Potter T.D., Colman B.R. (co-chief editors). The handbook of weather, climate, and water: dynamics, climate physical meteorology, weather systems, and measurements
Подождите немного. Документ загружается.

CCN due to human activities in the United States has been estimated at about 14%
of that from natural sources. Cloud and fog droplets sampled over land frequently
contain residue of combustion products and organic material, often of biogenic
origin. Forest fires and sugar cane burns, for example, produce large concentrations
of CCN. Studies of subequatorial and Saharan air over West Africa suggest that CCN
are produced from bush fire smoke, bacterial decomposition of plants and associated
sulfur-containing gases, and emission of droplets rich in soluble substances by
plants.
The surface of a pure water droplet consists of a layer of water molecules, each
with the potential to evaporate and enter the humid air above the surface. Vapor
molecules within the humid air also collide with and enter the drop. When a non-
volatile solute is present in the droplet, solute molecules or ions occupy some sites
on the droplet surface. Thus, fewer water molecules are available to evaporate into
the air. However, vapor molecules can enter the solution at the same rate as before.
Equilibrium can only be established when the vapor pressure decreases over the
solution so that the rate that water molecules leave the solution equals the rate at
which they enter the solution from the air. Raoult’s law,
e
0
r
e
r
¼
n
0
n
s
þ n
0
¼
4
3
pr
3
eq
r
w
m
s
=M
w
4
3
pr
3
eq
r
w
m
s
=M
w
þ im
s
=M
s
¼ 1 þ
im
s
M
w
M
s
4
3
pr
3
eq
r
w
m
s
"#
1
ð12Þ
which can be derived from principles of equilibrium thermodynamics, describes this
process formally. Raoult’s law states that the vapor pressure over a solution droplet
(e
0
r
) is reduced from that over a pure water dropl et (e
r
) by an amount equal to the
mole fract ion of the solvent. In (12), n
0
is the number of molecules of water in the
droplet, n
s
the number of molecules of solute, m
s
is the mass of the solute, and M
s
is
the molecular weight of the solute. In nature, soluble p articles are typically salts or
other chemicals that dissociate into ions when they dissolve. For solutions in which
the dissolved molecules dissociate, the number of moles of solute, n
s
, must be
multiplied by the factor i, the degree of ionic dissociation. Unfortunately, the
factor i, called the van’t Hoff factor, has not been determined for many substances.
Other quantities such as the rational activity coefficient, the mean activity coeffi-
cient, and the molal or practical osmotic coefficient have been used as alternate
expressions to the van’t Hoff factor and are tabulated in many chemical reference
books (Pruppacher and Klett, 1997).
The Kelvin equation (8) describes the equilibrium vapor pressure over a curved
water surface, e
r
, and Raoult’s law (12) describes the reduction in vapor pressure
e
0
r
=e
r
over a solution droplet. Multiplying (8) by (12) to eliminate e
r
, gives
S
s;v
¼
e
0
r
e
s;1
¼ 1 þ
im
s
M
w
M
s
ð
4
3
pr
3
eq
r
w
Þm
s
"#
1
exp
2M
w
s
w;v
R
Tr
w
r
eq
!
