Popov K.I., Djokic S.S., Grgur B.N. Fundamental Aspects of Electrometallurgy
Подождите немного. Документ загружается.

122
Chapter 4
4.1.5.2 The critical current density for dendritic growth initiation at
the edges
The polarization curve equation is given by:
for The critical overpotential for dendritic growth initiation, is
given by
Substitution of from Eq. 3.60 into Eq. 2.28 and further rearranging
produces:
where is the critical current density for the dendritic growth initiation. On
the other hand the edge current density is given by:
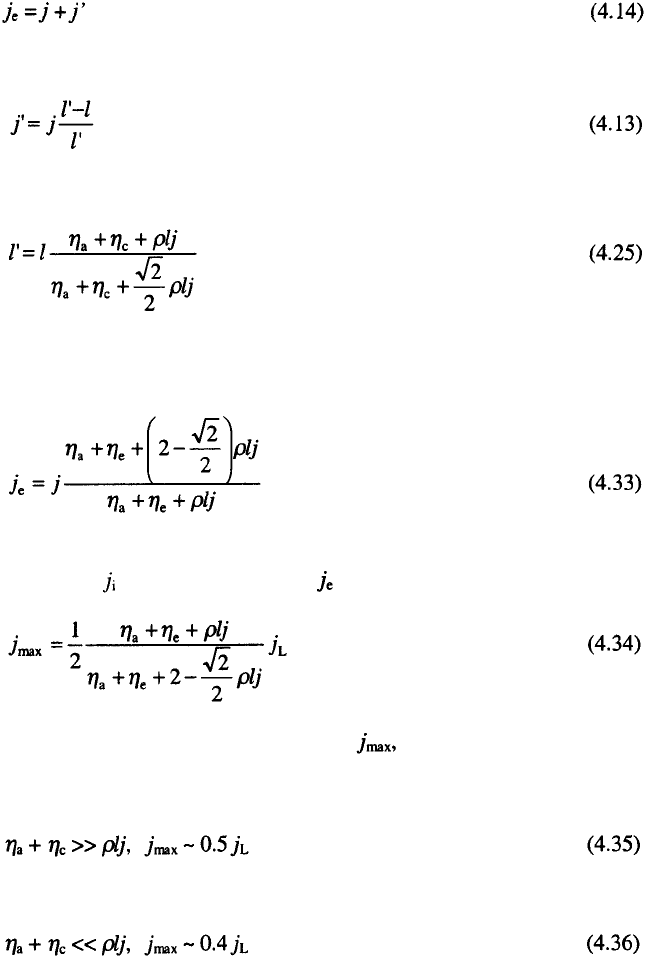
4. The Current Distribution in Electrochemical Cells
123
where
and
If
L
> L’, the edge current density could be obtained by combining Eqs.
4.13, 4.14
and
4.25
as:
Assuming that maximum edge current density is given by Eq. 4.32 the
substitution of in Eq. 4.33 instead of and further rearranging produce:
from which the maximum current density, in the homogenous field at
which dendrites at the edges do not grow can be calculated. It follows from
Eq. 4.34 that for:
and if
being in both cases larger than the current density corresponding to the end
of the Tafel linearity, which is the optimum current density for the
deposition of compact metal (see section 3.2.1.3.1). Hence, if deposition
current density corresponds to the end of the Tafel linearity dendrites will
not grow at the edges of the electrode. It should be noted that in metal
124
Chapter 4
electrorefinning working current density can be determined relative to the
initial concentration of depositing ions, because it remains constant or
increases during refining process. In electrowinning processes the working
current density must be determined relative to the final concentration of
depositing ions, because it is lower than initial one. The same reasoning is
valid in the case of L <L’, meaning, in general, that if the current density in
cell is lower than the dendrites and probably carrot like protrusion on
the electrode edges can not grow.
4.2 CELLS WITH LOW ANODE POLARISATION
Cells with a small cathode and a large anode are often used in
electroplating technology. In this case, a homogenous distribution of the
deposit over the entire cathode is required.
4.2.1 The dependence of the current density at the tip of a
stationary wire electrode on the current density in the middle
of the electrode
It can be seen from Fig. 4.21 that the dissipation of current lines from the
tip of a stationary wire electrode is more pronounced than in the case of the
edges of plane parallel electrodes. This is because the dissipation in the
former case occurs through the space, while in the latter case it takes place in
one plane, normal to the electrodes, to which two symmetrically positioned
points belong. Hence, it can be taken that the overall resistance between the
tip of the cathode and anode will be equal to an infinitely large number of
resistances, as in the case of the edges of two plane parallel electrodes
connected in parallel, being equal to zero.
