Potter T.D., Colman B.R. (co-chief editors). The handbook of weather, climate, and water: dynamics, climate physical meteorology, weather systems, and measurements
Подождите немного. Документ загружается.


ments, for example, usually have a finite field of view and a finite spectral sensitivity;
interpreting measurements from these sensors therefore requires calculations of
intensity, which may be broadband, narrowband, or essentially monochromatic,
depending on the detector.
Imagine, though, wanting to compute the rate at which radiation heats or cools a
layer of air in the atmosphere. Here we need to know the net amount of energy
remaining in the layer, which we can calculate by considering the decrease in both
upwelling and downwelling flu xes as they cross a layer between z
1
and z
2
:
E
in
¼ F
"
ðz
1
ÞF
"
ðz
2
ÞþF
#
ðz
2
ÞF
#
ðz
1
Þ
¼ F
"
ðz
1
ÞF
#
ðz
1
Þ½F
"
ðz
2
ÞF
#
ðz
2
Þ
¼ F
net
ðz
1
ÞF
net
ðz
2
Þ
ð7Þ
where we have defined the net flux as F
net
(z) ¼F
:
(z)F
;
(z). As z
1
and z
2
get very
close together, the difference in (7) becomes a differential, and the rate of heating
can be related to the divergence of the radiative flux though the air density r and heat
capacity c
p
:
dTðzÞ
dt
¼
1
rc
p
dF
net
ðzÞ
dz
ð8Þ
3 SOURCES OF RADIATION
Imagine setting a cast-iron skillet over a really powerful burner and turning on the
gas. At first the pan appears unchanged, but as it heats it begins to glow a dull red.
As the pan becomes hotter it glows more brightly, and the color of the light changes
too, becoming less red and more white.
In the idealized world inhabited by theoretical physicists, the cast-iron skillet is
replaced by a block. The block has a cavity inside it and a small hole opening into
the cavity. The block can be heated to any temperat ure. Measurements of the radia-
tion emerging from this cavity show that:
1. The spectral intensity B
l
emerging from the cavity at each wavelength depends
only on the temperature T of the block, and not on the material or the shape of
the cavity.
2. The total amount of energy emitted by the cavity increases as the temperature
increases.
3. The wavelength at which B
l
reaches its maximum value decreases with
temperature.
306 RADIATION IN THE ATMOSPHERE: FOUNDATIONS

The Planck Function
A theoretical explanation for cavity radiation was the single most compelling
unsolved problem in physics at the beginning of the twentieth century. The best
fit to observations had been suggested by Wien:
B
l
ðTÞ¼
c
1
l
5
1
e
c
2
=lT
ð9Þ
where c
1
and c
2
were determined experimentally. In October 1900 Max Planck
proposed a small modification to this relationship, namely
B
l
ðTÞ¼
c
1
l
5
1
e
c
2
=lT
1
ð10Þ
Planck’s relationship, now called the Planck function, was a better fit to the data
but was not an explanation for why radiation behaves this way. To develop a theo-
retical model, Planck imagined that the atoms in the cavity walls behave like tiny
oscillators, each with its own characteristic frequency, each emitting radiation into
the cavity and absorbing energy from it. But it proved impossible to derive the
properties of cavity radiation until he made two radical assumptions:
1. The atomic oscillators cannot take on arbitrary values of energy E. Instead, the
oscillators have only values of E that satisfy E ¼nhn where n is an integer
called the quantum number, n the oscillator frequency, and h a constant.
2. Radiation occurs only when an oscillator changes from one of its possible
energy levels to anothe r; that is, when n changes value. This implies that the
oscillators cannot radiate energy continuously but only in discrete packets, or
quanta.
By Dece mber of 1900 Planck had worked out a complete theory for cavity
radiation, including the values of c
1
and c
2
:
c
1
¼ 2hc
2
c
2
¼
hc
k
ð11Þ
In these equations k is Boltzmann’s constant, which appears in statistical mechanics
and thermodynamics, and h is Planck’s constant. Planck used observations of B
l
(T )
to determine the values h ¼6.63 10
34
J=s and k ¼1.38 10
23
J=K. Equations
(10) and (11) were the basis for the development of quantum mechanics, which
fundamentally changed the way physicists looked at the world.
3 SOURCES OF RADIATION 307

Blackbody Radiation and Its Implications
In the atmospheric sciences we refer not to cavity radiation but to blackbody radia-
tion, a blackbody being anything that radiates according to (10). Blackbody radiation
is isotropic, meaning that it is emitted in equal amounts in all direc tions.
