Potter T.D., Colman B.R. (co-chief editors). The handbook of weather, climate, and water: dynamics, climate physical meteorology, weather systems, and measurements
Подождите немного. Документ загружается.


Growth by Coalescence Two methods have been used for calculating growth
rates of cloud droplets by coalescence. The first, called continuous collection, is used
to calculate the growth of single drops that pass through a cloud of smaller droplets.
These single drops are assumed not to interact with drops of similar size so as to lose
their identity. The continuous collection equation is
dM
dt
¼
X
KðR; rÞw
L
ðrÞDr ð23Þ
where M is the mass of the drop, K is the collection kernel, and w
L
is the liquid water
content per unit size inter val Dr. The collection kernel, the volume of air swept out
per unit time by the larger drop, is given by K ¼ pEðR þ rÞ
2
½V
T
ðRÞV
T
ðrÞ, and
w
L
¼ nðrÞ4pr
3
r
w
=3, where E is the collection efficiency, V
T
(R) and V
T
(r) are the
terminal velocities of the large and small drops, r
w
is the liquid water content, and n
is the small droplet concent ration.
Calculations show that small raindrops can form in about 20 to 30 min provided
coalescence nuclei (R > 50 mm) are present in the c loud and the smaller cloud
droplets have radii of at least 7 mm. Szumowski et al. (1999) applied the continuous
growth equation in marine cumulus using a Lagrangian drop-growth trajectory
model in wind fields derived from dual-Doppler radar analyses. The evolution of
the radar-observed clouds in this study provided a strong constraint on the growth of
raindrops. Raindrops were found to grow to sizes ranging from 1 to 5 mm from
coalescence nuclei in about 15 to 20 min. Their calculations suggest that coalescence
growth is efficient in marine clouds that contain giant and ultragiant sea salt aerosol
and low (100 to 300 cm
3
) droplet concentrations. In continental clouds, which
generally have narrow droplet distributions and high (800 to 1500 cm
3
) droplet
concentrations, continuous growth may take significantly longer.
In nature, the droplets composing a cloud have a continuous distribution of sizes,
and droplets of all sizes have the potential to collide with drops of all other sizes. The
drops of most importance to the precipitation process occupy the large end tail of the
drop size distribution. To consider rain formation, the statistical aspects of interac-
tions between droplets must be considered. There is an element of probability
associated with the collection process, and, occasionally, collisions will occur
between larger droplets. A general method for calculating coalescence growth is
to assess the probability that droplets of one size will collect droplets of another size
for all possible droplet size pairs in the droplet distribution. The term stochastic
collection is applied when the collection probability is used to determine the number
of droplets of a given size, r, that are created by collisions of smaller pairs, r 7Dr
1
and r 7Dr
2
, and destroyed by collisions of r with other droplets. Stochastic collec-
tion considers rare collisions between larger droplets and consequently predicts
accelerated coalescence in warm clouds.
Possible interacti ons between drops include small droplets collecting small
droplets (autoconversion), large drops collecting small drops (accretion), and
large drops collecting large drops (large hydrometeor self-collect ion). Bulk
microphysical cloud models often parameterize these three proces ses. Another
286 MICROPHYSICAL PROCESSES IN THE ATMOSPHERE

outcome is droplet breakup where two drops collide with the result being a number
of smaller drops.
Collisions between large drops will result in a dramatic broadening of the tail of
the distribution and an increase in the size and number of precipitating drops.
However, these drops are in such low concentration that collisions of this type
must be considered a rare event from a statistical point of view. Simulations of
the collection process using stochastic approaches must accurately consider the
most rare events.
The continuous form of the stochastic coalescence equation is given by:
@Nðm; tÞ
@t
¼
1
2
ð
m
0
Nðm
0
; tÞNðm m
0
; tÞKðm
0
; m m
0
Þdm
ð
1
0
Nðm; tÞNðm
0
; tÞKðm; m
0
Þdm
0
ð24Þ
where N is the concentration of drops of mass m at time t and K is the collection
kernel. The first term describes the coalescence of two drops of mass m
0
and m 7 m
0
to form a droplet of mass m. The average concentration of drops of mass m will
increase for every coalescence of two smaller drops of mass es m
0
and m 7 m
0
.For
every two drops ‘‘destroyed’’ one is created, thus the factor of
1
2
. The second term
describes coalescence of drops of mass m with drops of mass m
0
. This ter m repre-
sents the ‘‘destruction’’ of drops of mass m, since any mass m drop that combines
with any other drop will no longer be mass m.