ð13Þ
266 MICROPHYSICAL PROCESSES IN THE ATMOSPHERE
which describes the equilibrium saturation ratio over a solution droplet. For a suffi-
ciently dilute solution, this equation can be simplified by approximating e
x
1 þ x
and (1 þ y)
1
1 7 y. Ignoring the small product x y, and m
s
compared to the
mass of water, one obtains
S
s;v
¼ 1 þ
a
r
eq
b
r
3
eq
ð14Þ
where a ¼2M
w
s
w,v
=R
Tr
w
¼3.3 10
5
=T, b ¼3im
s
M
w
=4pM
s
r
w
¼4.3im
s
=M
s
in
cgs units with T in kelvins. Equations (13) and (14) are forms of the Ko¨hler equation,
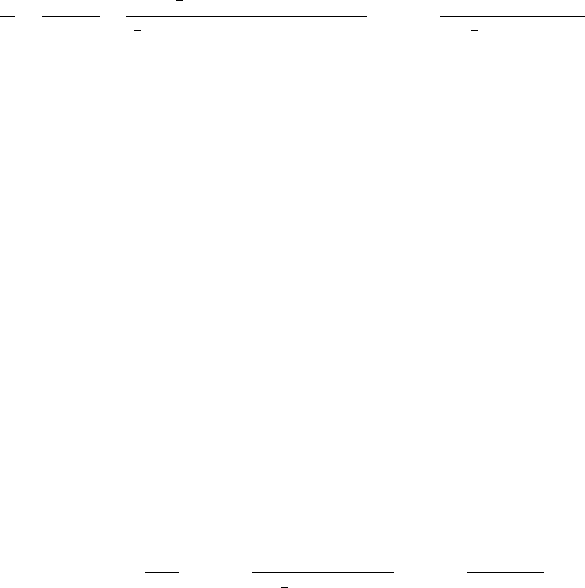
first derived by the Swedish meteorologist H. Ko¨hler in the 1920s. Curves 2 to 6 in
Figure 6 show solutions of the Ko¨hler equation for droplets containing fixed masses
of NaCl and NH
4
SO
4
, common components of CCN. Curve 1 shows the solution for
a pure droplet, the Kelvin equation. For a given S
s,v
, droplets on the left side of the
maximum in the Ko¨hler curves are in stable equilibrium. If a droplet in equilibrium
on the left side of the curve experiences a small increase in radius due to chance
collection of vapor molecules, the droplet would find the vapor pressure around itself
less than that required for equilibrium at its new radius. Physically, less water
molecules would be striking the droplet from the vapor field than would be evapor-
ating from the droplet. As a result, the droplet would shrink, returning to its position
on the equilibrium curve. The opposite would happen if the droplet lost water
molecules—it would grow back to its equilibrium size. Note that when S
s,v
1,
all droplets growing from CCN remain on the left side of the curves. The small
droplets in stable equilibrium on the left side of the curve are called haze droplets.
Over some cities, where soluble particles are abundant and relative humidities high,
haze droplets can severely restrict visibility.
Figure 6 Variations of the relative humidity and supersaturation of air adjacent to droplets of
(1) pure water and solution droplets containing the following fixed masses of salt: (2) 10
19
kg
of NaCl, (3) 10
18
kg of NaCl, (4) 10
17
kg of NaCl, (5) 10
19
kg of (NH
4
)
2
SO
4
, and (6)
10
18
kg of (NH
4
)
2
SO
4
(Wallace and Hobbs, 1977).
3 FORMATION OF INDIVIDUAL CLOUD DROPLETS AND ICE CRYSTALS 267
A droplet at the peak of a curve, gaining a few molecules by random collisions,
would find the vapor pressure around it greater than that required for equilibrium.
More molecules would strike the droplet than evaporate. Vapor would rapidly
deposit on the dropl et and it would quickly grow into cloud droplet. At the peak,
and on the right side of the curves, droplets are in unstable equilibrium. Droplets that
reach the peak in their Ko¨hler curves are said to be activated. Such droplets are
called cloud droplets. The peak of a curve (the critical radius) is the transition point
between the effect of the solute and drop curvature. When the supersaturation
(S
s,v
7 1) in the atmosphere exceeds a critical value, droplets containing solute of
a critical mass will rapidly grow from haze droplets into cloud droplets. As indicated
in Figure 6, larger nuclei, which produce stronger solution droplets, are much
more likely to become cloud droplets because they require lower supersaturation
to activate.
Homogeneous Nucleation of Ice Crystals in Humid Air
Equations analogous to (8) and (10) can be derived for the homogeneous nucleation
of ice in humid air by assuming that the embryonic ice particle is spherical. In (8),
for example, S
w,v
, s
w,v
, and r
w
are replaced by S
i,v
¼e
r,i
=e
si, 1
, the saturation ratio
with respect to ice, s
i,v
, the surface tension at the ice surface, and r
i
, the density of
ice. Numerical evaluation of the Kelvin equation for ice embryos shows that the
required supersaturations exceed those for water droplet nucleation. Calculations of J
for ice nucleation and the Kelvin equation both show that this process does not occur
in the atmosphere.
