Popov K.I., Djokic S.S., Grgur B.N. Fundamental Aspects of Electrometallurgy
Подождите немного. Документ загружается.

142
Chapter 4
dendritic growth at the edges of the electrodes can be avoided by keeping the
deposition current density below some critical value, probably little larger
than this corresponding to the end of the Tafel linearity. At this current
density the less coarse deposit in the homogenous field without the dendrites
at the edges of electrodes can be expected.
The current density distribution in electroplating and electroforming were
also treated semiquantitatively and it was shown that decrease of ohmic
resistance of electrolyte and increase of the Tafel slope for the cathodic
process improves it. Obviously, all above discussion is valid if the local
values of limiting diffusion current density do not varies along electrode
surface, i.e. if the effect of the hydrodynamics can be neglected. As a metter
of fact, the diffusion layer thickness may vary along the electrode interface
due to hydrodynamic conditions and cause the different deposition
conditions. This phenomenon is treated elsewhere
20,21
4.5 FURTHER READINGS
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
Ibl, Norbert. “Current Distribution.” In Comprehensive Treatise of Electrochemistry,
Vol. 6, Ernest E. Yeager, John O’M. Bockris, Brian E. Conway and S. Sarangapani, eds.
New York, NY: Plenum Press, 1983.
Ibl N., Current distribution in electrolysis (in French). Oberflähe-Surface 1975; 16:23-32
Wagner C. Theoretical analysis of the current density distribution in electrochemical
cells. J. Electrochem. Soc. 1951; 98:116-28
Newman, John., Electrochemical Systems. N.J: Engelwood Clifts, Prentice Hall, 1973
Marathe V., Newman J. Current distribution on a rotating disc electrode. J. Electrochem.
Soc. 1969; 116: 1704-19
Kasper C. The theory of the potential and the technical practice of electrodeposition. I.
The general problem and the case of uniform flow, Trans. Electrochem. Soc., 140;
77:131-42
Hoar T.D., Agar. Factors in throwing power illustrated by potential-current
dependencies. Disc. Faraday. Soc. 1947; 1:162-68
Haring H.E., Blum W.M. Current distribution and throwing power in electrodeposition.
Trans. Am. Electrochem. Soc. 1923; 43:365-97
Popov K.I., S.K., S.M. The current distribution in an electrochemical cell.
Part I: The current voltage relationship for a cell with parallel plate electrodes. J. Serb.
Chem. Soc. 1995; 60:307-16
Popov K.I., M.D., Totovski V.N. Some aspects of current
density distribution in electrolytic cells I: Dendritic growth of cadmium at the cathode
edge in galvanostatic electrodeposition. Surf. Technol. 1983; 19:173-80
Popov K.I., S.K., S.M. J. The current distribution in an electrochemical
cell. Part II:Qualitative considerations of the basis of polarization curve shape.J. Serb.
Chem. Soc. 1996; 61:583-90
Popov K.I., S.M., T.M. The current distribution in an electrochemical cell.
Part V: The determination of the depth of the current line penetration between the edges
of the electrodes and the side walls at the cell. J. Serb.Chem. Soc. 1999; 64:795-800

4. The Current Distribution in Electrochemical Cells
143
13.
14.
15.
16.
17.
18.
19.
20.
21.
Popov K.I., S.M., P.M. The current distribution in an electrochemical
cell. Part VII: The concluding remarks. J. Serb.Chem. Soc. in press
Popov K.I., S.M., P.M. The current distribution in an electrochemical
cell. Part VI: The quantitative treatment for cells with three plane parallele electrode
arangments. J. Serb.Chem. Soc. 2001; 66: 491-98
Popov K.I., M.G., E.R., Z.Ž. The current density
distribution on stationary wire electrodes during copper and lead electrodeposition.