Care must be taken to avoid numerical diffusion when integrating the stochastic
collection equation. Considerable efforts have been made to develop integration
schemes that accurately portray the evolution of the droplet spectra. Berry and
Reinhardt’s (1974) study indicated that the formation of raindrops in warm clouds
can be fairly rapid. In their numerical experiments, initial droplet sizes were speci-
fied using gamma distributions of their masses, with the liquid water content as
1g=m
3
and mean drop sizes ranging from 10 to 18 mm radius (see example in
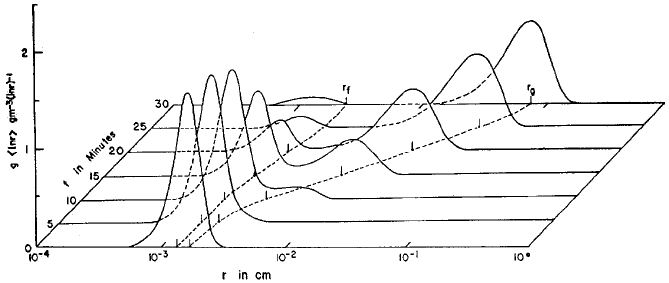
Fig. 18). Drizzle drops were produced in 10 to 22 min and raindrops in 20 to
30 min in different experiments. Faster growth occur red for larger mean initial
sizes. The growth of drops after they reached drizz le drop size was nearly indepen-
dent of the drop spectrum. Berry and Reinhardt’s studies show that growth of rain-
drops in warm clouds can occur by stochastic processes within time scales observed
in nature, provided the initial spectra has sufficiently large mean radii and the spectra
contain droplets with radii of at least 30 mm radius.
5 GROWTH OF ICE PARTICLES IN ATMOSPHERE
Ice crystals form in the atmospher e through either homogeneous or heterogeneous
nucleation. Once formed, ice particles grow by three mechanisms: diffusion of water
vapor, accretion of supercooled droplets, and aggregation with neighboring crystals.
5 GROWTH OF ICE PARTICLES IN ATMOSPHERE 287
Accretion leads to the formation of graupel and hail, while aggregation produces
snowflakes. Ice particles sometimes shatter during growth or evaporation, increasing
particle concentrations. Mechanisms that enhance ice particle concentrations in
clouds in this manner are called secondary ice particle product ion mechanisms.
Diffusional Growth of Ice Particles
The Clausius–Clapeyron equation describes the equilibrium condition between two
phases. By comparing the solution of the Clausius–Clapeyron equation for the cases
of water and vapor, and ice and vapor, it can be easily shown (e.g., Rogers and Yau,
1989; Pruppacher and Klett, 1997) that the saturation vapor pressure over water,
e
s, 1
, will always exceed the saturation vapor pressure over ice, e
si, 1
, for T < 0
C.
This is a consequence of the fact that the latent heat of sublimation, L
s
, is larger than
L
v
. This difference has major implications for the growth of ice in clouds. Air
saturated with respect to ice will always be subsaturat ed with respect to water.
Conversely, air saturated with respect to water will always be supersaturated with
respect to ice. As a consequence, superc ooled water droplets and ice crystals can
never exist in a cloud together in equilibrium. Ice crystals, always subject to greater
supersaturations, will rapidly grow, extracting vapor from the surroundings, while
coexisting supercooled droplets will evaporate, supplying vapor to the surroundings.
