Popov K.I., Djokic S.S., Grgur B.N. Fundamental Aspects of Electrometallurgy
Подождите немного. Документ загружается.

152
Chapter 5
It is obvious from Eqs. 5.2, 5.4, and 5.7 and Eqs. 5.21 and 5.26 that, in
both cases,
taking into account also Eq. 2.29
For j(t) given by Eq. 5.15, the surface concentration under periodic
conditions is approximately given by
16
Hence, at sufficiently small value of T and not extremely high values of
in AC will also be given by Eq. 5.27, implying that at sufficiently high
frequencies, surface concentration is determined by the average current
density regardless of the shape of the current wave.
In the case of a rectangular pulsating overpotential, as a function of
time, is given by
17
:
Assuming that the surface concentration is determined by the average
current density, Eq. 5.16 can be rewritten in the form
For a sufficiently high value of Eq. 5.30 reduces during the on
periods to:
and during the off periods to:
5. Electrodeposition at a Periodically Changing Rate
153
The average current in PO deposition can easily be determined by
The overpotential amplitude is then given by
and
where
and
Polarization curves for the average values for the copper deposition
have been successfully calculated from the stationary polarization curve
using Eq. 5.35
17
for This is a good evidence that in PO deposition,
the average current density also determines the surface concentration of the
depositing ion.
The overpotential amplitude is larger than in the DC regime for one and
the same average current density. At the same time, the diffusion overpo-
tential remains constant, depending on the average current density only.
Hence, the part of activation control in the overall amplitude overpotential
increases with increasing pause to pulse ratio.
The situation is similar in pulsating or reversing current electrode-
position.
154
Chapter 5
5.2.2
Capacitance effects
From the above discussion, it can be concluded that the useful range of
frequencies is limited by mass-transfer effects at low frequencies. At high
frequencies, the useful range is limited by the effect of the capacitance of
the electrical double layer
15
. This is shown here for PC deposition. The
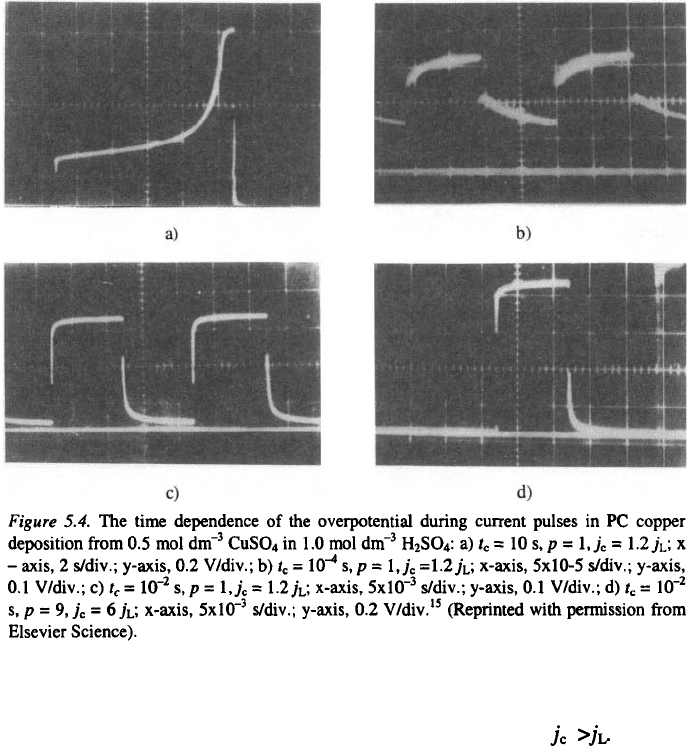
time dependencies of the overpotential during the current pulses are shown
in
Fig. 5.4.
