Popov K.I., Djokic S.S., Grgur B.N. Fundamental Aspects of Electrometallurgy
Подождите немного. Документ загружается.

182
Chapter 7
7.1.1
Selectivity of metal dissolution and deposition
The main principle of electrorefining is based on the anodic dissolution
of a metal containing impurities and its cathodic deposition with an as much
as possible reduced amount of impurities. The selectivity principle of metal
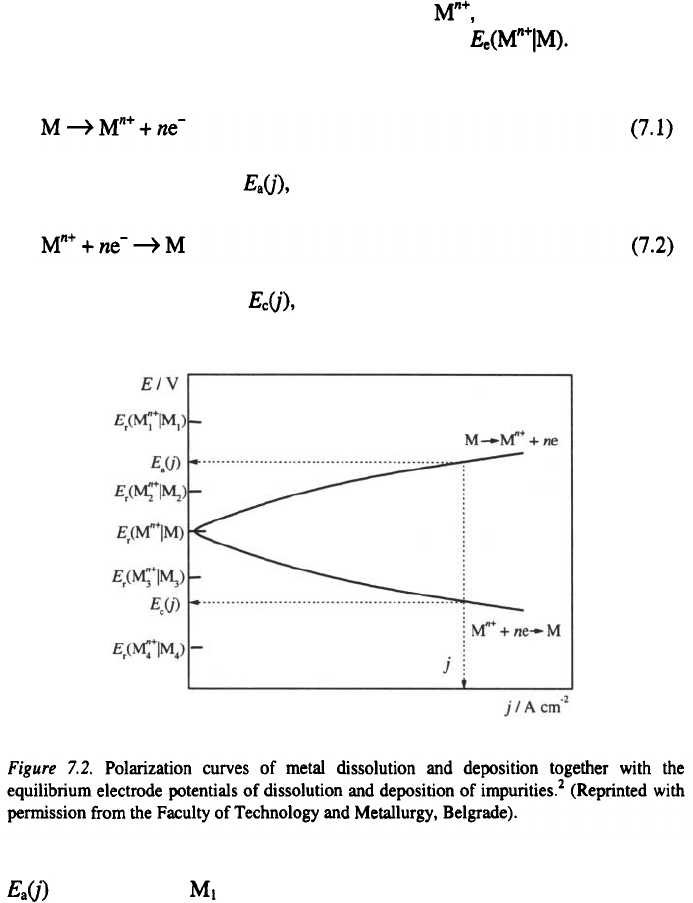
dissolution and deposition can be explained starting from a metal M placed
in a suitable solution containing its ions which leads to the
establishment of the equilibrium electrode potential Under some
value of current density, j, the base metal is anodically dissolved
having an anodic potential and cathodically deposited
having a cathodic potential as shown in Fig. 7.2
2
.
All metal-impurities having an equilibrium potential more positive than
(such is metal ) will not be anodically dissolved, but owing to the
dissolution of the base metal (erosion) they will be transferred into the
6. Electrowining
183
electrolyte and fall down to the bottom of the reactor forming the anodic
slime.
Metal-impurities which have an equilibrium potential more negative than
(such as: and will be dissolved together with the base metal
M, and transferred into the electrolyte in the form of metal ions. Exceptions
are those metals whose cations can react with the anions in the electrolyte
forming sparingly soluble salts which sediment as anodic slime
2
.
During cathodic deposition of a metal, all the metals whose equilibrium
potentials are more positive than the potential of the cathode (metals
and could be deposited, depending on their concentrations and
deposition overpotentials. In the example given in Fig. 7.2. metal will be
anodically dissolved, but not cathodically deposited, while metal will not
be anodically dissolved, but transformed into the anodic slime
2
.
It can be concluded that the selectivity principle of electrorefining is
based on such electrolysis conditions which ensure that metal impurities that
could be cathodically deposited will not be anodically dissolved (anodic
slime) and impurities that could be anodically dissolved will not be
cathodically deposited, but remain in the electrolyte.