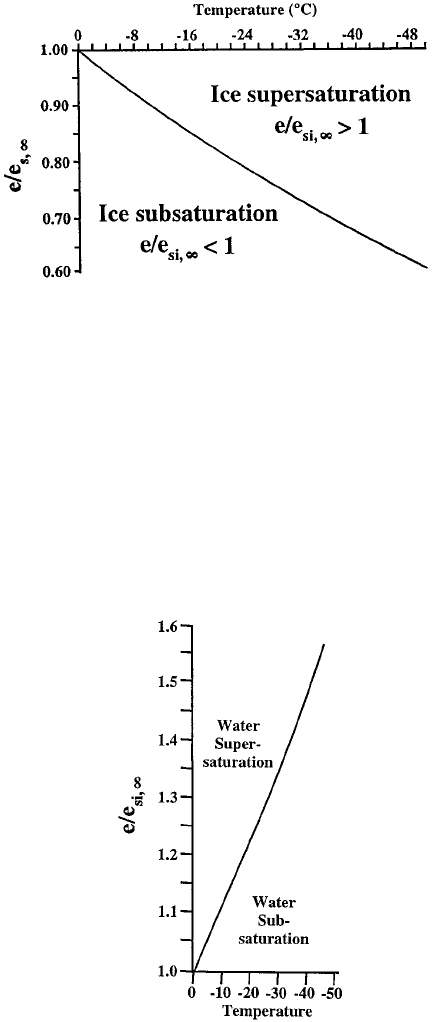
The differences in relative humidity with respect to ice and water as a function of
temperature can be found by taking the ratios e=e
s, 1
and e=e
si, 1
and plotting them
as a function of temperature. Figures 19 and 20 show that, at cold temperatures, air
can be significantly below saturation with respect to water (relative humidity wi th
respect to water, RH
w
65 to 70%) and still be supersaturated with respect to ice.
High in the atmosphere, at cirrus cloud levels, ice crystals can grow at moderate
relative humidities with respect to water. Even at lower altitudes, particularly in
Figure 18 Example of development of a droplet spectrum using stochastic coalescence
(from Berry and Reinhardt, 1974). The radius r
f
denotes the mean mass, which follows the
cloud droplet peak, and r
g
denotes the radius of mean-square mass, which follows the raindrop
peak.
288
MICROPHYSICAL PROCESSES IN THE ATMOSPHERE
wintertime, ice particles can grow at RH
w
< 100%. On the other hand, a cloud that
has a relative humidity with respect to water of 100% will be supersaturated with
respect to ice by a substantial amount. For example, at 10
C, the relative humidity
with respect to ice will be about 112%. At 20
C, it will be 120%. In an environ-
ment where RH
w
¼100% exactly, water d rops will not grow, yet ice crystals in this
same environment will experience enormous supersatura tions. As a consequence,
they grow very rapidly to become large crystals.
Figure 19 Ice saturation as a function of saturation ratio with respect to water (adapted from
Pruppacher and Klett, 1997).
Figure 20 Water saturation as a function of saturation ratio with respect to ice (adapted from
Pruppacher and Klett, 1997).
5 GROWTH OF ICE PARTICLES IN ATMOSPHERE 289
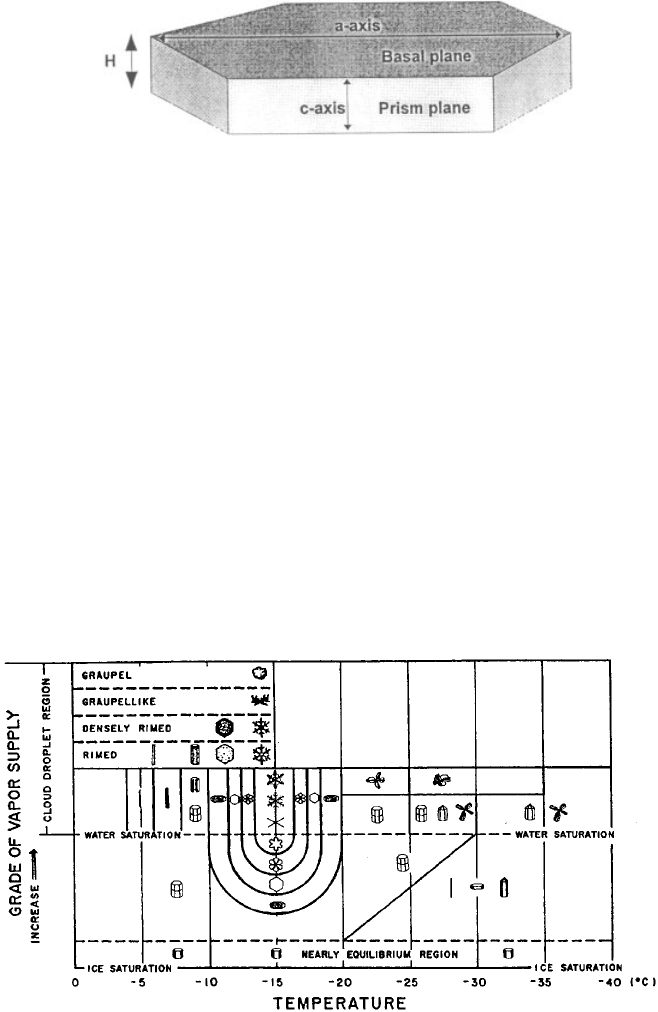
From a crystallographic point of view, ice crystal growth normally occurs on two
planes, the basal and prism planes. The basal plane of an ice crystal refers to the
hexagonal plane illustrated in Figure 21. The axis that passes from the outside edge
of the crystal through the center along the basal plane is called the a axis. The a axis
can be thought of as the diameter of the circle that circumscribes the basal plane. The
