Ortiz de Montellano Paul R.(Ed.) Cytochrome P450. Structure, Mechanism, and Biochemistry
Подождите немного. Документ загружается.


124
Mark J.I. Paine et al.
in relation to hydride transfer. Non-conservative
substitutions of
Ser457,
Asp675, and Cys630 pro-
duce large decreases in cytochrome c reductase
activity^^' ^^' ^^' ^^. These parallel changes in the
rate of flavin reduction are associated with large
NADPD isotope effects^ ^ thus pointing to
impaired hydride transfer from NADPH. Based on
spectral analysis of flavin reduction of mutants
at residues Ser457, Asp675, and Cys630 and
analysis of the kinetics and pH dependence of
cytochrome c reduction, it was proposed that
Cys630 acts a proton donor/acceptor to FAD, and
Ser457 and Asp675 interact to stabilize both the
transition state and the FAD semiquinone^^. This
is supported by crystallographic analysis of
CPR mutants, which shows that both Ser457 and
Asp675 are directly involved in interaction with
the nicotinamide group of NADP(H) and likely to
orient the C-4 atom of NADP(H) into an optimal
position for hydride transfer^^. The catalytic triad
of Ser457, Asp675, and Cys630 is highly con-
served in all diflavin oxidoreductases apart from
NRl, which has a nonconservative Ala549 corre-
sponding to Cys630^^ and a greatly decreased
(~ 100-fold) rate of reduction relative to CPR.
2.6. The Electron Transfer
Mechanism
CPR catalyzes the transfer of electrons along
the pathway NADPH-> FAD-> FMN-> P450,
and there are two striking features of its involve-
ment in the monooxygenase system. First, the
flavin redox potentials are such that electron trans-
fer from NADPH to FAD is moderately unfavor-
able (the physiological -direction of electron
transfer in the structurally related ferredoxin
reductase is in the direction of NADPH forma-
tion).
Second, NADPH is an obligate two-electron
donor, but these two electrons must be delivered to
P450s individually at the appropriate points in the
catalytic cycle. CPR orchestrates the electron sup-
ply from NADPH to the P450 cytochromes by sta-
bilizing the one-electron reduced form of the flavin
cofactors FAD and FMN. This stabilization gives
rise to the blue semiquinone species (FADH7
FMNH*), which is observed in both kinetic and
equilibrium studies of CPR^^' ^^-84 cPR and the
aforementioned related structural isoforms of
nitric oxide synthase^^, methionine synthase reduc-
tase^"^,
and protein NRl^^ can accept a maximum
of four electrons. The different redox states of
these enzymes have distinct spectral characteris-
tics.
However, the fact that the oxidised, semi-
quinone and reduced states of both FAD and FMN
have virtually indistinguishable spectra means that
kinetic and equilibrium studies of the diflavin
reductases is complicated. This is alleviated, how-
ever, by using the genetic approaches discussed
previously to dissect the enzymes into their com-
ponent domains^^'
^^' ^^^ ^^.
This strategy has been
used successfiilly with human CPR, human
methionine synthase reductase, and human oxi-
doreductase NRl. Molecular dissection of these
diflavin enzymes removes ambiguity in spectral
assignment during redox titration, potentiometry,
and kinetic analysis^^'
^'^' ^^-^^.
With CPR, the do-
mains have also formed a basis for more detailed
study of the kinetic and thermodynamic proper-
ties of the frill-length enzymes^^'
^^' ^^.
The use of
FMN-depleted frill-length enzyme has also facili-
tated assignment of reduction potentials and indi-
vidual kinetic phases in other mammalian CPRs^^.