Mass-transfer limitations cause an increase of the overpotential at
deposition times longer than the transition time as shown in Fig. 5.4a; the
system enters full diffusion control at low frequencies if This is
followed by an increase in the average overpotential
10,15
. At high
frequencies, the PC is used both for double-layer charging and discharging,
and for the deposition process, as illustrated by Fig. 5.5. The capacitance
current during periodic charging and discharging of the double layer, at
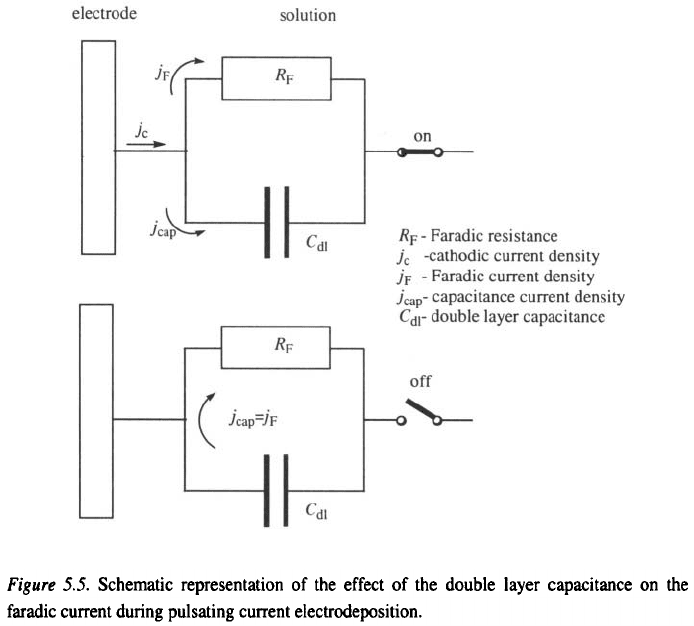
5. Electrodeposition at a Periodically Changing Rate
155
frequencies at which the effect of the double layer cannot be neglected,
produces a smearing effect on the Faradic current wave, as illustrated by
Fig. 5.4b.
Hence, as the frequency increases, the faradic current wave flattens,
approaching a DC shape, and gives the same quality of deposit as DC even
though the overall current appears to be pulsating. This is also followed by
an increase in the average overpotential. Hence, the minimum average
overpotential is a good indicator of the optimum frequency range of
pulsation
3,10,15
in PC deposition. This range depends on the average current
density and p, but, in general, the frequency lies in the range between 10 and
100 Hz, as illustrated in Figs. 5.4c and 5.4d.
In PO deposition, the effect of the double layer capacitance becomes less
pronounced at higher frequencies compared to the other cases
10
. Also, at
very high frequencies, the shape of the PO wave changes; for example, a