Hydrometallurgy 1997; 46: 321-36
Popov K.I., Z.P., N.V., S.D. Fundamental aspects of
plating technology, V. The effect of strongly adsorbed species on the morphology of
metal deposits. Surf. Technol. 1985; 25:217-22
Popov K.I., N.V., Popov S.R. Fundamental aspects of plating technology, III.
The effect of electrodeposition from complex salt solutions on metal distribution over
macroprofiles. Surf.Technol. 1984; 22:245-50
Spiro, Peter, Electroforming. Teddington, Robert Draper Ltd, 1968.
Popov K.I., R.M. A new line division concept for the determination of the
current distribution in electrochemical cell. Part I. Theoretical background of the “corner
weaknes” effect in electroforming. J.Serb.Chem.Soc. 2000; 65:905-14
Levich, Veniamin, “Physicochemical Hydrodunamics” N.J: Engelwood Clifts, Prentice
Hall, 1962.
Ibl, Norbert. Dossenbach, O “Convective Mass Transport.” In Comprehensive Treatise
of Electrochemistry, Vol. 6, Ernest E. Yeager, John O’M. Bockris, Brian E. Conway and
S. Sarangapani, eds. New York: Plenum Press, 1983.
This page intentionally left blank
Chapter 5
ELECTRODEPOSITION AT A PERIODICALLY
CHANGING RATE
5.1 BASIC DEFINITIONS
It has been known for a relatively long time that the application of a
periodically changing current in metal electrodeposition practice leads to
improvements in the quality of electrodeposits. Three types of current
variation have been found useful: reversing current (RC); pulsating current
(PC); and sinusoidal, alternating current superimposed on a direct current
In recent years, the beneficial effects of pulsating overpotential
(PO) have also been discussed
3
. Even though this kind of electrodeposition
at a periodically changing rate (EPCR) is important from a theoretical point
of view and offers a variety of experimental possibilities, it is as yet not
frequently used in metal electrodeposition practice.
5.1.1 Reversing current
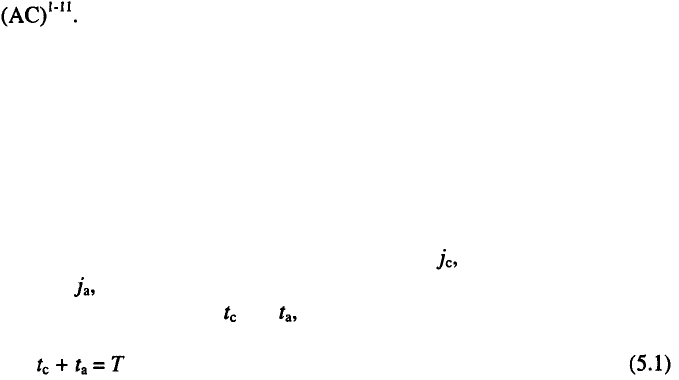
Reversing current is represented schematically in Fig. 5.1. It is
characterized by the cathodic current density, and the anodic current
density, as well as by the duration of flow of the current in the cathodic
and the anodic direction, and respectively. Naturally,
where T is the full period of the RC wave.