Each isolated flavin-binding domain of human
CPR (and of related family members) is soluble,
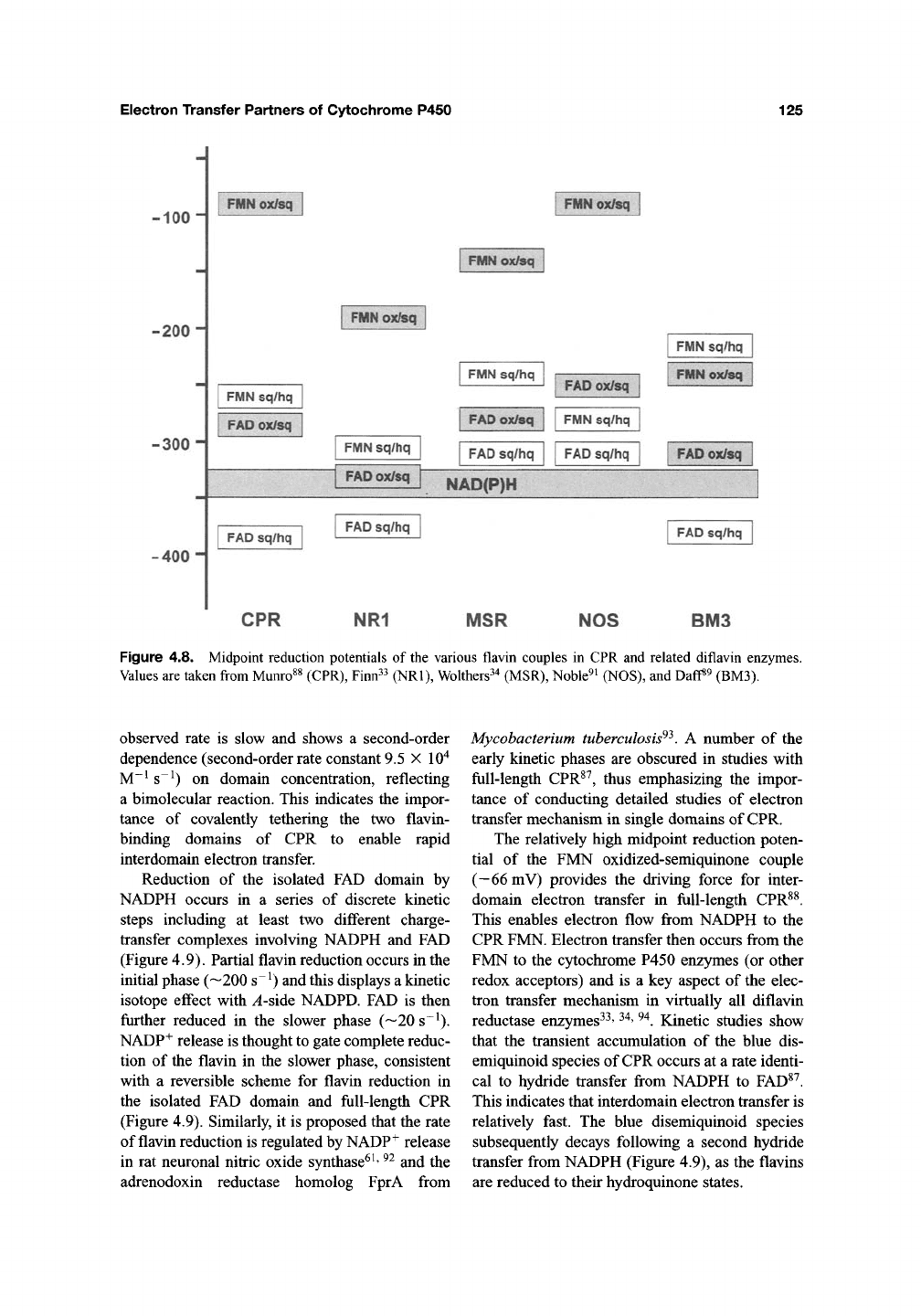
binds flavin, and is redox active^^' ^^. The mid-
point reduction potentials of the flavin couples are
similar in the isolated domains and ftill-length
CPR^^, indicating that the isolated domains are
good mimics of domain properties in ftiU-length
CPR. The blue semiquinone on the FMN is both
kinetically and thermodynamically stabilized, and
the reduction potential of the oxidized-semi-
quinone redox couple is much more positive than
those of the other redox couples in CPR (Figure
4.8).