square-wave PO becomes similar to a triangular one
10,17
.
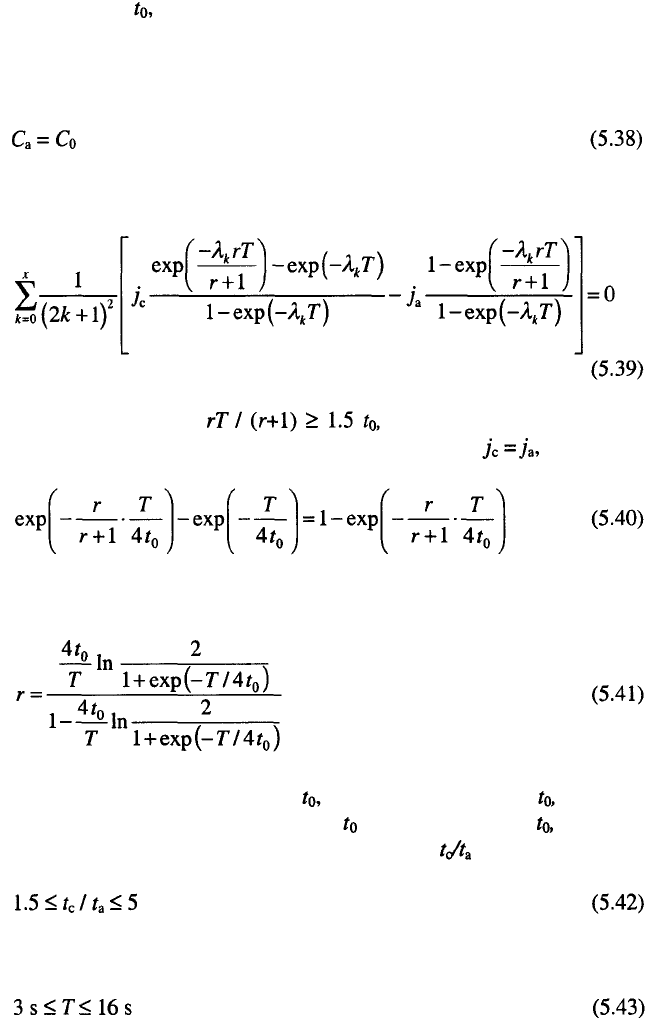
156
Chapter 5
5.2.3 Reversing current in the second range
For T close to the behaviour of the system under RC conditions has to
be analyzed using Eq. 5.18
7
. In this case, the concentration distribution
inside the diffusion layer at the end of the anodic pulse is close to that given
by Eq. 5.10. It follows from Eq. 5.18 that this will occur at:
or
It is known
14
that for the series in Eq. 5.39 can be
approximated using only the first term
(
k = 0). Hence, for
or
It is easy to show that for T = 3 r = 0.7 and for T = 16 r = 0.2 by
assuming that Eq. 5.40 is valid for T > 3 and that for T > 16 the system
behaves as under DC conditions. The optimum ratio is given by
for periods T such that
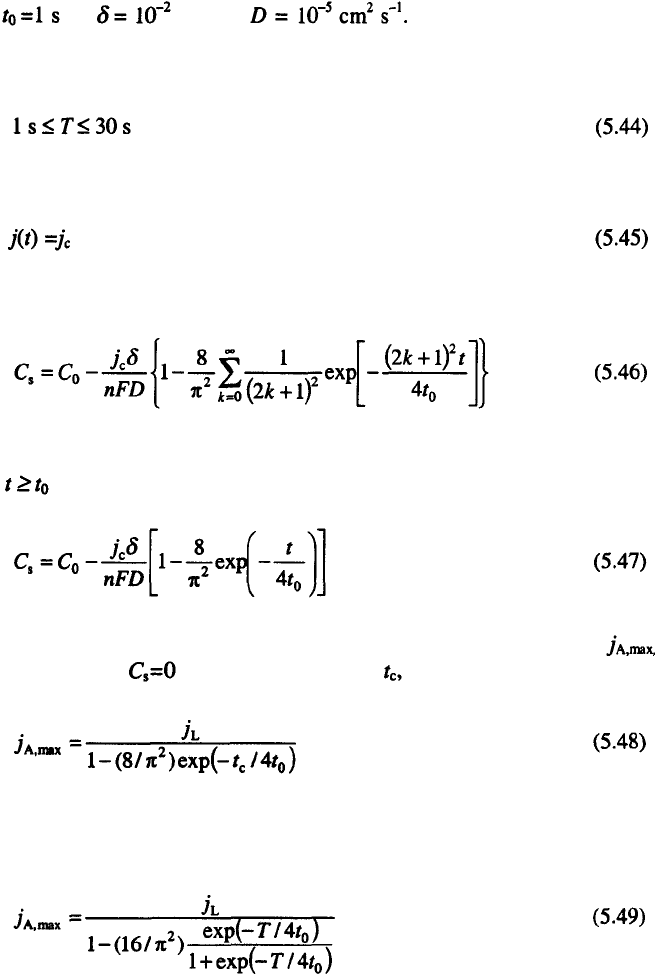
5. Electrodeposition at a Periodically Changing Rate
157
if for cm and A good agreement between
the shape and the frequency of the RC calculated in this way and literature
data is obtained, because in practically all cases, according to Bakhvalov
2
,
On the other hand, the solution of Eqs. 5.9 to 5.12 for
is given by
18
It follows from Eq. 5.46 that the surface concentration of depositing ions
for can be given by:
The maximum amplitude of the current density variation,
corresponding to after a deposition time is given by
It is obvious that using Eqs. 5.1 and 5.4 and r =f(T) given by Eq. 5.41,
Eq. 5.48 can be rewritten in the form
In this way the complete RC wave can be estimated, or precisely
calculated without approximations using a computer.