7.2
THEORETICAL ASPECT OF COPPER
ELECTROREFINING
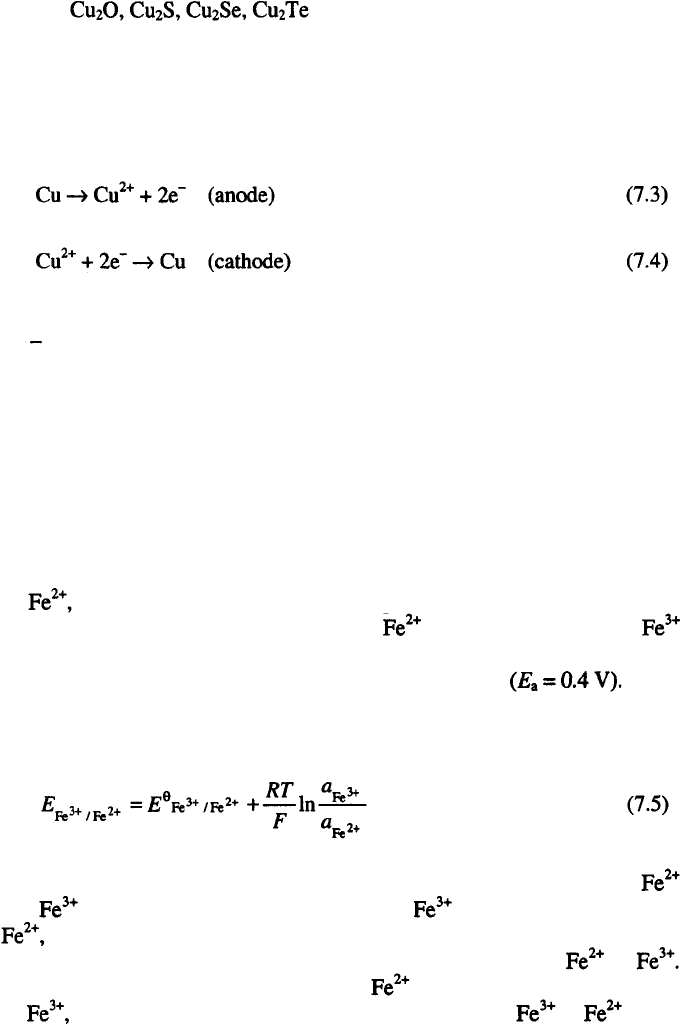
Copper produced by metallurgical processes contains significant amount
of impurities, between 0.5 and 2 %, which have a bed influence on the
mechanical and electrical properties of copper
3
. Amounts of 0.15 % of
phosphorous or 0.5 % of arsenic, dramatically decrease the electrical
conductivity of copper, as shown in Table 7.1.
3
Hence, the improvement of
the electrical properties of copper is the main reason for electrorefining.
The second reason for electrorefining is the extraction of some noble
impurities, normally present in non-refined copper (anodic metal), such as:
gold, silver, platinum and palladium etc
2
.
As stated previously, anodic copper contains between 0.5 and 2 % of
admixtures that can be divided into four groups
2
:
184
Chapter 7
1.
2.
3.
4.
Ni, Co, Fe, Sn, Zn, Pb
Au, Ag, Pt, Pd, Se, Te
As, Sb, Bi
The main reactions occurring at the electrodes, which determines the
potentials of anode and cathode, during electrorefining of copper are as
follows:
while the impurities can react in the following manners
2,3
.
Metals of the first group, whose equilibrium potentials are more
negative (less noble) than copper (see Table 2.1) will during anode
dissolution be transferred together with copper into solution. Due to the
presence of sulfate ions in the electrolyte, lead ions will form lead sulfate
and fall into the anodic slime. Other metals, such as Ni, Co, Sn and Zn, will
be concentrated in the electrolyte, since their deposition potential is more
negative than the potential of the cathode, whose value is around 0.3 V. The
presence of these metals in the electrolyte has no bad influence, but it
necessitates periodical purification of the electrolyte. The presence of iron
ions is completely undesirable. Iron can be anodically dissolved in the form
of
but can not be deposited since its equilibrium potential is much more
negative than the potential of the cathode. ions can be oxidized to
at the anode, but the value of the standard electrode potential of this reaction
(+0.77 V) is more positive than the potential of the anode
Bearing in mind the Nerst relation (Eq. 2.2) for the equilibrium electrode
potential:
it could be concluded that the potential is dependent on the ratio of the
and concentration. If the concentration of is much lower than that of
the equilibrium potential will become more negative than the actual
potential of the anode which could result in the oxidation of to
This would decrease the concentration of and increase the concentration
of which leads to a possibility for the reduction of to at the
cathode. This unwanted closed circle would consume a part of the current
during the electrorefining process.

6. Electrowining
185
All the metals with which the electrolyte is enriched lead to a
consumption of free acid.
Insoluble impurities from the second group, such as and
can not be anodically dissolved, but sediment and form anodic slime.
Only can be dissolved, according to:
Hence, half of the copper is dissolved into the electrolyte in the form of
ions, while the other half forms a fine powder which partly reacts with
the air oxygen and sulfuric acid in the electrolyte, and partly sediments to
form anodic slime.