The cell voltage, U, for the part of the system where the current density is
homogeneously distributed is given by:
where R is the ohmic resistance of the electrolyte and
I
current in the cell,
and for the tip of wire electrode:
where and are the overpotential at the tip of wire electrode and at the
edge of the cylindrical anode, respectively, or after elimination of U-E from
Eqs. 4.37
and
4.38

4. The Current Distribution in Electrochemical Cells
125
In this way the ohmic potential drop in a homogenous field transforms
into the electrochemical overpotential for points at the tip of a wire electrode
or in a similar position. This means a larger tip current density than in a
homogenous field. Finally, if the anode surface area is much larger than that
of the cathode i.e., if:
Eq 4.38 can be rewritten in the form:
The ability of an electrode to distribute uniformly current density on a
whole cathode can be easily estimated by comparing the cathodic
polarization curve with the cathodic current density-cell voltage dependance.
The lower is the difference between them, the better distribution of the
current density should be expected.
On the other hand, Eq. 4.41 can be rewritten in the form:
126
Chapter 4
assuming that in mixed control deposition:
or in activation controlled deposition:
where
It can be taken to the first approximation that the same relation is valid
also for the edge of a small, square stationary vertical cathode placed in the
middle of a large cylindrical anode because the current line distribution is
similar to the one from tip of wire electrode.
It follows that for Eq. 4.42 can be rewritten in the form:
This means that the lower the resistance of the electrolyte the is lower the
difference in the current densities in the middle and at the tip of the
electrode. The increase of leads to a more uniform current distribution,
also. This can happen in the presence of strongly adsorbed species or during
deposition from some complex salt solution.
It is also seen that current density distribution on a macroprofile becomes
uniform if but in this case rough and dendritic deposit appears, being
unuseful for plating purpose.