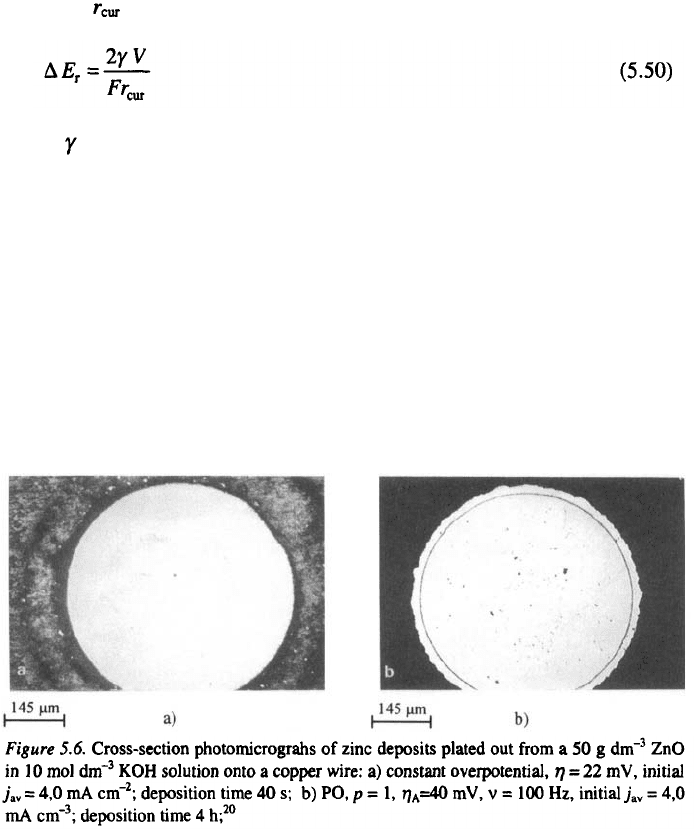
158
Chapter 5
5.3 PREVENTION OF THE FORMATION OF
SPONGY DEPOSITS AND THE EFFECT ON
DENDRITIC PARTICLES
EPCR is used in the charging of silver-zinc storage batteries, to prevent, or to
delay, the formation of spongy and dendritic deposits of zinc
19,20
. It is impossible
to obtain smooth deposits of zinc from alkaline zincate solutions during
prolonged deposition at a constant rate
20,21
, because at lower overpotentials,
spongy deposits arise while at higher overpotentials, dendritic zinc is formed.
It is well known that the reversible potential of a surface with radius of
curvature would depart from that of a planar surface by the quantity
22
where is the interfacial energy. The filaments which form spongy deposits
have extremely small tip radii. This makes the equilibrium potential of the
spongy deposit 7 to 10 mV more cathodic than that of zinc foil
23,24
.
Spongy deposit formation can, however, be completely prevented by PO
deposition
20
, as illustrated in Fig. 5.6.
Obviously, the more negative filaments dissolve faster during the off
period than the flat surface, resulting in a smooth deposit. This is also valid
for deposition using all current or overpotential waveforms that are
characterized by some anodic current flow
3,7
.
If spongy deposit formation is prevented or delayed the charging of the
silver -zinc batteries is considerably improved
3
.