Metals of the third group as Au, Ag, Pt, Se and Te also sediment as the
anodic slime. They are more noble than copper and according the rule that
reactions with lower electrode potentials occur first (see section 2.3.1) at the
anode, these metals act as insoluble inert inclusions and fall down as the
anodic slime.
The most undesirable impurities are the metals of the fourth group: As,
Sb, and Bi. Their presence drastically decreases the quality of the cathode
copper deposit, as shown in Table 7.1. These metals are the most difficult to
be removed becouse of the values of their reduction potential which are near
the potential of copper, precisely between the equilibrium potential of copper
and hydrogen. However, their concentration in the electrolyte is usually very
small, so their deposition potential is more negative compared with the
potential of the cathode, and under normal conditions they are not deposited.
If for some reason the concentration of copper ions decreases, their
deposition could be expected. On the other hand, Sb and Bi could react with
As forming sparingly soluble arsenates, which floats in the form of fine foam
on the electrolyte and make electrolysis process difficult.
4
The effects of electrorefining could best be illustrated by Table 7.2,
2
which gives the distribution of impurities in the anodic slime, electrolyte and
cathode metal
2
.
186
Chapter 7
7.2.1
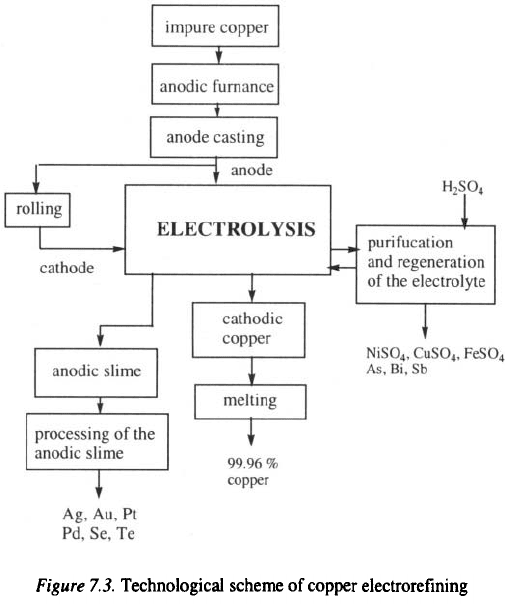
Technological scheme of copper electrorefining
The process of copper electrorefining is schematically illustrated in Fig
7.3.
Anodes of 99 – 99.5 % pure copper obtained, by pyrometallurgical
treatment, are prepared by casting after melting in the anode furnace. The
surface area of the anode depending on the capacity of the electrolytic cell, is
between 1 and their width is between 40 and 50 mm, while their
weight is between 300 and 350 kg
2,4
. A part of the anodes is prepared to
cathodes by rolling into 1 mm thick copper sheets 5 kg in weight
2,4
. During
the manufacture of the anodes and cathodes, hooks for hanging the
electrodes onto electrode tracks are also produced.
After manufacture, the electrodes are placed into electrochemical cells,
the number of which can, depending on the capacity of the plant, be a few
tens connected in series or parallel.
Electrorefining of copper takes place in an electrolyte, a typical
composition of which is given in Table 7.3
3
.
Thiourea and gelatine (10-100 g per 1 t of copper) are used as additives
for enhancing the quality of the deposit and preventing the formation of
dendrites which can produce short circuits during the electrolysis process
3
.
The conditions of the copper electrorefining process are
2,4
:
cathodic current density:
current efficiency
cell voltage:
temperature of the electrolyte:
specific electrical energy consumption:
94 – 96
%
0.25–0.35V
55–60°C
6. Electrowining
187
After the electrorefining process, cathodic copper of 99.97 – 99.99 %
purity is dried, and further processed by melting, rolling into thin metal
sheets, wires or similar commercial products.
During electrolysis process the electrolyte is enriched in Ni, Sb, and As,
which are removed by regeneration, while the anodic slime is removed from
the electrochemical cell and transported for further processing. It is
important to mention that the process of the anode slime can bring in such
high incomes that the entire cost of electrorefining is covered.
As previously stated, copper electrorefming is an electrolysis process
with a soluble anode and theoretically the composition of the electrolyte
should not change during the process. However, in a real system some
composition changes do occur. First by the acid electrolyte dissolves some
amount of anodic copper in the presence of air oxygen, which results in an
increase in the concentration of copper ions, and a concurrent decrease in the
free acid concentration. Secondly, impurities from the anodic copper, which
did not sediment into the anodic slime, remain in the electrolyte and
additionally decrease the concentration of free acid.

