thermal breakdown of poly-
isobutylene and saves energy. The major drawback of the chlorine process is that the resulting
dispersants contain residual chlorine as organic chlorides. Their presence in the environment is
becoming a concern because they can lead to the formation of carcinogenic dioxins. A number
of strategies are reported in the literature to decrease the chlorine content in dispersants [49–54].
The thermal process does not suffer from the presence of chlorine, although it is less energy-ef -
cient and requires the use of predominantly a terminal ole n, that is, the polyisobutylene of high
vinylidene content.
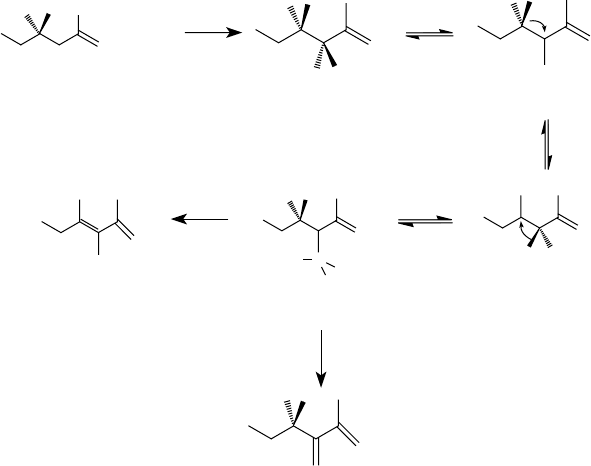
The mechanism by which the two processes proceed is also different [46,47,50–52]. The ther-
mal process is postulated to occur through an ene reaction. The chlorine-mediated reaction is postu-
lated to proceed through a Diels–Alder reaction. The mechanism of the diene formation is shown in
Figure 5.5. Chlorine rst reacts with polyisobutylene 1 to form allylic chloride II. By the loss of the
chloride radical, this yields the intermediate III, which through C
4
to C
3
methyl radical transfer is
converted into the intermediate IV. A C
3
to C
4
hydrogen shift in the intermediate results in the for-
mation of the radical V. This radical can lose hydrogen either from C
4
to yield the diene VI or from
C
5
to result in the diene VII. The resulting dienes then react with maleic anhydride through a 4 + 2
addition reaction, commonly called a Diels–Alder reaction [55], to form alkenyltetrahydrophthalic
anhydrides [50,52]. These reactions are shown in Figure 5.6.
These anhydrides can be converted into phthalic anhydrides through dehydrogenation by
using sulfur [50–52]. These compounds can then be transformed into dispersants by reacting with
polyamines and polyhydric alcohols [51,52]. During the thermal reaction of polyisobutylene with
maleic anhydride, that is, the ene reaction, the vinylidene double bond moves down the chain to
the next carbon. Since thermal reaction requires a terminal ole n, further reaction of the new
ole n with another mole of maleic anhydride will not occur if the double bond internalizes, and
+
O
O
O
O
O
O
Polyisobutylene
Polyisobutenyl
Maleic anhydride
Polyisobutenylsuccinic
anhydride
Heat
or CI
2
FIGURE 5.4 Alkenylsuccinic anhydride formation.
CRC_59645_Ch005.indd 151CRC_59645_Ch005.indd 151 10/31/2008 2:00:41 PM10/31/2008 2:00:41 PM
152 Lubricant Additives: Chemistry and Applications
the reaction will stop at this stage. This is shown in reaction 5.3 of Figure 5.6. If the new double
bond is external, the reaction with another molecule of maleic anhydride is possible [45]. This is
shown in reaction 5.4.