The difference, between the current density at the tip (edge) and in the
middle of the electrode is obviously:
4. The Current Distribution in Electrochemical Cells
127
and
It follows from Eq. 4.42 that the exchange current density does not effect
the current distribution, but there is an exception in the cases of
electrodeposition processes characterized by very large value of the
exchange current density. When the deposition can be under diffusion or the
ohmic control and the deposit will be formed only at the edges or in a similar
position, where ohmic resistance is low. Decreasing the exchange current
density by complexing the depositing ion leads to a more homogenous
distribution. The above discussion is illustrated by the following examples.
4.2.2
Experimental evidence
4.2.2.1 The effe
ct of ohmic resistance
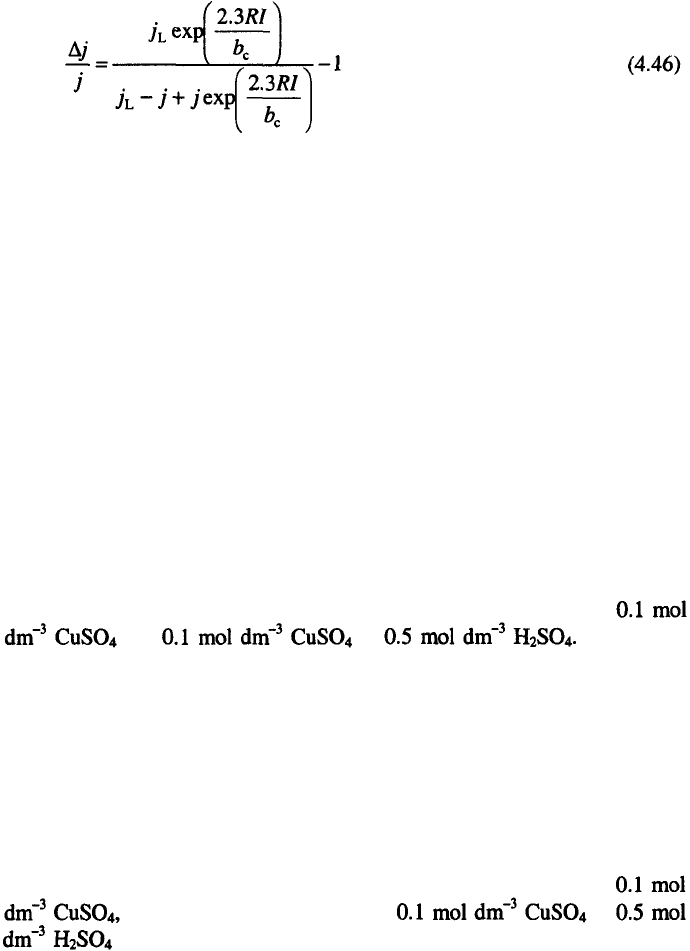
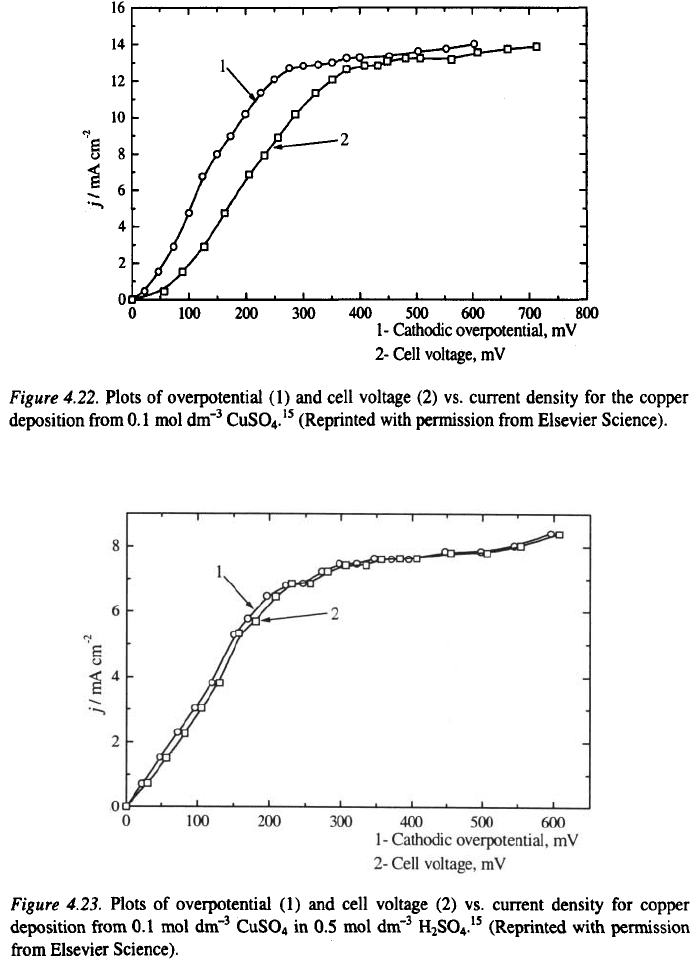
In order to illustrate the effect of the ohmic resistance of a cell, deposition
of copper was performed at room temperature on to a stationary copper wire
electrodes (length 40 mm and diameter 0.8 mm) placed in the middle of a
cylindrical cell (length 5 cm and diameter 6 cm). The surface of the cell was
covered by a high purity copper plate from electrolytes containing
and in The plot of
cell voltage and overpotential vs. current density for the copper deposition
are shown in Figs. 4.22 and 4.23.
The relation between the current density at the electrode tip and in the
homogenous field can be estimated by considering the cathodic overpotential
– current density and cell voltage –current density dependencies. According
to Eq. 4.41, the tip overpotential is equal to the cell voltage for a wire
electrode.
It could be seen that the tip overpotential (cell potential) is larger than the
overpotential in the middle of the electrode during deposition from
while during deposition from in
the overpotentials are practically the same. In the former case
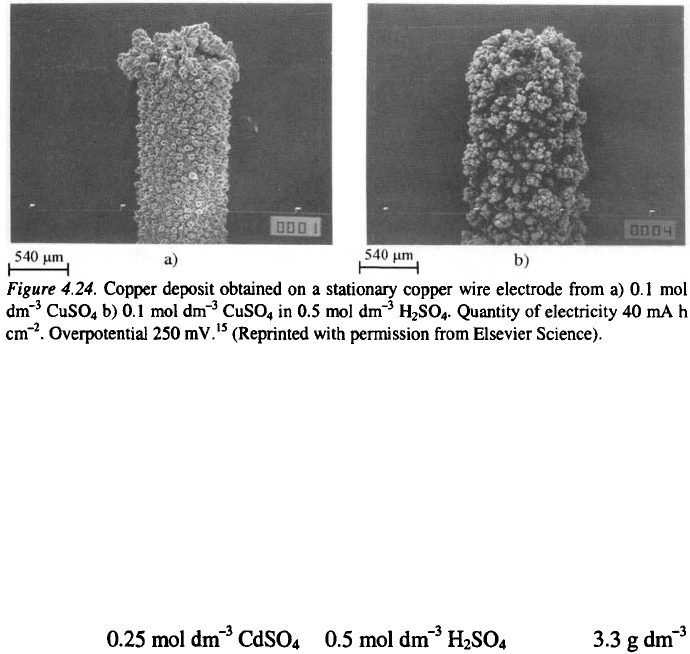
there is a large difference in the morphology of the deposit at the tip and the
rest of the electrode (Fig. 4.24a), while in the latter case the quality of the
deposit is the same over the whole surface (Fig. 4.24b).