Homogeneous Nucleation of Ice Particles in Supercooled
Water Droplets
Homogeneous nucleation of ice in a water droplet requires that a stable icelike
molecular structure form within the droplet through statistical fluctuations in the
arrangement of the water molecules. The development of such an ice embr yo is
favored compared to homogeneous nucleation of ice in humid air because the water
molecules in the droplet will be in direct contact with any icelike molecular struc-
tures created through statistical fluctuations. Unlike homogeneous nucleation of ice
in moist air, the process of homogeneous nucleation in water involves two energy
barriers. The first, DF
i
, is associated with the increase in free energy at the ice
crystal–liquid surface interface. The second, DF
0
, exists because energy is required
to break the bonds between individual water molecules before they can realign
themselves to join the ice embryo. The nucleation rate of ice in water therefore
depends on the number of liquid molecules per unit volume per unit time that will
contact an ice structure of critical size, the probability that an icelike structure will
exist in a droplet, and the probability that one of these liquid molecules will over-
268 MICROPHYSICAL PROCESSES IN THE ATMOSPHERE
come the energy barrier and become free to attach to the ice structure. The rate
equation becomes
J ¼ 2N
c
r
w
kT
r
i
h
s
w;i
kT
1=2
exp
DF
0
R
T
þ
DF
i
kT
ð15Þ
where h is Planck’s const ant, s
w;i
is the interface energy per unit area between the ice
surface and the water, and N
c
is the number of water molecules in contact with a unit
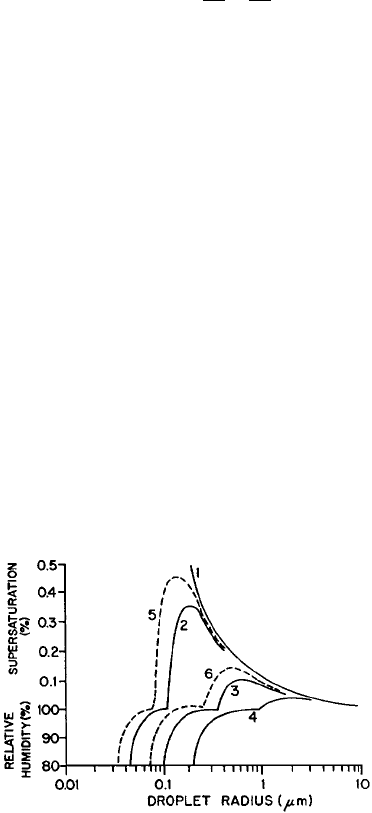
surface area of ice. Figure 7 shows measurements of J from several experiments. J
increases from about 1 to 1020 cm
3
=s as the temperat ure decreases from 32 to
40
C. Until recently, insufficient information was available on the properties of
supercooled water and on DF
0
. Pr uppacher (1995) showed that incorrect extrapola-
tions of data for these properties to large supercoolings, and a lack of understanding
of the behavior of water molecules at large supercooli ngs led to the disagreement
between J calculated from classical theory and experimental data (Fig. 7).
Pruppacher (1995) resolved the discrepancies between the classical nucleation
equation, laboratory data, and the results of an earlier molecular theory. Pruppacher
noted that because water molecules become increasingly bonded at colder tempera-
tures, DF
0
might be expected to increase with decreasing temperature. However,
earlier cloud chamber experiments found that DF
0
decreases sharply at temperatures
Figure 7 Variation of the rate of homogeneous nucleation in supercooled water. Data are
from different experiments. The solid line (1) is from early classical theory. The solid line (2)
is from the revision of classical theory discussed by Pruppacher (1995). The dashed line is
from molecular theory (Pruppacher, 1995).
3 FORMATION OF INDIVIDUAL CLOUD DROPLETS AND ICE CRYSTALS 269
colder than 32
C. Pruppacher used nucleation rates measured in laboratory
experiments and inferred from field observations, and recent measurements of the
physical properties of supercooled water, to calculate DF
0
and showed using the
classical nucleation equation that DF
0
indeed decreases at temperatures colder than
32
C. This behavior was attributed to the transfer of clusters of water molecules,
rather than individual water molecules, across the ice–water interface. With clusters,
the only hydrogen bonds that must be broken are those at the periphery of the cluster.
Pruppacher (1995) summarized the experiments of many investigators to deter-
mine the homogeneous nucleation temperature threshold for ice in water droplets.