prism plane refers to any plane along the vertical axis of the crystal, the c axis. Prism
planes appear rectangular in Figure 21. The height of a crystal, H, is shown in
Figure 21. Growth along the basal plane implies that the c axis wi ll lengthen and
H will become larger. Growth along the prism plane implies that the a axis will
lengthen and the diameter of the crystal will become larger.
Laboratory experiments have shown that the rate of growth along the c axis
relative to growth along the a axis of crystals varies significantly as a function of
temperature and supersaturation. These variations occur in a systematic manner,
resulting in the characteristic shapes, or ‘‘habits,’’ of ice crystals, summarized in
Figure 22. As temperature decreases, the preferred g rowth axis changes from the a
axis (platelike crystals, 0 to 4
C) to the c axis (columnar crystals, 4to9
C), to
the a axis (platelike crystals, 9 to about 22
C), to the c axis (columnar crystals,
Figure 21 Geometry of a simple ice crystal.
Figure 22 Natural ice crystal habits that form when crystals grow in different temperature
and humidity conditions (Magono and Lee, 1966).
290
MICROPHYSICAL PROCESSES IN THE ATMOSPHERE

22
C and colder). The first two trans itions are well defined, while the latter is
diffuse, occurring between about 18 and 22
C. At high supersaturations with
respect to ice (water supersatur ated conditions), the transition as a function of
temperature is, in the same order as above, a plate, a needle, a sheath, a sector, a
dendrite, a sector, and a sheath. The tem peratures of these transitions are evident
from Figure 22. At low supersaturations with respect to ice (below water saturation),
the transition in habit as a function of temperature is from a plate, to a hollow (or
solid) column, to a thin (or thick) plate to a hollow (or solid) column, depending on
the level of saturation. Observations of ice particles in the atmosphere show that the
same basic habits appear in nature, although the structure of the particles is often
more complicated. This can be understood when one considers that the particles fall
through a wide range of temperatures and conditions of saturation.