This accounts for the "blue/green" appear-
ance of purified CPR prior to chemical treatment
with exogenous oxidants. The midpoint reduction
potentials measured for human CPR^^ are in broad
agreement with earlier studies performed with
rabbit CPR^^. The reductive half-reaction of the
isolated FAD domain has been studied by
stopped-flow methods^^. Hydride transfer is
reversible, and reduction of NADP^ by FADH2 is
more rapid (8 s ') than FAD reduction by
NADPH (3 s~^), consistent with the relative val-
ues of the midpoint reduction potentials for the
2-electron couples for FAD/FADH2 and NADPH/
NADP^. The more rapid reduction of NADP^
probably reflects the physiological role of the
ancestral FNR-like protein, which evolved to
catalyze NADP^ reduction in photosynthetic
electron transfer. The isolated FAD domain trans-
fers electrons to the isolated FMN domain, but the
Electron Transfer Partners of Cytochrome P450
125
-100 i
-200'
-300 i
-400 i
n^m^li
jMf^'^s&^ki^-
mm mm
mmmtm
FMN sq/hq
FMN sq/hq
FMN sq/hq
fMUQ^'Di^liil
iFlffNIl^tiNi
'W0^mm
fmmm
FMN sq/hq
FMN sq/hq
FMIi^i^
FAD sq/hq FAD sq/hq
•PM^mm
NAE^PP
FAD sq/hq
FAD sq/hq
FAD sq/hq
CPR NR1 MSR NOS BM3
Figure 4.8. Midpoint reduction potentials of the various flavin couples in CPR and related diflavin enzymes.
Values are taken from Munro^^ (CPR), Finn^^ (NRl), Wolthers^^ (MSR), Noble^i (NOS), and Daff«9 (BM3).
observed rate is slow and shows a second-order
dependence (second-order rate constant 9.5 X 10"^
M"^ s~^) on domain concentration, reflecting
a bimolecular reaction. This indicates the impor-
tance of covalently tethering the two flavin-
binding domains of CPR to enable rapid
interdomain electron transfer.
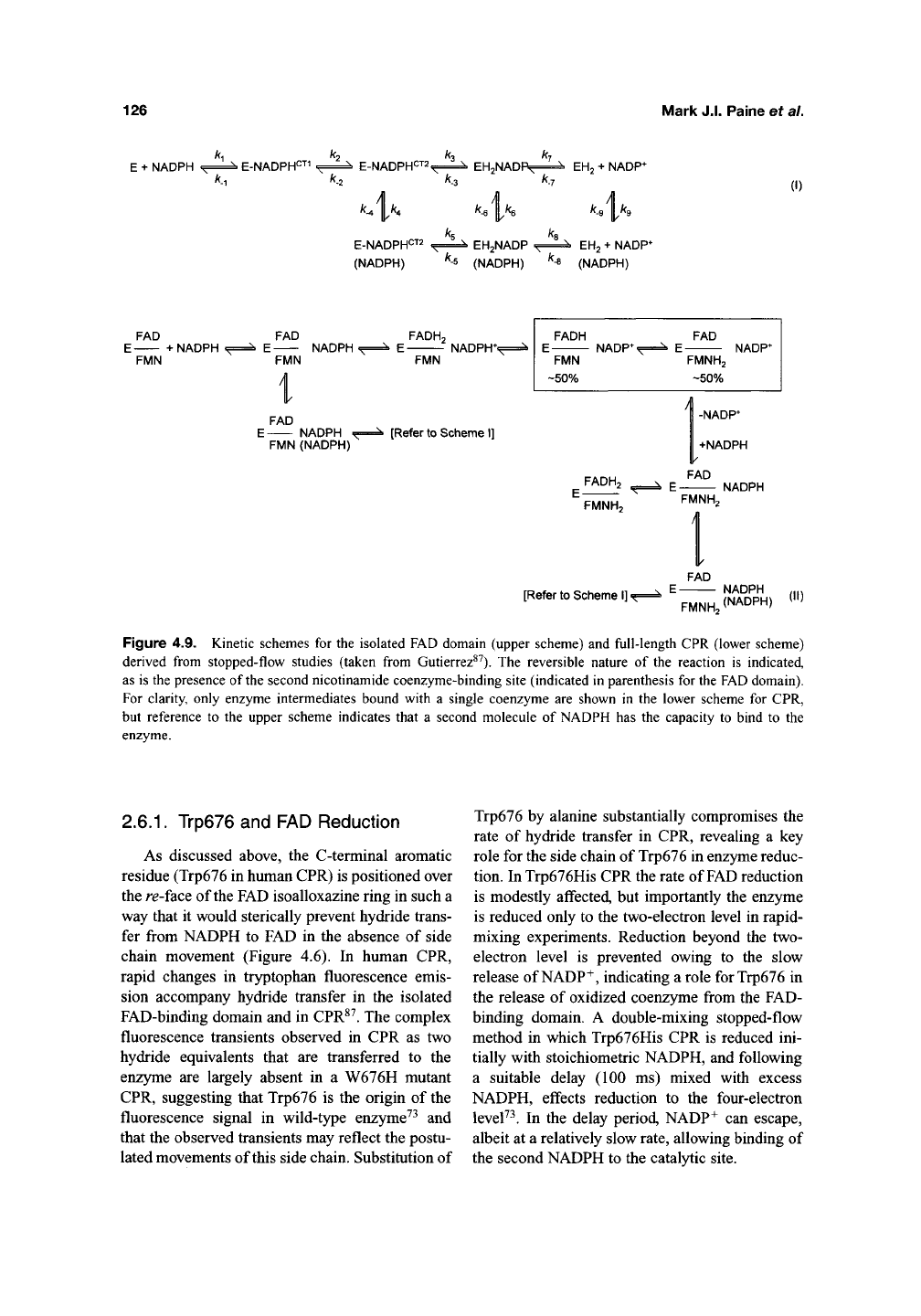
Reduction of the isolated FAD domain by
NADPH occurs in a series of discrete kinetic
steps including at least two different charge-
transfer complexes involving NADPH and FAD
(Figure 4.9). Partial flavin reduction occurs in the
initial phase (—200 s~^) and this displays a kinetic
isotope effect with ^-side NADPD. FAD is then
further reduced in the slower phase (~20s~^).
NADP+ release is thought to gate complete reduc-
tion of the flavin in the slower phase, consistent