Wavelength of Maximum Emission
The wavelength at which blackbody radiation reaches its maximum intensity can be
found by taking the derivative of B
l
(T ) with respect to wavelength, setting the result
to 0, and solving for l. That is, we solve
@B
l
ðTÞ
@l
¼ 0 ð12Þ
for the wavelength l
max
, which yields Wien’s displacement law
l
max
¼ 2898 mm K=T ð13Þ
At temperatures typical of Earth’s atmosphere (say, T ¼288 K), the maximum wave-
length is about 10 mm in the infrared portion of the spectrum, while the sun (at a
temperature about 5777 K) is brightest at about 0.5 mm, where visible light is green.
Total Amount of Energy Emitted
How much energy does a blackbody radiate at a given temperature? This quantity
is the blackbody broadband intensity and is computed by integrating over all
wavelengths:
BðTÞ¼
ð
1
0
B
l
ðTÞdl ¼
sT
4
p
ð14Þ
Equation (14) is the Stefan–Boltzmann relation and makes use of the Stefan–
Boltzmann constant s ¼5.67 10
8
W=m
2
K
4
. Because blackbody radiation is
isotropic, the total amount of radiation lost by an object into each hemisphere is
FðT Þ¼
ð
2p
0
ð
1
0
BðTÞm dm dj ¼ sT
4
ð15Þ
Figure 2 shows B
l
(l, T ) plotted for two values of T, roughly corresponding to the
average surface temperatures of Earth and sun. The curves are each normalized,
since otherwise the much greater intensity produced at solar temperatures would
swamp the intensity produced by terrestrial objects. Almost all the sun’s energy is
produced at wavelengths less than about 4 mm, while almost all the energy emitted
308 RADIATION IN THE ATMOSPHERE: FOUNDATIONS
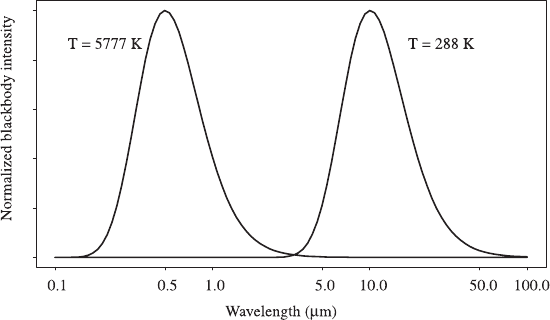
by Earth is produced at wavelengths longer than 4 mm; this convenient break lets us
treat solar radiation and terrestrial radiation as independent.
Blackbody radiation helps us understand the light produced by our cast-iron
skillet. The pan emits radiation even at room temperature, although the emission
is mostly in the infrared por tion of the spectrum. As the skillet is heated, the total
amount of radiation emitted increases (as per the Stefan–Boltzmann relation) and the
wavelength of maximum emission gets shorter (as per Wien’s displacement law)
until some of the emission is in the longer (red) part of the visible spectrum. If the
pan were made even hotter, it would glow white, like the filament in an incandescent
light bulb.
Emissivity, Energy Conservation, Brightness Temperature
The radiation emitted by any object can be related to the blackbody radiation
I
l
ðTÞ¼e
l
B
l
ðTÞð16Þ
where e
l
is the emissivity of the object, which varies between 0 and 1. If e
l
does not
depend on wavelength, we say that the object is a gray body; a blackbody has a value
of e
l
¼1.
How are emission and absorption related? Imagine an object that absorbs
perfectly at one wavelength but not at all at any other wavelength (i.e., an object
with e
l
¼1 at one value of l ¼l* and e
l
¼0 everywhere else), illuminated by
broadband blackbody radiation from a second body. The object absorbs the incident
radiation and warms; as it warms the emission (which occurs only at l *, remember)
increases. Equilibrium is reached when the amount of energy emitted by the particle
E
out
is equal to the amount absorbed E
in
. At wavelength l the body acts as a
Figure 2 Blackbody intensity B
l
as a function of wavelength for temperatures correspond-
ing to the surfaces of Earth and sun. The curves are arbitrarily normalized.