He reasoned that (1) the larger the volume of a droplet, the larger is the probability of
a density fluctuation in the droplet and the larger the probability that an ice embryo
will be produced and (2) the probability for ice formation in a given sized droplet
increases with increasing time of exposure of the droplet to a given range of
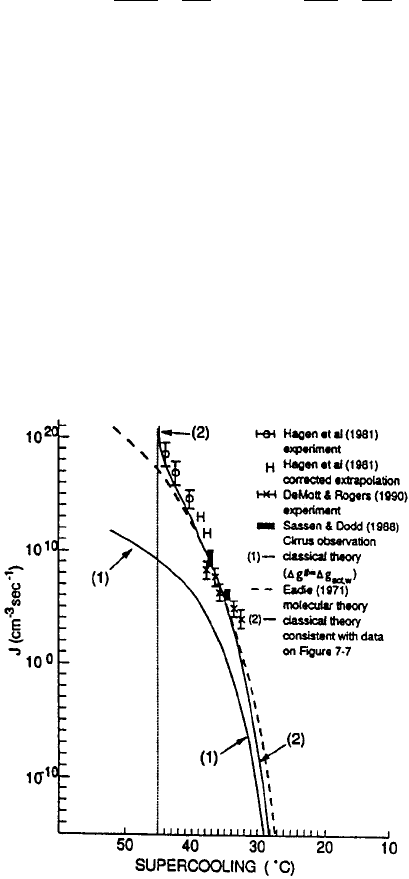
temperatures. Reexamining the available experimental data, he showed that the
lowest temperature at which virtually all pure water droplets froze was a function
of droplet diameter, with the spread in the data attributable to the different tech-
niques used in the experiments to support the drops (Fig. 8). Figure 8 shows that
freezing occurs at 40
C for 1-mm droplets and 35
C for 100-mm droplets.
Pruppacher’s results apply to cloud droplets, which are nearly pure water droplets,
their solute concentrations typically less than 10
3
mol=L.
Unactivated haze droplets consist of much stronger solutions whose chemical
consistencies depend on the parent CCN. Recent interest in understanding the
importance of polar stratospheric clouds in ozone depletion and the role of cirrus
in climate change has fueled investigations of the homogeneous nucleation of haze
droplets. The homogeneous nucleation tem perature in strong solution haze droplets
Figure 8 Lowest temperature to which extra pure water drops of a given size and exposed to
cooling rates between 1
C=min and 1
C=s have been cooled in various laboratory experi-
ments, indicated by different letters. Lines 1 and 2: Temperature at which 99.99% of a
population of uniform-sized water drops freezes when exposed to cooling rates of 1
C=min
and 1
C=s, respectively (Pruppacher, 1995).
270
MICROPHYSICAL PROCESSES IN THE ATMOSPHERE
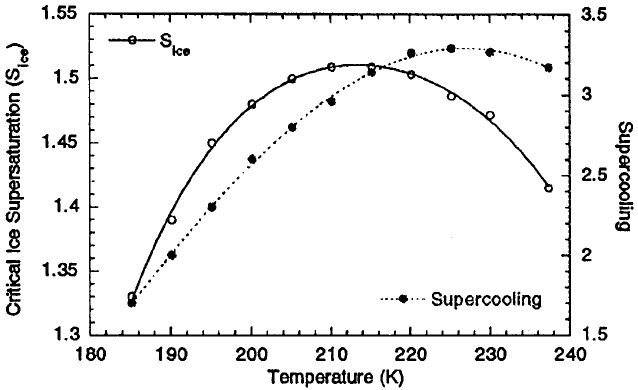
is depressed in proportion (nonlinearly) with increased solution molality. The equi-
librium size of a solution haze droplet increases and solution molality decreases with
increasing ambient relative humidity. As a result, the homogeneous nucleation
temperature is depressed most at low relative humidities. Two factors of interest
are the ice supers aturation where homogeneous nucleation occurs and the extent to
which a sulfate solution can be supercooled above ice saturation over the solution
drop before ice nucleation is possible. Figure 9 shows calculations of homogeneous
nucleation of droplets con taining sulfate aerosols made by Tabazadeh and Jensen
(1997). Their results, which correspond to J ¼1cm
3
=s, show that supercoolings of
about 3 K (below the equilibrium freezing temperature of a strong H
2
SO
4
solution)
and ice supersaturation between 40 and 50% are required for homogeneous nuclea -
tion at ambient temperatures between 33 and 63
C (240 and 210 K). These
conditions can exist in upper tropospheric clouds, suggesting that the high ice
particle concentrations observed in some cirrus clouds are due to homogeneous
nucleation of haze droplets.