with a reversible scheme for flavin reduction in
the isolated FAD domain and full-length CPR
(Figure 4.9). Similarly, it is proposed that the rate
of flavin reduction is regulated by NADP^ release
in rat neuronal nitric oxide synthase^
^' ^^
and the
adrenodoxin reductase homolog FprA from
Mycobacterium tuberculosis^^. A number of the
early kinetic phases are obscured in studies with
full-length CPR^^, thus emphasizing the impor-
tance of conducting detailed studies of electron
transfer mechanism in single domains of CPR.
The relatively high midpoint reduction poten-
tial of the FMN oxidized-semiquinone couple
(—66mV) provides the driving force for inter-
domain electron transfer in full-length CPR^^.
This enables electron flow from NADPH to the
CPR FMN. Electron transfer then occurs from the
FMN to the cytochrome P450 enzymes (or other
redox acceptors) and is a key aspect of the elec-
tron transfer mechanism in virtually all diflavin
reductase enzymes^^'
^^' ^^.
Kinetic studies show
that the transient accumulation of the blue dis-
emiquinoid species of CPR occurs at a rate identi-
cal to hydride transfer from NADPH to FAD^^.
This indicates that interdomain electron transfer is
relatively fast. The blue disemiquinoid species
subsequently decays following a second hydride
transfer from NADPH (Figure 4.9), as the flavins
are reduced to their hydroquinone states.
126
Mark J.I. Paine et al.
k k k k
E + NADPH ., "* ^ E-NADPHCT1 ^ ^
N
E-NADPHCT2
^
^ \ EHgNADP^ ^ ^ EH2 + NADP*
'^-l
'^-2 ^^-3 '^-l
k^\k.
4'^
k k
E-NADPHCT2 ^i^ EH2NADP .^i
(NADPH) ^-^ (NADPH) ^-8
.i«
EH2 + NADP^
(NADPH)
(I)
FAD
E + NADPH '
FMN
FAD
E NADPH.
FMN
i
FAD
E NADPH .^
FMN (NADPH)
FADHo
E NADPH**
FMN
[Refer to Scheme I]
FADH
E NADP*
FMN
-50%
FAD
E NADP*
FMNH2
-50%
FADH2
' FMNHo
[Refer
to
Scheme I].,
I
-NADP*
+NADPH
FAD
E NADPH
FMNHo
1
FAD
E NADPH
FMNH (NADPH)
(II)
Figure 4.9. Kinetic schemes for the isolated FAD domain (upper scheme) and full-length CPR (lower scheme)
derived from stopped-flow studies (taken from Gutierrez^^). The reversible nature of the reaction is indicated,
as is the presence of the second nicotinamide coenzyme-binding site (indicated in parenthesis for the FAD domain).