3 SOURCES OF RADIATION 309

blackbody, so emission depends only on the equilibrium temperature and
E
out
¼I
l*
(T ) ¼B
l*
(T ). The body is exposed to broadband blackbody radiation, so
E
in
¼B
l*
(T ). But since equilibrium implies that E
in
¼E
out
, the absorption at every
wavelength other than l* must be zero. This chain of reasoning, known as Kirchoff’s
law, tells us that the absorptivity and emissivity of objects is the same at every
wavelength.
The Planck function finds another application in the computation of brightness
temperature. If we make measurements of monochromatic intensity I
m
at some
wavelength l, and assume that e
l
¼1, we can invert the Plank function to find
the temperature T
b
at which a blackbody would have to be in order to produce
the measured intensity
T
b
¼
c
2
l
1
lnðc
1
=I
m
l
5
þ 1Þ
ð17Þ
where T
b
is called the equivalent blackbody temperature or, more commonly, bright-
ness temperature. It provides a more physically recognizable way to describe
intensity.
4 ABSORPTION AND EMISSION IN GASES: SPECTROSCOPY
The theory of blackbody radiation was developed with solids in mind, and in the
atmospheric sciences is most applicable to solid and liquid materials such as ocean
and land surfaces and cloud particles. The emissivity of surfaces and suspended
particles is generally high in the thermal part of the spectrum, and tends to change
fairly slowly with wavelength.
In gases, however, emissivity and absorptivity change rapidly with wavelength, so
blackbody radiation is not a useful model. Instead, it is helpful to consider how
individual molecules of gas interact with radiation; then generalize this understand-
ing by asking about the behavior of the collection of molecules in a volume of gas.
We will find that the wavelengths at which gases absorb and emit efficiently are
those that correspond to transitions between various states of the molecule; under-
standing the location and strength of these transitions is the subject of spectroscopy.
Spectral Lines: Wavelengths of Absorption and Emission
Imagine the simplest possible atom, a single elect ron of mass m
e
circling a single
proton. In a mechanical view the electrostatic attraction between the unlike charges
balances the centripet al acceleration of the electron, so the velocity v of the electron,
and therefore its angular momentum, are related to the distance r between the
310 RADIATION IN THE ATMOSPHERE: FOUNDATIONS

electron and the proton. In 1913 Niels Bohr postu lated that the angular momentum
of the electron is quantized, and can take on only certain values such that
m
e
vr ¼
nh
2p
ð18Þ
where n is any positive integer. This implies that the electron can only take on certain
values of v and r, so the total energy (kinetic plus potential) stored in the atom is
quantized as well. If the energy levels of the atom are discrete, the changes between
the levels must also be quantized.
It is these specific changes in energy levels that determine the absorption and
emission lines, the wavelengths at which the atom absorbs and emits radiation. The
energy of each photon is related to its frequency and so its wavelength
E ¼ hn ¼
hc
l
ð19Þ
A photon striking an atom may be absorbed if the energy in the photon corre-
sponds to the difference in energy between the current state and another allowed
state. In a population of atoms or molecules (i.e., in a volume of gas) collisions
between molecules mean that there are molecules in many states, so a volume of gas
has many absorption lines.
In the simple Bohr atom the energy of the atom depends only on the state of the
electron. Polyatomic molecules can contain energy in their electronic state, as well as
in their vibrational and rotational state. The energy in each of these modes is
quantized, and photons may be absorbed when their energy matches the difference
between two allowable states in any of the modes.
The largest energy differences (highest frequencies and shortest wavelengths,
with absorption=emission lines in the visible and ultraviolet) are associated with
transitions in the electronic state of the molecules. At the extreme, very energetic
photons can completely strip electrons from a molecule. Photodissociation of ozone,
for example, is the mechanism for stratospheric absorption of ultraviolet radiation.
Energy is also stored in the vibration of atoms bound together in a stable mole-
cule. Vibrational transitions give rise to lines in near-infrared and infrared, between
those associated with electronic and rotational transitions. The amount of energy in
each vibrational mode of a molecule depends on the way the individual atoms are
arranged within the molecule, on the mass of the atoms, on the strength of the bonds
holding them together, and on the way the molecule vibrates. Vibrational motion can
be decomposed into normal modes, patterns of motion that are orthogonal to one
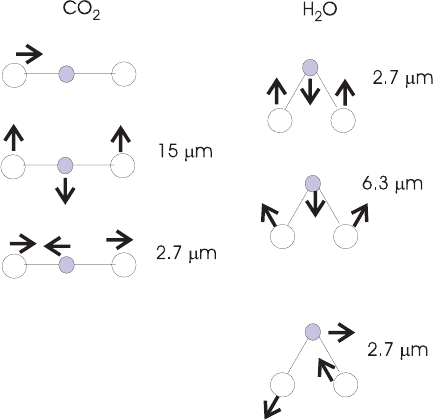
another. In a linear symmetric molecule such as CO
2
, for example, the patterns are
symmetric stretch, bending, and antisymmetric stretch, as shown in Figure 3. The
state of each normal mode is quantized separately.