Ice particles possess complex shapes and, as a result, are more difficult to model
than spherical droplets. However, the diffusional g rowth of simple ice crystals can be
treated in a similar way as drops by making use of an analogy between the governing
equations and boundary conditions for electrostatic and diffusion problems. The
electrostatic analogy is the result of the similarity between the equations that
describe the electrostatic potential about a conductor and the vapor field about a
droplet. Both the electrostatic potential function outside a charged conduct ing body
and the vapor density field around a growing or evaporating droplet satisfy Laplace’s
equation. The derivation of the growth equation for ice crystals proceeds exactly as
that for a water droplet, except the radius of a droplet, r, is replaced by the electro-
static capacitance, C, the latent heat is the latent heat o f sublimation, L
s
, and the
saturation is that over ice, S
i,v
, rather than water. In the case of a spherical ice crystal
C ¼r. The result is
dM
dt
¼
4pCðS
i;v
1Þ
L
s
=KT ðL
s
=R
v
TÞ1
þ R
v
T=De
si;1
ð25Þ
The problem in using this equation is determining the capac itance factor C, which
varies with the shape of the conductor. For a few shapes, C has a simple form. These
shapes are not the same as ice crystals but have been used to approximate some ice
crystal geometries. To use Eq. (25) to determine the axial growth rate of ice parti-
cles, the term dM=dt must be reduced to a form containing each of the axes. In
general, this will depend on the crystal geometr y. To solve for a or c, an additional
relationship is required between da=dt and dc=dt. This relationship is generally
obtained from measurements of the axial relationships determined from measure-
ments of a large number of crystals. Laboratory experiments conducted to determine
the importance of surface kinetic effects in controlling ice crystal growth have shown
that surface kinetic effects are important in controlling crystal habit since kinetic
effects control how vapor molecules are transported across the crystal surface and
incor porated into the crystal lattice. Ryan et al. (1976) has measured the growth rates
of ice crystals in the laboratory. Figure 23 from their study shows that the a axis
5 GROWTH OF ICE PARTICLES IN ATMOSPHERE 291
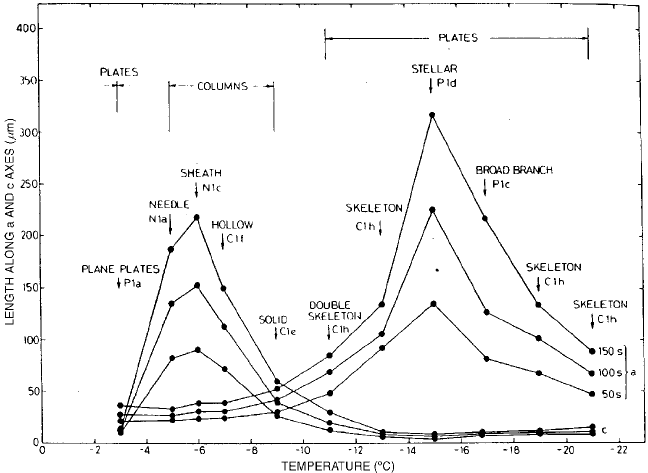
grows most rapid ly at T > 9
C, with a peak at 15
C, while the c axis grows most
rapidly at T < 9
C, with a peak at 6
C.
Ice Particle Growth by Accret ion
Growth by accretion occurs when ice particles collide with supercooled water
droplets, causing them to freeze on to the ice surface. For supercooled water to be
present in substantial quantities in a mixed-phase cloud, it is necessary for the
condensate supply rate to exceed the bulk diffusional growth rate of the ice particles,
after accounting for any dry air entrainment that may be occurring. High condensate
supply rates require sustained large vertical velocities. For this reason, accretion is an
important growth process in clouds with localized regions of sustained high vertical
velocities.
The most common type of cloud that meets this requirement is a cumulus cloud,
particularly cumulus clouds with large vertical development. Regions of other types
of cloud systems also have sustained high vertical velocities. Exa mples include
orographic clouds, par ticularly near the maximum terrain slope, and convective
cores of frontal bands. A second way supercooled water can appear in a cloud is
through vertical transport of droplets upward through the 0
C level. This process can
occur in any type of cloud with a moderate updraft near the melting level. The
supercooled water in this case will generally be confined to the warmer range of
Figure 23 Variation of crystal axial dimensions with temperature for 50, 100, and 150 s after
seeding (Ryan et al., 1976).
292
MICROPHYSICAL PROCESSES IN THE ATMOSPHERE

subfreezing temperatures. Clouds that generally do not contain much supercooled
water are those with weak vertical velocities, such as stratus clouds. In such clouds,
the accretion process will not be as important, if it occurs at all.