For dispersants, polyisobutylphenol is the alkylphenol of choice. It is synthesized by reacting
polyisobutylenes with phenol in the presence of an acid catalyst [56–58]. Lewis acid catalysts, such
as aluminum chloride and boron tri uoride, are often employed. Boron tri uoride is preferred over
aluminum chloride because the reaction can be carried out at low temperatures, which minimizes
acid-mediated breakdown of polyisobutylene [58]. This is desired because dispersants derived from
low-molecular-weight phenols are not very effective. Other catalysts, such as sulfuric acid, meth-
anesulfonic acid, and porous acid catalysts of Amberlyst
®
type, can also be used to make alkylphe-
nols [59,60]. Polyisobutylene also reacts with phosphorus pentasul de through an ene reaction, as
described in Chapter 4. The resulting adduct is hydrolyzed by the use of steam to alkenylphosphonic
and alkenylthiophosphonic acids [2,3]. The methods to synthesize alkylphenols and alkenylphos-
phonic acids are shown in Figure 5.7.
A new carboxylate moiety derived from glyoxylic acid to make dispersants has been reported in
the literature [61–65]. However, at present, no commercial products appear to be based on this
chemistry.
5.6.3 THE POLAR MOIETY
The two common polar moieties in dispersants are based on polyamines and polyhydric alco-
hols. The structures of common amines and alcohols used to make dispersants are shown in
Figure 5.8.
The polyamines are manufactured from ethylene through chlorination, followed by the
reaction with ammonia [66]. The reaction scheme is given in Figure 5.9. As shown, polyamines
R
4
3
2
H
3
C
H
3
C
H
3
C
H
3
C
CH
3
CH
3
CH
3
CH
3
H
3
C
CH
3
CH
2
CH
2
CH
3
CH
3
CH
3
CH
2
CH
2
CH
3
CH
2
CH
3
CH
3
CH
3
CH
3
CH
2
CH
3
CH
3
CH
3
CH
2
1
R
4
3
2
1
I
VI
+ CI
2
−CI
•
−H
•
−H
•
(From C
5
)
CI H
II
R
4
1
3
2
H
•
III
•
transfe
r
H
•
transfer
R
4
3
2
1
(From C
4
)
H
3
4
2
1
C
H
H
H
•
5
V
VII
IV
R
R
4
3
2
1
H
•
H
R
4
3
2
1
FIGURE 5.5 Mechanism of chlorine-assisted diene formation.
CRC_59645_Ch005.indd 152CRC_59645_Ch005.indd 152 10/31/2008 2:00:41 PM10/31/2008 2:00:41 PM
Dispersants 153
contain piperazines as a by-product. Examining the structures of various amines, one can see
that they contain primary, secondary, and tertiary amino groups. Each type of amino group has
different reactivity toward alkenylsuccinic anhydride. The primary amino group reacts with
the anhydride to form a cyclic imide, the secondary amino group reacts with the anhydride to
form an amide/carboxylic acid, and the tertiary amino group does not react with the anhydride
at all [67].
However, it can make a salt if a free carboxylic acid functionality is present in the molecule,
as is the case in amide/carboxylic acid. These reactions are shown in Figure 5.10. New high-
molecular-weight amines derived from phosphoric acid–catalyzed condensation of polyhydroxy
compounds, such as pentaerythritol, and polyalkylene polyamines, such as triethylenetetramine,
are known [68]. These amines are claimed to form high total base number (TBN) dispersants with
Diels−Alder reaction
Ene reaction
O
H
H
O
O
H
3
C
R
H
H
3
C
H
3
C
R
H
H
3
C
CH
2
CH
2
O
O
O
H
3
C
H
3
C
CH
2
C
O
R
O
O
CH
3
O
H
3
C
H
3
C
CH
3
H
H
O
O
R
R
H
3
C
O
CH
2
H
3
C CH
3
O
O
H
H
R
H
3
C
H
O
O
O
CH
3
H
3
C
C
H
R
H
O
O
O
CH
2
H
3
C
H
3
C
H
H
H
H
C
H
H
O
R
H
O
O
H
3
C
H
3
C
(5.1)
(5.2)
(5.3)
(5.4)
FIGURE 5.6 Mechanism of alkenylsuccinic anhydride formation.
CRC_59645_Ch005.indd 153CRC_59645_Ch005.indd 153 10/31/2008 2:00:42 PM10/31/2008 2:00:42 PM