The way atoms are arranged within a molecule also plays a role in how energy is
stored in rotational modes. Carbon dioxide, for example, contains three atoms
arranged in a straight line, and thus has only one distinct mode of rotation about
4 ABSORPTION AND EMISSION IN GASES: SPECTROSCOPY 311
the center molecule and one quantum number associated with rotation. Complicated
molecules like ozone or water vapor, though, have three distinct axes of rotation, and
so three modes of rotation and three quantu m numbers associated with the rotational
state, each of which may change independently of the others. For this reason the
absorption spectrum of water is much more complicated than that of CO
2
.
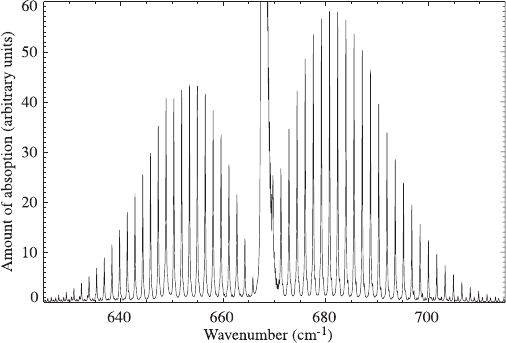
Changes in vibrational and rotational states may also occur simultaneously, lead-
ing to a very rich set of absorption lines. Becau se the changes in energy associated
with rotation are so much smaller than those associated with vibration, the absorp-
tion spectrum appears as a central line with many lines (each corresponding to a
particular rotat ional change) clustered around it, as shown in Figure 4. More
energetic lines (with higher wavenumbers and shorter wavelengths) are associated
with simultaneous incre ases in the rotational and vibrational state, while the lines to
the left of the central line occur when rotational energy is decreased while vibra-
tional energy increases.
What gives rise to the family of absorption lines flanking the central wavenumber
in Figure 4? Carbon dioxide has only one axis of rotation, so the different energies
are all associated with the same mode, but the family of lines implies that there is a
set of rotational transitions, each with a different energy. If J is the quantum number
describing the rotational states, and DJ the change between two states, there are two
possibilities. The different lines might correspond to different values of DJ, but this
is prohibited by quantum mecha nics. In fact, each line corresponds to DJ ¼1, but
neighboring states differ in energy by various amounts depending on J.
Figure 3 Vibrational normal modes of CO
2
and H
2
O molecules. Any pattern of vibration
can be projected on to these three modes, which are all orthogonal to one another. Also shown
are the wavelengths corresponding to each vibrational mode. See ftp site for color image.
312
RADIATION IN THE ATMOSPHERE: FOUNDATIONS
Line Strength
The quantum mechanics of electronic, vibrational, and rotational transitions deter-
mines the location of absorption and emission lines in gases. Molecules do not float
freely in the atmosphere, however, but rather are members of a large ensemble, or
population. The temperature of the gas determines the mean energy of the mole-
cules, and random collisions between them redistribute the energy among the possi-
ble states. The amoun t of absorption due to any particular transition, then, is the
product of how likely the transition is (as determined by quantum mechanics) and
the fraction of molecules in the originating state. The effectiveness of absorption is
called the line strength S and has dimensions of area per mass or per molecule.
Line strength depends in part on temperature. The atmosphere is almost every-
where in thermal equilibrium, which means that the average amount of energy per
molecule is determined by the temperature, but this energy is constantly being
redistributed among molecules by frequent collisions. The collisions also transfer
energy between different states, so that a fast-moving molecule may leave a collision
moving more slowly but rotating more rapidly. The abundance of molecules in a
state with energy E depends on the ratio of E to the average kinetic energy kT. This
explains the increase and decrease of line strengths to either side of the central line in
Figure 4: The most common rotational state is J ¼7orJ ¼8, and the number of
molecules in other states, and so the line strength, decreases the more J differs from
the most common value.