145
146
Chapter 5
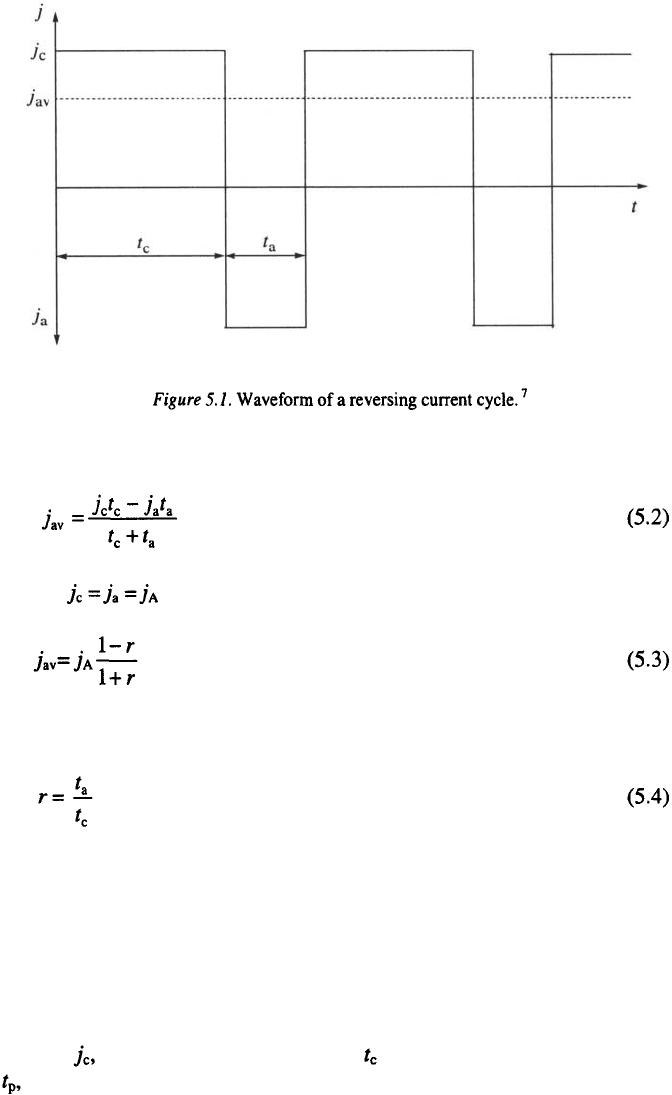
The average current density is then given by:
and for
where
RC is used in the second and millisecond range
7
.
5.1.2 Pulsating current
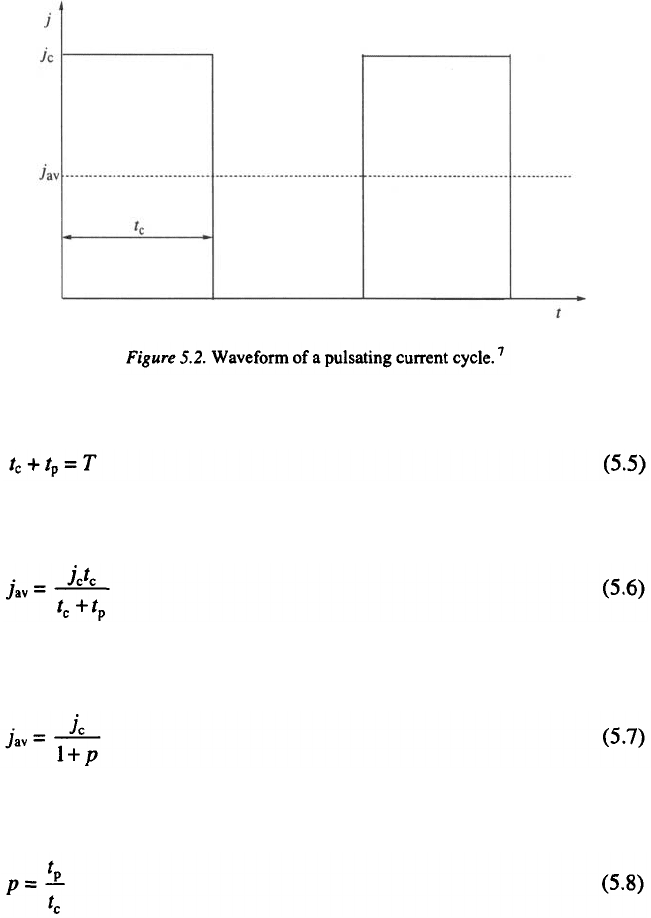
Pulsating current consists of a periodic repetition of square pulses. It is
similar in shape to RC except for the absence of the anodic component, as is
shown in Fig. 5.2. PC is characterized by the amplitude of the cathodic
current, the cathodic deposition time, (on period), and the time interval
in which the system relaxes (off period).