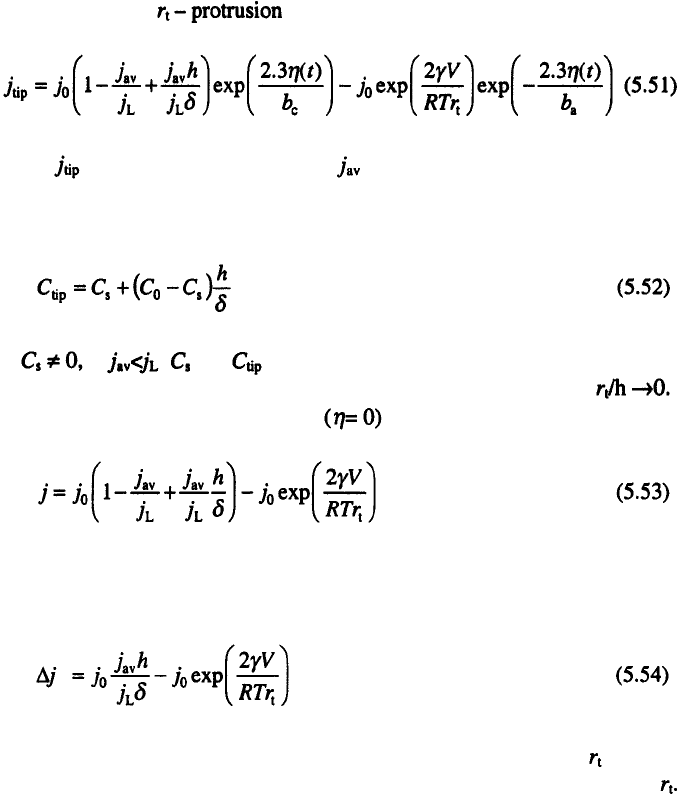
5. Electrodeposition at a Periodically Changing Rate
159
The quantitative treatment of the selective dissolution during pauses can
be performed as follows
25
. Equation 5.30 is valid for a flat electrode surface
or protrusions with sufficiently large tip radii, where the surface energy term
can be neglected. If it can not be neglected in zinc deposition, Eq. 5.30. can
be rewritten for the tip of a protrusion inside the diffusion layer (h – height
of protrusion and tip radius) in the form:
where is the tip current density and is the average current density to the
macroelectrode.
This is because:
if or ( and are the concentration of the depositing ions on
the electrode surface and on the tip of a protrusion, respectively), if
The output current during pauses become
It is easy to show that the difference between the current density on the
tip of a dendrite and the flat surface during the "off ” period can be given by
This means that the dissolution of the a protrusion with tip radius is faster
relative to the flat surface or a protrusion with a sufficiently large value of
It is obvious that spongy filaments can be completely (Fig. 5.6), and
dendrites with low tip radii partially or completely dissolved during the
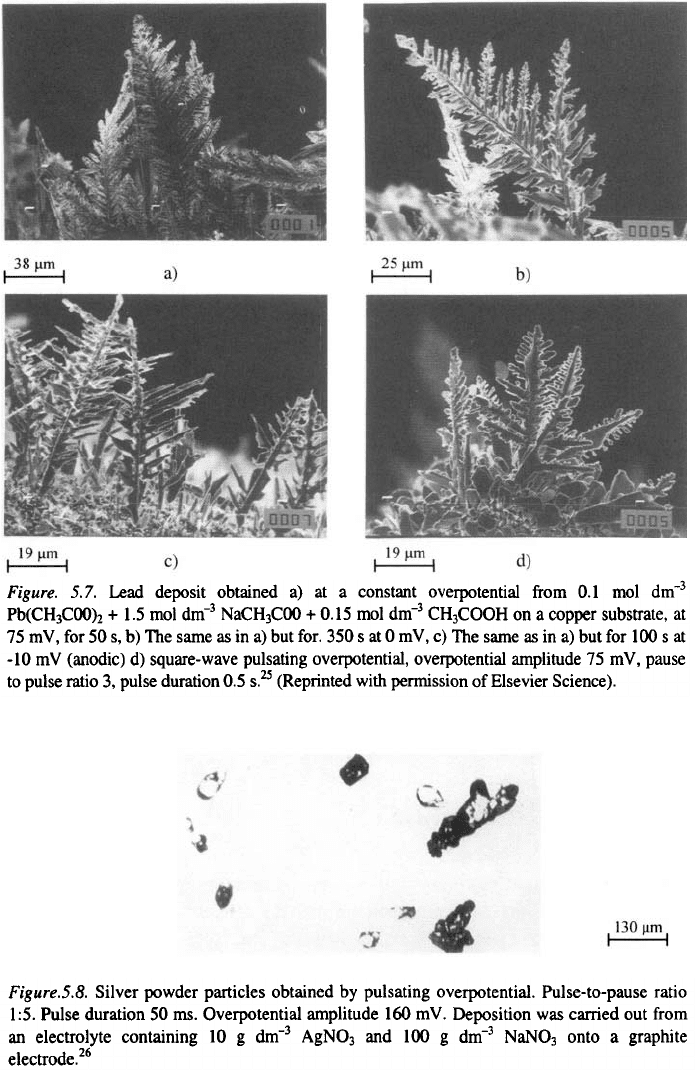
pause, (Fig 5.7.). This means that both branching of dendrites and the