Line Shape
Each molecular transition, be it electronic, vibrational, or rotational, corresponds to a
particular change in energy, which implies that absorption and emission take place at
Figure 4 Pure vibration and vibration-rotation lines near the 15 mm (666 cm
1
) absorption
line of carbon dioxide. The spectrum is computed for gases at 300 K and 1000 mb.
4 ABSORPTION AND EMISSION IN GASES: SPECTROSCOPY 313
exactly one frequency. But as Figure 4 shows, absorption and emission occur in a
narrow range of wavelengths surrounding the wavelength associated with the transi-
tion. We say that absorption lines are affected by natural broadening, pressure
(or collision) broaden ing, and Doppler broadening. We describe these effects in
terms of a line shape function f ðn n
0
Þ, which describes the relative amount of
absorption at a frequency n near the central transition frequency n
0
. Line broadening
affects the spectral distribution of absorption but not line strength.
Natural Broadening of Absorption Lines
Natural broadening of absorption lines has a quantum mechanical underpinning.
Because molecules spend a finite amount of time in each state, the Heisenberg
uncertainty principle tells us that the energy of the state must be somewhat uncertain.
This blurring of energy levels implies a similar blurring in the transition energies
between levels, though the effect is small compa red to other broadening
mechanisms.
Doppler Broadening of Absorption Lines
If we stand beside a racetrack and listen to an approaching car, the whine of the
engine will seem to increase in pitch, then decrease as the car passes us. This change
in frequency is known as the Doppler shift and occurs whenever an object and
observer move relative to one another. The frequency n of the emitted sound or
light appears to increase or decrease from its unperturbed value n
0
depending on how
fast the object moves v relative to the speed of the wave c:
n ¼ n
0
1
v
c
ð20Þ
Because the atmosphere is almost always in thermal equilibrium, the velocities of
a collection of gas molecules follow a Maxwell–Boltzmann distribution. The pro-
bability p(v) that a molecule will have a radial velocity v depends on the temperature
T, since molecules move faster at higher temperatures, and on the mass m of the
molecules
pðvÞ¼
ffiffiffiffiffiffiffiffiffiffiffi
m
2pkT
r
exp
mv
2
2kT
ð21Þ
We can compute the Doppler line shape f
D
ðn n
0
Þ around an absorption line by
combining (20) and (21):
f
D
ðn n
0
Þ¼
1
a
D
ffiffiffi
p
p
exp
ðn n
0
Þ
2
a
2
D
!
ð22Þ
314 RADIATION IN THE ATMOSPHERE: FOUNDATIONS
where we have defined the Doppler line width
a
D
¼
n
0
c
ffiffiffiffiffiffiffiffi
2kT
m
r
ð23Þ
Absorption lines associated with heavier molecules are broadened less than those
associated with light molecules, since for the same mean thermal energy (as
measured by temperature) heavier molecules move more sluggishly than light
ones. Doppler broadening also increases in importance at shorter wavelengths. It
has the greatest effect high in the atmosphere, where pressure is low.
Pressure Broadening of Absorption Lines
When pressure is high, however, collision or pressure broadening is the most import-
ant process determining the shape of absorption and emission lines. The exact
mechanisms causing pressure broadening are so complicated that there is no exact
theory. What is clear, however, is that the width of the absorption line increases as
the frequency of collisions between molecules increases. Pressure-broadened spec-
tral lines are well-characterized by the Lorentz line profile:
f
L
ðn n
0
Þ¼
a
L
p
1
ðn n
0
Þ
2
þ a
2
L
ð24Þ
The Lorentz line width a
L
depends on the mean time t between collisions:
a
L
¼
1
2pt
ð25Þ
In practice the value of a
L
is determined for some standard pressure and temperature
p
0
and T
0
, then scaled to the required pressure and temperature using kinetic theory:
a
L
¼ a
L0
p
p
0
T
0
T
n
ð26Þ
where n varies but is near
1
2
. When molecules collide with others of the same
species, we say that the line is self-broadened; when the collisions are primarily
with other gases we say the line is subject to foreign broadening. For the same
width pressure broadening affects the line wings, those frequencies further from
the central frequency, more dramatically than Doppler broadening, as Figure 5
demonstrates.
Both pressure and Doppler broadening can occur simultaneously. The line shape
that results, the convolution of f
L
ðn n
0
Þ and f
D
ðn n
0
Þ, is called the Voigt line
shape, which does not have an exact analytic for m. It is instructive, though, to
4 ABSORPTION AND EMISSION IN GASES: SPECTROSCOPY 315