Studies of accretion have focuse d primarily on the conditions required for riming
onset, and the late stages of riming, when the particles reach the graupel or ha il stage
of growth. The onset of riming has been studied theoretically and in field and
laboratory experiments. These studies generally show that platelike crystals must
grow to a threshold size of about 150 mm radius before any droplets accret e to their
surfaces. Droplets must be larger than about 5 mm radius before they are collected.
Collection efficiencies increase with increasing ice particle and cloud droplet size,
reaching about 0.9 when the ice particle radius approaches 400 mm and the droplet
radius reaches 35 mm.
Graupel develops in a cloud when small supercooled cloud droplets collect in
large numbers on falling ice particles. The parent ice pa rticle, or embryo, of a
graupel particle can have various shapes. Certain types of ice particles serve as
graupel embryos more effectively than others. Studies have shown that aggregates
of ice crystals can provide excellent graupel embryos in hailstorms because of their
ability to rapidly collect water droplets. Aggregate embryos can be entrained into the
main updraf t core of hailstorms from the debris clouds of previously active convec-
tive towers. Large drops present in the core updraft can also serve as graupel
embryos upon freezing. Graupel characteristics, including density, mass, and
terminal velocities have been measured in a number of field studies. In general,
the data show a great deal of spread, since the observations come from very different
cloud systems.
Hail develops when extreme g rowth by accretion occurs in thundersto rms. Hail
growth occurs in one of two ways, depending on the surface temperature of the
hailstone and the rate of supercooled water accumulation. As a hailston e collects
supercooled water droplets while falling through a thunderstorm, latent heat is
released at the hailstone’s surface as the droplets freeze, raising the surface tempera-
ture, T
s
, of the hailstone. If T
s
< 0
C, droplets continue to freeze immediately on the
hailstone’s surface. If, however, T
s
reaches 0
C, water impacting on the hailstone will
no longer immediately freeze. Water then spreads across the surface of the stone,
drains into porous regions, and can shed from the stone’s surface. The former
situation, when T
s
< 0
C, is called the dry growth regime. The latter situation,
when T
s
¼0
C, is called the wet growth regime. For dry growth, the g rowth rate
of the spherical hailstone due to collection of water drops can be approximated by
the continuous collection equation:
dM
dt
dry
¼ EpR
2
H
V
T;H
w
L
ð26Þ
where R
H
is the radius of the hailstone and V
T,H
is its terminal velocity. The terminal
velocity and radius of cloud drops are neglected because they are small relative to R
H
and V
T,H
[compare Eqs. (23) and (26)]. The same equation applies in the wet growth
5 GROWTH OF ICE PARTICLES IN ATMOSPHERE 293
regime provided the hailstone does not shed water. If the hailstone grows in the wet
growth regime, and sheds excess liquid water from its surface, its growth rate will be
determined by the rate at which the collected water can be frozen. Calculations show
that at 5
C, the effective liquid water content for wet growth is less than 1 g=m
3
.At
10
C the effective liquid water content is near 1 g=m
3
. Higher liquid water content
is required for wet growth at colder temperatures. The threshold values of liquid
water content are well within the range of values found in cumulonimbus, implying
that shedding of drops and wet growth are important processes in thunderstorms.
Hailstones exhibit alternating layers of higher and lower density. The density
variations are due to variations in the concentration of bubbles trapped in the
stone. Generally bubbles are grouped in concentric layers of higher and lower density,
which in turn are associated with the different growth regimes. Field studies have
shown that hailstone embryos can be conical graupel, large frozen drops, and occa-
sionally large crystals. Hailstones sometimes exhibit irregular structure while others
have distinct conical or spherical shapes . The largest known hailstone, collected near
Coffeyville, Kansas, weighed 766 g and had a circumference of 44 cm.
Ice Particle Aggregation
Snowflakes form through aggregation of ice crystals. Several mechanisms have been
proposed to explain the adhesion of ice particles to one another. Snowflakes of sizes
greater than 1 cm are often compo sed of planar and spatial dendritic crystal s, needle-
like crystals or, at higher cloud levels, radiating assemblages of bulletlike crystals.
All of these crystals have shapes that easily interlock during collisions, suggesting
that mechanical interlocking is initially important for crystals to attach to each other.