formation of agglomerates can be prevented in square-wave pulsating
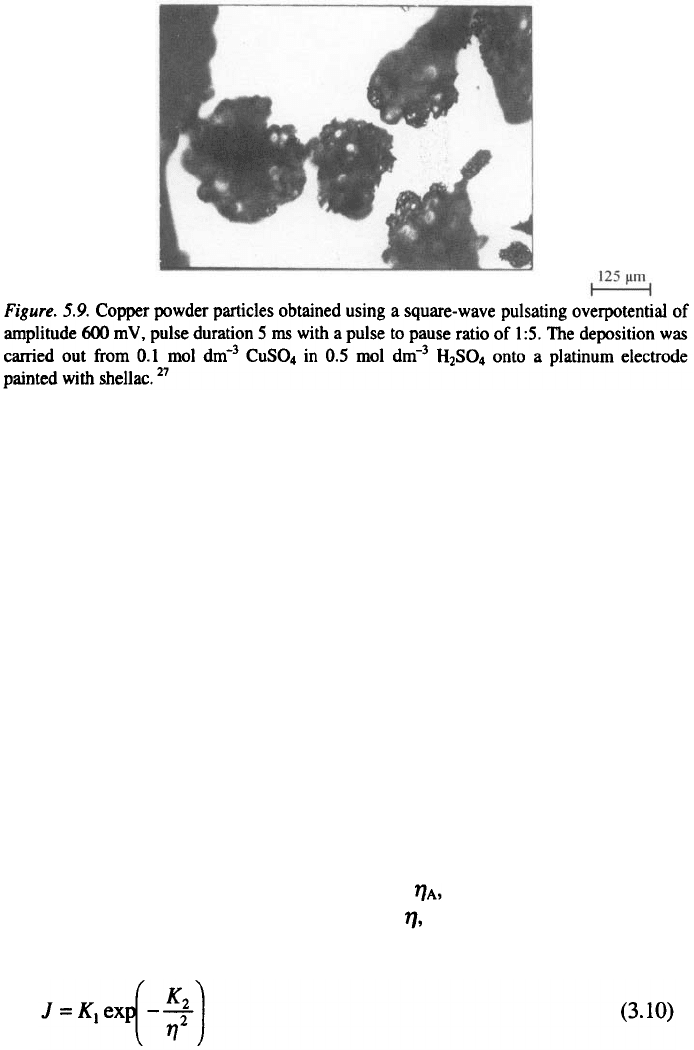
overpotential deposition. In this way, even powder particle like that in Figs.
5.8 and 5.9 can be obtained.
160
Chapter 5
5
. Electrodeposition at a Periodically Changing Rate
161
The monocrystal surfaces of silver powder particles can be explained by
the assumption that during the off period of PO the adatoms in nonstable
positions will dissolve faster than atoms in stable position in lattice. The
similar effect on the morphology of powder particles can be seen in RC
deposition
28,29
, which leads to the strong effect on the apparent density of
copper powders
29
.
5.4
COMPACT DEPOSITS
5.4.1
Surface film
The first stage of metal film formation is nucleation on a foreign
substrate. The nucleation rate, J, is given by Eq. 3.10 and depends strongly
on the deposition overpotential. The nucleation overpotential is larger than
the stationary one in galvanostatic deposition, and stationary values of the
overpotential can be used in discussions of the effect of EPCR in
galvanostatic as well as in potentiostatic deposition. It is obvious that in all
cases of EPCR, the overpotential amplitude, is larger than in constant-
current or constant overpotential deposition, for the same average current
density.
30
Nucleation rate, J, in DC regime is given by:
Therefore,