188
Chapter 7
7.2.1.1
Refining of the electrolyte
In practice, two methods of correcting the electrolyte composition and
refining are in common use
2
.
The first way consists of removing copper, As and Sb by cathodic
deposition in separate electrochemical cells with insoluble anodes. This
procedure increases the concentration of the acid after which the solution is
concentrated by vaporization whereby crystallization of nickel, iron and
copper sulfates occurs.
The second procedure consists of the neutralization of the free sulfuric
acid by dissolving copper. Copper sulfate crystallizes from the saturated
solution. The rest of the electrolyte is freed from Cu, As and Sb by
electrolysis in a separate cell and from Ni, Zn and Fe by evaporation.
After this refining, the electrolyte, practically contains only sulfuric acid,
which is further, corrected by dissolving copper.
7.2.1.2
Processing of the anodic slime
The anodic slime is a precious crude from which noble and rare metals
and metalloids such as the platinum group metals, gold, silver, selenium,
tellurium etc are extracted
1,2
.
Copper is removed from the anodic slime by dissolution in hot dilute
sulfuric acid with air circulation, after which selenium and tellurium are
removed. Subsequently, by melting after the addition of quartz sand, sodium
carbonate and sodium nitrate, the anodic slime is transformed into an alloy
composed of 93 % silver, 3% gold, 1% copper, 0.05% palladium, 0.03%
platinum, and traces of other metals
3
.
Silver of 99.99 % purity is extracted from the alloy by the process of
anodic dissolution in a solution of silver nitrate. Owing to the high value of
the exchange current density which leads to the poor adhesion of the silver
deposit, some specific constructions of the refining cell are required which
enable the separation of the anodic slime collected in polypropylene bags
containing the anodes from the crystals of silver that drop from the cathode
to the bottom of the cell
2
.
After the process of silver electrorefining, the anodic slime is composed
of 95% gold, 5% silver and about 1% copper, palladium, platinum and some
other metals. After melting and casting, the anodes are subjected to a
electrorefination in the electrolyte containing and free hydrochloric
acid. Owing to the high value of the electrode potential of gold deposition of
about 1.4 V, all of impurities, except silver which forms insoluble silver(I)
chloride, are dissolved and remain in the electrolyte
2
.
When the amount of platinum and palladium reaches a value of about
the electrolyte is replaced. These metals are subsequently recovered
6. Electrowining
189
in the form of ammonium salts: and By the
reduction of these salts, palladium and platinum are obtained
2
.
It is important to mention that the price of noble metals salts are a few
times higher than the price of the noble metals themselves.
7.3 FURTHER READINGS
1.
2.
3.
4.
Pletcher, Derek, Industrial Electrochemistry, New York: Chapman and Hall, 1984.
Strahinja; Snežana; Branislav, Electrochemical Engineering
(In Serbian), Belgrade: Faculty of Technology and Metallurgy, 2001.
Spasoje, Electrometallrgy (In Serbian), Belgrade: Faculty of Technology and
Metallurgy, 1972.
Kubasov, Vladimir; Bannikov, Vladimir, Electrochemical Technology of Inorganic
Compounds (In Russian), Moscow: Khimia, 1989.
This page intentionally left blank

Chapter 8
OPTIMUM CONDITIONS FOR
ELECTROPLATING
Metal coating represents a metal electrodeposit which changes the surface
properties from those of the basic metal to those of electrodeposited one. It
should be adherent, nonporous and without internal stresses. A good adhesion
depends mainly on the preparation of the substrate for electrodeposition, resul-
ting in a clean surface amenable to accepting a satisfactory deposit. Poor surface
preparation can cause peeling of the coating making it useless. Crashing and
peeling of a deposit can also be caused by internal stresses, which arise mainly
from the incorporation of foreign materials in the lattice. If lattice of an electro-
deposited metal is free of such inclusions the mechanical properties of the
electrodeposit are practically the same as those of thermally prepared metal.
Finally, the surface properties of the basic metal can not be completely trans-
formed into the properties of the electrodeposited one if the deposit is porous.
1
The preparation of a substrate for electrodeposition is not connected with
metal electrodeposition and is not treated in this book. It is treated in details
elsewhere
1,2
. The appearance of stresses, which is partially connected with the
electrodeposition process, but has not yet been clarified is also not treated here.
On the other hand the effect of cementation on the adhesion of a coating
and the effect of deposition condition on the porosity of the metal deposits
can be treated in a semi-quantitative way.
8.1
CEMENTATION AND DEPOSITION FROM THE
COMPLEX SALT SOLUTIONS
If zinc is immersed in a copper sulfate solution the reaction
191