For clarity, only enzyme intermediates bound with a single coenzyme are shown in the lower scheme for CPR,
but reference to the upper scheme indicates that a second molecule of NADPH has the capacity to bind to the
enzyme.
2.6.1.
Trp676 and FAD Reduction
As discussed above, the C-terminal aromatic
residue (Trp676 in human CPR) is positioned over
the re-face of the FAD isoalloxazine ring in such a
way that it would sterically prevent hydride trans-
fer from NADPH to FAD in the absence of side
chain movement (Figure 4.6). In human CPR,
rapid changes in tryptophan fluorescence emis-
sion accompany hydride transfer in the isolated
FAD-binding domain and in CPR^^. The complex
fluorescence transients observed in CPR as two
hydride equivalents that are transferred to the
enzyme are largely absent in a W676H mutant
CPR, suggesting that Trp676 is the origin of the
fluorescence signal in wild-type enzyme^^ and
that the observed transients may reflect the postu-
lated movements of this side chain. Substitution of
Trp676 by alanine substantially compromises the
rate of hydride transfer in CPR, revealing a key
role for the side chain of Trp676 in enzyme reduc-
tion. In Trp676His CPR the rate of FAD reduction
is modestly affected, but importantly the enzyme
is reduced only to the two-electron level in rapid-
mixing experiments. Reduction beyond the two-
electron level is prevented owing to the slow
release of NADP^, indicating a role for Trp676 in
the release of oxidized coenzyme from the FAD-
binding domain. A double-mixing stopped-flow
method in which Trp676His CPR is reduced ini-
tially with stoichiometric NADPH, and following
a suitable delay (100 ms) mixed with excess
NADPH, effects reduction to the four-electron
leveF^.
In the delay period, NADP^ can escape,
albeit at a relatively slow rate, allowing binding of
the second NADPH to the catalytic site.

Electron Transfer Partners of Cytochrome P450
127
2.6.2.
Binding of Two Coenzyme
IVIolecules
Rapid-mixing studies of the kinetics of
hydride transfer in both the isolated FAD-binding
domain and in CPR have led to a kinetic model
(Figure 4.9) that invokes the existence of two
kinetically distinct binding sites for NADPH^^.
Only one site is catalytic; binding to the second
site attenuates FAD reduction, probably by inter-
fering with NADP^ release from the catalytic site.
In wild-type CPR, NADP+ release from the cat-
alytic site is sufficiently rapid that binding to the
second noncatalytic site does not prevent reduc-
tion to the four-electron level in direct-mixing
stopped-flow experiments with excess NADPH.
Following the identification of two kinetically
distinct coenzyme binding sites in CPR and the
unusual mechanism for electron transfer to FAD,
similar dual binding-site models have been pro-
posed for nitric oxide synthase^^ and the adreno-
doxin reductase homolog FprA isolated from M
tuberculosis^^.
The presence of two coenzyme-binding sites is
unexpected since they cannot be inferred solely
from the crystal structure of
CPR^^.
Kinetic stud-
ies with wild type and W676H CPR at different
concentrations of NADPH have, however, pro-
vided further support for the existence of two
sites^^'
^^.
The rate of flavin reduction in the iso-
lated FAD domain and CPR increases as NADPH
is decreased from molar excess to stoichiometric
concentrations. At stoichiometric concentration,
the second noncatalytic site is predominantly
vacant and the partial inhibition on the rate of
flavin reduction from the catalytic site is therefore
relieved (Figure 4.9). Occupation of the noncat-
alytic site occurs at NADPH concentrations in
excess of the enzyme concentration, and impairs
NADP^ release from the catalytic site. This in
turn partially inhibits flavin reduction, the rate of
which is gated by NADP^ release. Preincubation
of the enzyme with a stoichiometric amount of
adenosine 2',5'-diphosphate does not lead to
inhibition of the flavin reduction rate^^. We infer
that the binding of adenosine 2',5'-diphosphate
prevents NADPH from binding to the noncatalytic
site.