Two mechanisms have been identified that bond ice particles together once in
contact. The first, called sintering, occurs because ice particles in point contact
form a nonequilibrium system. To minimize the total free surface energy of the
system, a requirement for equilibrium, water molecules must diffuse from ice
surfaces toward the point of contact, strengthening the bond between the particles.
The second bonding mechanism appears to be associated with the presence of a
liquid layer on the surface of ice crystals. This layer is thought to enhance a crystal’s
sticking efficiency. Experiments have shown that the surface electrical conductivity
of ice increases significantly at temperatures warmer than 10
C, suggesting the
surface contains a quasi-liquid layer. Studies using optical and magnetic resonance
techniques support the existence of the layer. However, the existence of a liquid layer
on the surface of ice crystals at subfreezing temperatures is still not universally
accepted.
Larger aggregates typically first develop between 15 and 12
C, the tem pera-
ture range where dendritic crystals form, although aggregates have been observed at
colder temperatures in cirrus. Aggregates form most rapidly near the melting level.
Enhanced aggregation near the melting level is apparently due to increased collec-
tion efficiency as a liquid layer develops on the surface of the crystals. The rate of
aggregation in clouds also depends on ice particle concentrations. Calculations and
294 MICROPHYSICAL PROCESSES IN THE ATMOSPHERE
field observations both suggest that aggregation is enhanced in the presence of high
crystal concentrations.
Ice Particle Concentrations and Evidence for Ice Multiplication
Ice particles first appear in clouds when ice nucleation occurs. Measurements of ice
nuclei suggest that they should be active primarily at cold temperatures (<20
C)
and that the concentration of active nuclei should be an exponential function of
temperature. According to measurements of ice nuclei (Fig. 10), few ice particles
should present in clouds whose tops are warmer than 10
C.
Measurements of ice particle concentrations in clouds have not shown this
expected relationship. Ice particle concentrations often exceed by several orders of
magnitude the concentrations expected on the ba sis of ice nuclei measurements. This
is particularly true at warm temperatures (>10
C). Conversely, at temperatures
between 20
C and the homogeneous nucleation threshold (35 to 40
C) ice
particle concentrations are often less than expected on the basis of ice nuclei
measurements.
Determining the origin of ice particles in clouds remains one of the major
unsolved problems in cloud physics. It is possible that ice nucleation occurs in
clouds under conditions that are not well understood. It is also possible that second-
ary mechanisms for ice enhancement occur. Three mechanisms have been studied
extensively: (1) fragmentation of colliding ice particles, (2) ice splintering during the
riming process, and (3) shattering of drops during freezing.
Ice particles that fragment during collisions are typically more delicate crystals
such as dendrites, sectors, side planes, and needles and sheaths. Particles, that
seldom fragment are thick and thin plates, solid and hollow columns, and scrolls.
Experiments to examine fragmentation show that dendritic crystals readily fragment
in conditions expected in clouds. On the other hand, breakup of rime accumulat ed on
crystals during collisions is unlikely in clouds. Overall, data suggest that only clouds
containing dendritic particles are likely to have a significant increase in measured ice
particle concentrations due to fragmentation during collisions.
The high ice particle concentrations observed in clouds provoked a large number
of research groups in the early 1970s to try to find a strong ice multiplica tion
mechanism to explain the observations. A breakthrough on the problem was
reported by Hallett and Mossop (1974), who showed that splintering of ice during
the riming process led to copious ice production under a limited range of conditions.
Subsequent studies determined the conditions required for splinter production and
clarified the mechanism for splintering.
These studies together showed that copious numbers of ice splinters could be
produced during riming when: (1) the temperature was between 3 and 8
C; (2)
large ice particles were present in the cloud to co llect cloud droplets, (3) droplets
with diameters >24 mm were present, and (4) droplets smaller than 13 mm diameter
were present. The small droplets, upon freezing onto a falling ice particle, provide
sites for the larger droplets to come into point contact with the ice, attach, and freeze.
Laboratory experiments indicate that supercooled drops accreting on to another
5 GROWTH OF ICE PARTICLES IN ATMOSPHERE 295