Heterogeneous Nucleation of Ice Crystals
Ice particles form in the atmosphere through homogeneous nucleation of super-
cooled water droplets, heterogeneous nucleation processes that involves aerosol
particles called ice nuclei (IN), and shattering of existing ice particles during
Figure 9 Critical ice supersaturation and the extent of supercooling required to achieve a
nucleation rate J ¼1cm
3
=s for strong H
2
SO
4
solution droplets. The ice supersaturation is
defined as the ratio of the ambient water vapor pressure over the ice saturation vapor pressure
when J ¼1cm
3
=s. Supercooling is defined as the critical nucleation temperature minus the
temperature of ice that has a vapor pressure equal to the ambient water vapor pressure
(Tabazadeh and Jensen, 1997).
3 FORMATION OF INDIVIDUAL CLOUD DROPLETS AND ICE CRYSTALS 271
collisions or droplet freezin g events. Homogeneous nucleation of ice particles in
supercooled water is limited to temperatures colder than about 33
C. At warmer
temperatures, primary ice particle formation requires ice nuclei.
Most ice nuclei are composed of clay particles such as vermiculite, kaolinite, and
illite and enter the atmosphere during wind erosion of soils. Combustion, volcanic
eruptions, and airborne microorganisms are also sources of IN. Ice nuclei funct ion in
four modes: (1) deposition or sorption-nuclei adsorb water vapor directly on thei r
surfaces to form ice; (2) condensation-freezing nuclei act first as CCN to form drops
and then as IN to freeze the drops; (3) immersion nuclei become incorporated into a
drop at T > 0
C and act to initiate freezing after the drop has been transported into a
colder region; and (4) contact nuclei initiate freezing of a supercooled drop on
contact with the drop surface. The fact that IN can function in these different
modes has made their measurement difficult. Instruments designed to measure IN,
which include rapid and slow expansion chambers, mixing chambers, thermal preci-
pitation devices, membrane filters, and other devices, typically create conditions
favoring one mode, and often only measure the dependence of IN concentration
on one variable, such as temperature [see reviews by Vali (1985) and Beard (1992)].
In addition, the time scales over which ice nucleation can occur in natural clouds
often differs substantially from those characterizing the measurements. Measure-
ments made with different instruments at workshops in controlled conditions with
air samples drawn from the same source have shown considerable scatter. Absolute
values of concentrations of ice nuclei should therefore be viewed with some caution.
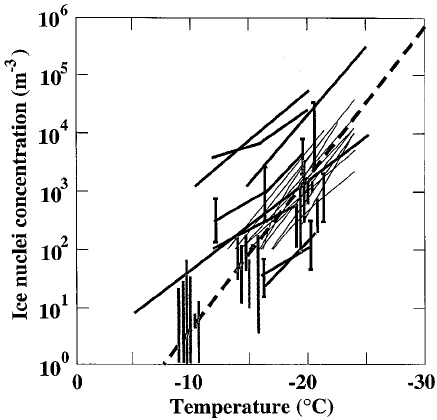
The concentration of ice nuclei in the atmosphere is highly variable (Fig. 10). In
general, ice nuclei concentrations increase by an order of magnitude with each 4
C
decrease in temperature but can vary by nearly an order of magnitude at any
temperature. The equation
N
IN
¼ A expðb DTÞð16Þ
where A ¼10
5
per liter, b ¼0.6=
C, N
IN
the number of active ice nuclei per liter
active at temperatures warmer than T and DT ¼T
0
7 T is the supercooling in degrees
centigrade, reasonably approximates this behavior. Ice nucleation can occur at rela-
tive humidities below water saturation, provided that the air is supersaturated with
respect to ice. Experiments have shown that at a given temperature, N
IN
increases
with increasing supersaturation with respect to ice (s
i
) according to the relationship
N
IN
¼ C
k
s
i
ð17Þ
where the values of C and k depend o n the air mass. The concentration of IN can
undergo orders of magnitude variations at a single location from day to day. Expla-
nations forwarded for these rapid changes include advection of dust from desert
windstorms, downward transport of stratospheric IN created from meteor bombard-
ment, IN production from evaporation of cloud and precipitation particles, and
preactivation of IN in the cold upper troposphere followed by transport to the
surface. Studies of the vertical profile of IN concentrations have found that
272 MICROPHYSICAL PROCESSES IN THE ATMOSPHERE
concentrations generally decrease with height in the troposphere above the surface
mixed layer, although evidence exists for higher concentrations of IN in the vicinity
of the jet stream.