5. Electrodeposition at a Periodically Changing Rate
147
The full period, T, is given by
and the average current density by
or
where
It should be noted that rectified sinusoidal AC, especially half-rectified
sinusoidal AC, often termed pulsating current in the literature, shows similar
effects to those of PC
7
.
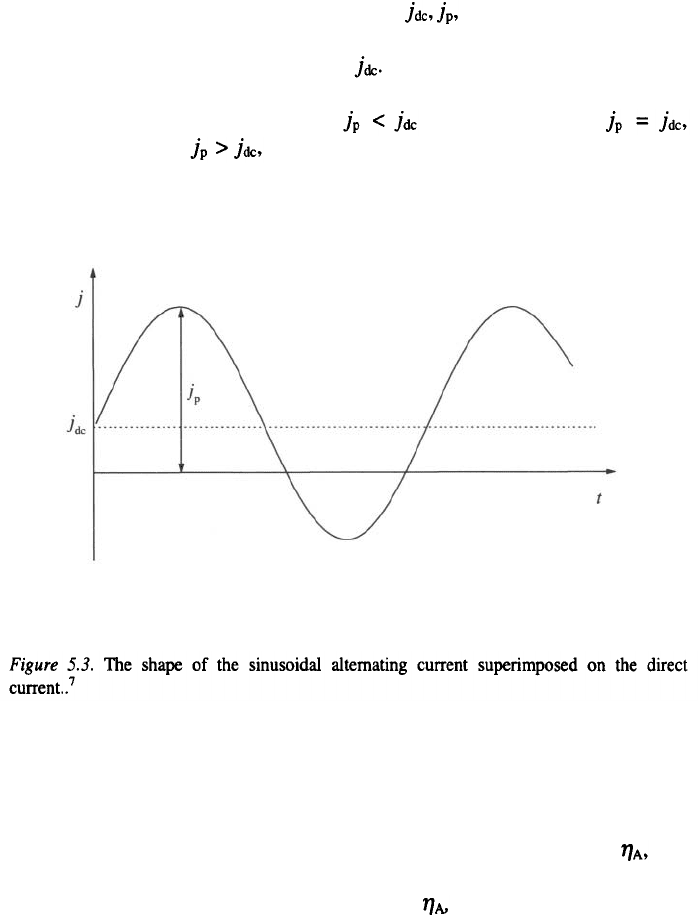
148
Chapter 5
5.1.3 Alternating current superimposed on direct current
Sinusoidal AC superimposed on a direct cathodic current (DC) is
represented in Fig. 5.3. It is characterized by and the frequency, which
is usually 50 or 60 Hz. The resultant is termed an asymmetric sinusoidal
current. The average current is equal to
At a given DC value, three different types of current can be obtained,
which can be denoted as follows: "rippling current";
“pulsating current"; "current with an anodic component." The last
type is mainly used in plating practice.
5.1.4 Pulsating overpotential
Pulsating overpotential consists of a periodic repetition of overpotential
pulses of different shapes. Square-wave PO is defined in the same way as PC
except that the overpotential pulsates between the amplitude value and
zero instead of current density. Non-rectangular pulsating overpotential is
defined by the amplitude of the overpotential, frequency, and overpoten-
tial waveform
7
.
There are a number of different current and overpotential waveforms
used in EPCR
12,13
, but the most important have been mentioned above.
5. Electrodeposition at a Periodically Changing Rate
149
5.2 SURFACE CONCENTRATION OF DEPOSITING
IONS IN THE PERIODIC CONDITION
5.2.1 Electrodeposition with periodically changing range in the
millisecond range
Electrodeposition with a periodically changing rate can be described in
terms of time-and distance-dependent concentrations:
Equations 5.9 to 5.12 are solved for different j(t) shapes and the solutions
applied to different types of problems
7
.