128
Chapter 4
4. The Current Distribution in Electrochemical Cells
129
This is in perfect agreement with Eq. 4.44.
4.2.2.2 Deposition in the presence of strongly adsorbed organic
additives (effect of increased cathodic Tafel slope)
In order to illustrate the effect of a strongly adsorbed organic additive
16
,
cadmium was deposited onto a stationary vertical flat copper electrode of
area 1 cm x 1 cm placed in the middle of a cylindrical cell of diameter 6 cm,
and height 5 cm. The cell surface was covered by the anode, which was
made from high purity cadmium plate. The reference electrode was a high
purity cadmium wire. The electrolyte used in all the experiments was a
solution of
in to which
polyoxyethylene alkylphenol (9.5 mol ethylene oxide) was added.
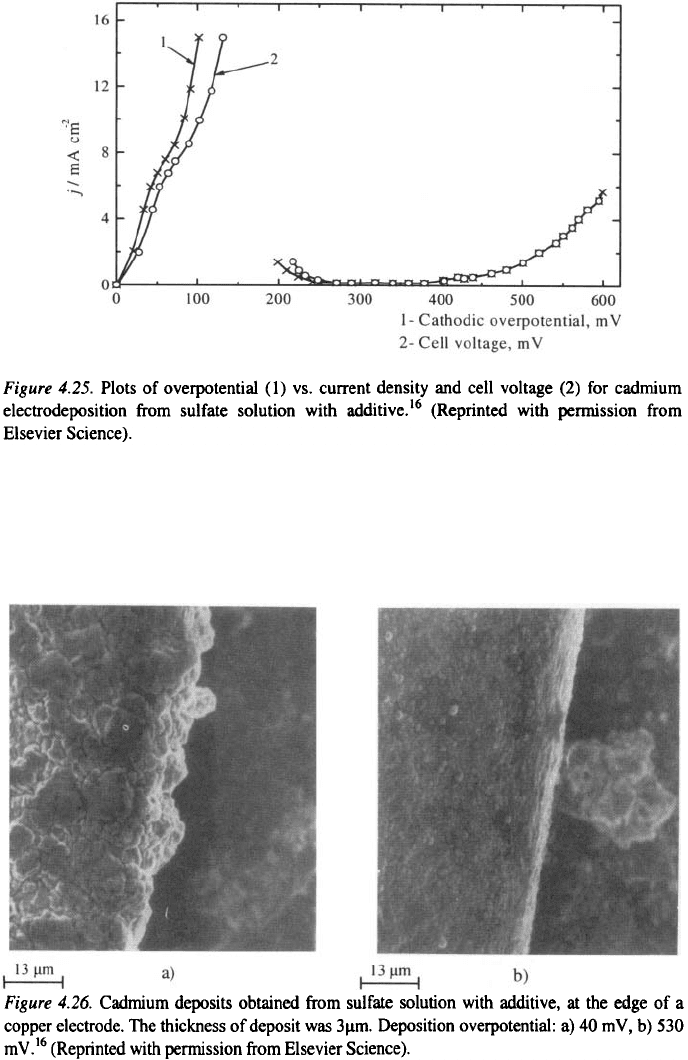
The overpotential-current density and cell voltage-current density plots
for cadmium deposition are presented in Fig. 4.25. The cathodic polarization
curve obtained from potentiostatic polarization measurements has a similar
shape to that found for an anode which can become passive; above a certain
overpotential, increasing the cathode polarization leads to a decrease in the
cathodic current followed by a range of potential in which the overpotential
has little effect on the current. Current oscillations were observed at the
beginning of this plateau in some cases (see also section 3.1.6.2).