Because ice nuclei must provide a stable solid substrate during the growth of ice
embryos, they are normally water insoluble. Nucleation occurs when an ice crystal
with a specific lattice structure first forms o n a substrate that has a different lattice
structure. The probability of an ice nucleation event increases when the lattices of
atoms composing the ice crystal and ice nuclei closely align. For the IN atoms and
ice atoms to bond, a strain in the bonds must be accommodated between the out-of-
place atoms in the IN and ice lattices. The surface free energy of the interface
increases in response to increased elastic deformation. The greater the lattice
mismatch, the higher the surface free energy and the less likely the ice crystal
will form. Particle surfaces normally contain con taminants, cracks, crevices, and
particular growth patterns and may possess specific electrical properties due to
ions or polar molecules. Certain of these locations are much more effective at
adsorbing water molecules onto the surface of the aerosol and enhance the nucleat-
ing capability of the substance. Since the water molecule is polar, and the ice lattice
is held together by hydrogen bonding, substances that exhibit hydrogen bonds on
their surfaces act as more effective nucleants. For this reason, some organics exhibit
strong ice nucleating behavior. Ice nuclei are large aerosols, typically having radii
larger than 0.1 mm.
Figure 10 Mean or median worldwide measurements of ice nuclei concentrations from Bigg
and Stevenson (1970) (vertical gray bars), compilations of 11 studies by various authors in
Go¨tz et al. (1992) (thick lines), and compilations of 10 additional studies by Pruppacher and
Klett (1997) (thin lines). Bigg and Stevenson’s data at 10, 15 and 20
C are spread over a
small temperature range for clarity. The heavy dashed line is N
IN
¼10
5
exp(0.6DT).
3 FORMATION OF INDIVIDUAL CLOUD DROPLETS AND ICE CRYSTALS 273
The theory governing the nucleation rate, J, of an ice embryo in a saturated vapor,
or of an ice embryo in a supercooled water drop, proceeds in a similar way to the
theory concerning heterogeneous nucleation of a drop. The theory assumes an ice
embryo forms a spherical cap with a contact angle with the surface substrate.
Hydrophobic substances have large contact angles and act as poor ice nucleants.
The equations are analogous to those used for nucleation of a water droplet, except
that all terms applying to water and vapor now apply to ice and vapor, or ice and
water. A discussion of this theory, and more complicated extensions of the classical
theory, is presented by Pruppacher and Klett (1997). The theory predicts that at
5
C, particles with radii smaller than 0.035 mm and a contact angle of 0 will not be
effective as ice nuclei. The threshold is 0.0092 mmat20
C. Few if any particles
will exhibit contact angles of zero, so actual ice nuclei will have to be somewhat
larger than these values.
Inside a water droplet, nuclei sizes can be somewhat smaller, with the threshold at
least 0.010 and 0.0024 mmat5 and 20
C, respectively. Experiments have shown
that the exact value of the cutoff is also dependent on the chemical composition of
the particle and on its mode of action (deposition, freezing, or contact). In the case of
deposition nuclei, it also depends on the level of supersaturation with respec t to ice.
Some organic chemicals have been found to have somewhat smaller sizes and still
act as ice nuclei.