The current density j(t) is the periodic function of time, which for
periodic reverse currents
14
is given by:
for pulsating currents
15
by:
and for AC superimposed on DC
16
by:
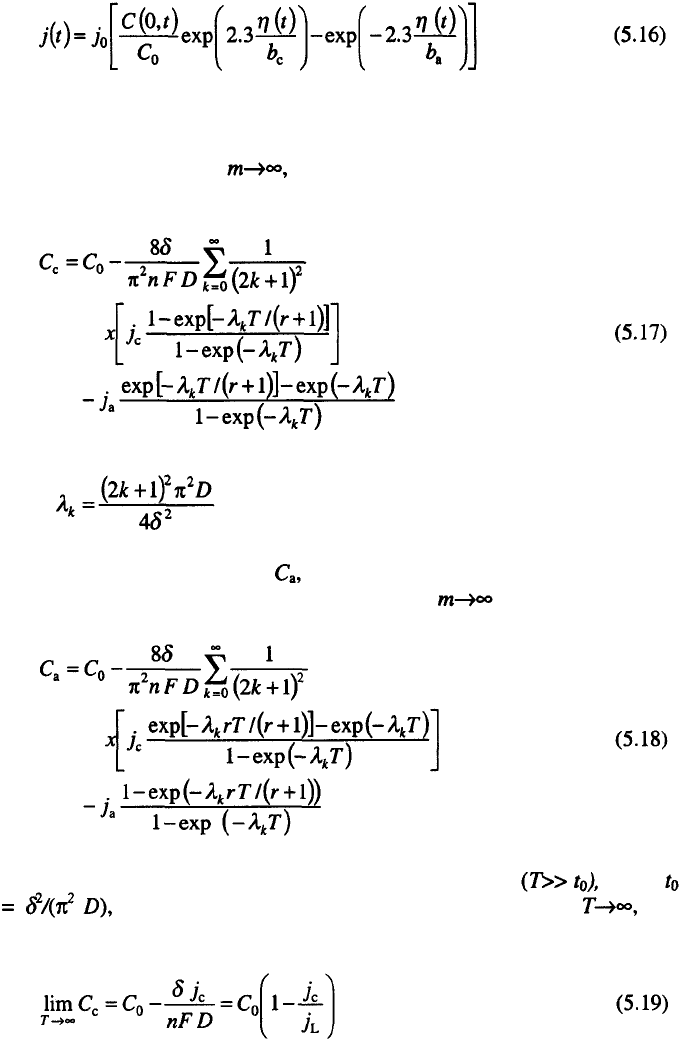
150
Chapter 5
In the case of pulsating overpotential
3
, j(t) is given by:
The surface concentration under periodic conditions can be evaluated as
follows. For j(t) given by Eq. 5.13, the solution of Eqs. 5.9 to 5.12 for x = 0,
t = [m + 1(r + 1)] T, and i.e., at the end of the cathodic pulses, under
the periodic conditions is given by
14
:
where
The surface concentration, at the end of the anodic pulses under the
same conditions, i.e., for x = 0, t =
(
m + 1) T
,
and is given by:
It is easy to show that for a sufficiently long period T, where
the system behaves as under DC conditions. For and
taking into account Eq. 2.29, Eqs. 5.17 and 5.18 become

5. Electrodeposition at a Periodically Changing Rate
151
and
For a sufficiently small value of T,
For j(t) given by Eq. 5.14, solution of Eqs.5.9 to 5.12 for x = 0, t=[m +
1/(p + 1)] T, and i.e., at the end of the cathodic pulses under periodic
conditions, is given by
15
:
The surface concentration at the end of pauses, under the same
conditions [x = 0, (m + 1) T, is given by:
As in the previous case for the system behaves as under DC
conditions where
and
For it follows from Eqs. 5.22 and 5.23 that