It was shown in a section 3.2.1.3.1 that the optimum plating overpotential
is determined by the upper limit of validity of the Tafel equation for the
deposition process. In this case, as can be seen from Fig. 3.13 that the
optimum deposition overpotentials for cadmium deposition are about 40 and
530 mV in the absence and in the presence of additive, respectively.
130
Chapter 4
Figure 4.25 shows that there is a large difference between the deposition
overpotential and the cell voltage (tip overpotential) at low overpotentials
which becomes negligible at high overpotentials, indicating a uniform current
density distribution
16
, due to the additive adsorption, as illustrated in Fig 4.26.
4. The Current Distribution in Electrochemical Cells
131
4.2.2.3 Deposition from a complex salt solution
(
effect of exchange
current density
)
In order to illustrate the effect of deposition from complex salt solutions
silver was deposited from simple and complex salt solutions
17
. The
electrolytes used throughout the experiments were in
solution and in
to which was added ammonium hydroxide to dissolve the precipitate of silver
sulfate. The conductivities of the above solutions were almost the same. Silver
was deposited onto a stationary vertical platinum cathode (1 cm x 1 cm)
placed in the middle of a cylindrical cell (diameter, 6 cm, height 5 cm); the
surface of the cell was covered by the anode, a high purity silver plate.
Polarization curves were obtained for the platinum wire cathode, which had
previously been plated with silver from the ammonium complex salt solution.
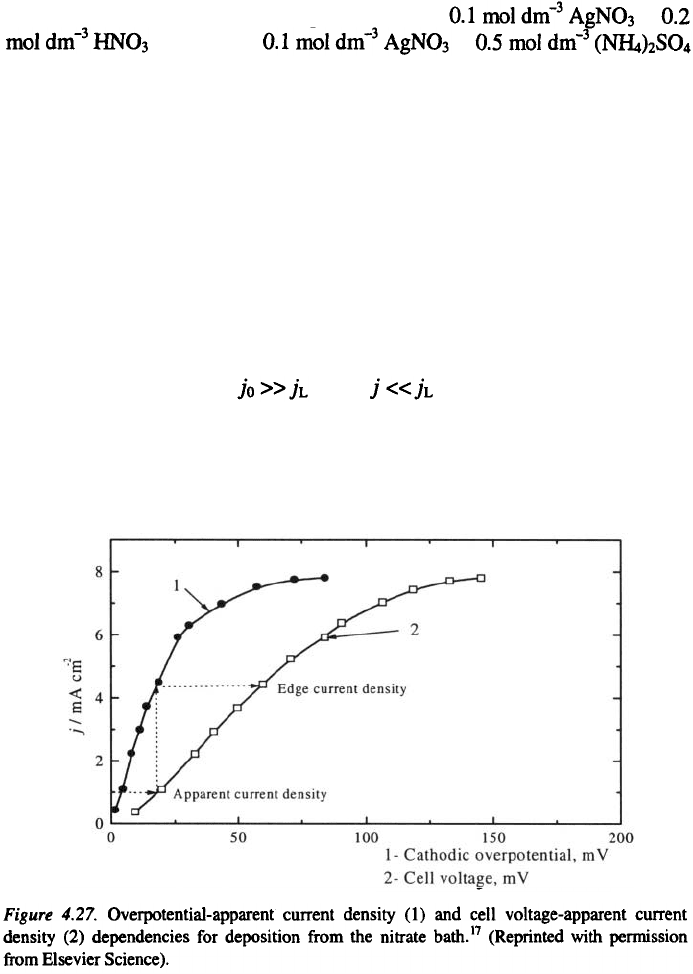
The overpotential-apparent current density and cell voltage (edge
overpotential)-apparent current density plots for silver deposition from the
nitrate solution and from the ammonium salt solution are presented in Figs.
4.27 and 4.28, respectively.
Silver deposition from the nitrate bath is under pure diffusion control at
all overpotentials because and at the nucleation in the middle
of the electrode does not occur because of ohmic control before nucleation.
Hence, deposition from the nitrate bath is expected only at the edges where
ohmic resistance is better, as predicted by Fig. 4.27. This is illustrated in Fig.
4.29a and b.