There is considerable experimental evidence showing that atmospheric ice nuclei
can be preactivated. Preactivation describes a process where an ice nucleus initiates
the growth of an ice crystal, is subjected to an environment where complete subli-
mation occurs, and then is involved in another nucleation event. The particle is said
to be preactivated if the second nucleation event occurs at a significantly warmer
temperature or lower supersaturation. Ice nuclei can also be deactivated, that is lose
their ice nucleating ability. This effect is due to adsorption of certain gases on to the
surface of the nucleus. Pollutants such as NO
2
,SO
2
, and NH
3
have been found to
decrease the nucleation ability of certain aerosols. There has also been evidence
from laboratory and field experiments that the nucleating ability of silver iodide
particles decreases when the aerosols are exposed to sunlight.
The very poor correspondence between ice nucleus measurements and ice particle
concentrations in clouds has yet to be adequately explained. Hypotheses forwarded
to explain these observations generally focus on more effective contact nucleation,
particularly in mixed regions of clouds where evaporation can lead to the formation
of giant ice nuclei, and secondary ice particle production, which involves shattering
of existing ice particles during collisions or droplet freezing events. The relative
importance o f each of these mechanisms in real clouds is still uncertain.
4 FORMATION OF RAIN IN WARM CLOUDS
Raindrops form through one of two microphysical paths. The first occurs when ice
particles from high, cold regions of clouds fall through the melting level and become
raindrops. In cloud physics literature, clouds that support this process are called
274 MICROPHYSICAL PROCESSES IN THE ATMOSPHERE

‘‘cold’’ clouds. ‘‘Warm’’ clouds are clouds that develop rain in the absence of ice
processes. In the tropics, shallow clouds such as trade wi nd cumulus and stratocu-
mulus produce rain entirely through warm rain processes. Deep tropical convective
clouds and summertime midlatitude convection also support active warm rain
processes, although melting ice also contributes to rainfall.
The warm rain process occurs in three steps: (1) activation of droplets on CCN,
(2) growth by condensation, and (3) growth by collision and coalescence of droplets.
The rapid production of warm rain in both maritime and continental clouds remains
one of the major unsolved problems in cloud physics. The centr al problem lies in the
transition from step 2 to 3. Theory predicts that g rowth by condensation will create
narrow drop spectra in clouds. Growth by coalescence, on the other hand, requires
large and small cloud droplets within droplet spectra. Determining how broad
droplet spectra develop in clouds has been a central theme of cloud physics research.
Growth of a Single Droplet by Condensation
Cloud droplets form when the supersaturation in the atmosphere becomes suffi-
ciently large so that haze droplets reach their critical radii a nd begin to grow in
the unstable regime to the right of the Ko¨hler curves (Fig. 6). The equation for the
growth of a pure water droplet, first derived by Maxwell in 1890, is obtained by
assuming that (1) heat transfer and vapor concentration in the vicinity of the droplet
both satisfy the diffusion equation, (2) an energy balance exists at the surface of the
droplet such that the latent heat added during condensation balances the diffusion of
heat away from the droplet, and that (3) the concentration of droplets in the vapor
field is independent of direction outwards from the droplet. Modifications must be
made to account for curvature and solute effects. Additionally, one must account for
kinetic effects near the drop surface. These include vapor mol ecules striking the
surface of the droplet and rebounding rathe r than condensing, and the direct heat
transfer that occurs at the droplet–vapor interface as water molecules cross the
interface between the liquid and gas phases.
Accounting for these effects, the equation describing the diffusional growth of a
droplet is
r
dr
dt
¼
S
w;v
1 a=r þ b=r
3
ððL
v
=R
v
TÞ1ÞðL
v
r
w
=KT
2
f ðaÞÞ þ ðr
w
R
v
T=De
s;1
gðbÞÞ
ð18Þ
where a and b are defined in Eq. (14), L
v
is the latent heat of vaporization, R
v
is the
gas constant for water vapor, D is the diffusion coefficient, and K, the thermal
conductivity. The quantities f (a) and g(b) are given by:
f ðaÞ¼
r
r þ½ðK=apÞð2pR
d
TÞ
1=2
=ðC
v
þðR
d
=2ÞÞ
¼
r
r þ l
a
ð19Þ
gðbÞ¼
r
r þðD=bÞð2p=R
v
TÞ
1=2
¼
r
r þ l
b
ð20Þ
4 FORMATION OF RAIN IN WARM CLOUDS 275