This observation also suggests that it is the
nicotinamide-ribose-phosphate portion of NADPH
bound at the second site that hinders NADP^
release from the catalytic site. Clearly, these new
data indicate that the coenzyme plays a role in the
control of CPR catalytic activity as well as acting
as a substrate. Models that assume a single
coenzyme-binding site for steady-state turnover
to explain competitive inhibition by coenz>ine
fragments might be inappropriate. The effect of
the second coenzyme-binding site on steady-state
turn-over and inhibition might lead to revised, and
by necessity more complex, steady-state kinetic
models.
2.6.3. Internal Electron Transfer
Although a thermodynamic and kinetic frame-
work for electron transfer in CPR has emerged
from rapid-mixing kinetic and potentiometry
studies^^' ^^, alternative approaches have been
required to access the rate of internal electron
transfer between the flavins. Tollin and coworkers
have used flash photolysis studies employing pho-
toexcited 5-deazariboflavin to investigate the
kinetics of inter-flavin electron transfer in rabbit
CPR^^. CPR is reduced rapidly by photoexcited
5-deazariboflavin (6.8 X lO^M-^s"^) and this
phase is followed by a slower (70 s~^) partial loss
of blue semiquinone signal that reports on inter-
flavin electron transfer from FAD to FMN^^.
Pre-reduction of rabbit CPR prior to flash photol-
ysis yields a rate constant for internal electron
transfer of
15
s~
^.
This slower rate for pre-reduced
enzyme (FAD^^/FMN ) is consistent with the
smaller driving force for electron transfer from
FAD^
to FMN following electron donation by
5-deazariboflavin. Studies with human CPR have
used temperature-jump relaxation kinetic methods
to follow inter-flavin electron transfer^^' ^^. As
with flash photolysis, relaxation kinetic methods
bypass preceding steps that might limit the rate of
inter-flavin electron transfer in rapid-mixing
approaches (e.g., the preceding FAD reduction
step).
Application of relaxation kinetic methods to
CPR has allowed direct measurement of the rate
of inter-flavin electron transfer in enzyme reduced
at the two- and three-electron level, and also
opened up studies of conformational gating and
ligand effects on this reaction^^. A key require-
ment of the temperature-jump approach is to poise
the equilibrium of a reaction, such that perturba-
tion of the equilibrium by rapid heating, effects
an absorption change that can be assigned to an

128
Mark
J.I.
Paine
ef a/.
individual reaction step (i.e., internal electron
transfer from FAD to FMN in the case of CPR).
This is especially difficult with CPR where slow
thermodynamic relaxation would lead to an equi-
librium mixture of several different redox forms
of the enzyme. The experimental conditions were
selected carefiilly to minimize any uncertainty
arising from the identity of
the
enzyme species in
the final equilibrium distribution. Potentiometry
studies indicate that in two-electron-reduced
enzyme the electrons are distributed as an equilib-
rium between the two enzyme species FAD^^/
FMN^^ and FAD^/FMN. , and that -50% of each
sq ox nq'
enzyme species is populated^^. A rapid tempera-
ture increase shifts this equilibrium toward the
FAD^^/FMNH2 form, and the kinetics of this
process report on the rate of inter-flavin electron
transfer^^. Inter-flavin electron transfer in human
CPR is relatively slow. For CPR reduced at the
two-electron level with NADPH, two kinetic
phases are seen in temperature-jump experiments.
Fluorescence and absorbance studies with
dithionite-reduced CPR indicate that the fast
phase
(1/T
= 2,200 ± 300 s~^) is not associated
with electron transfer, but is attributed to local
conformational change in the vicinity of
the
FAD
when CPR is reduced with NADPH. The slow
phase
(1/T
=
55±2S~^)
reports an internal elec-
tron transfer, FAD^^-FMN^^ ^ FAD^^-FMN^^q.
An intrinsic electron transfer rate of
—10^^
s"^ is
predicted based on the separation distance of the
two flavins in rat CPR^^' ^^. The modest transfer
rates observed in the temperature-jump studies
indicate that electron transfer is gated. Possible
origins of the gating reaction have been consid-
ered. For example, the reaction involves the
deprotonation of the FAD blue semiquinone, but
solvent isotope effects do not accompany the reac-
tion, ruling out a rate-limiting deprotonation reac-
tion. Electron transfer is compromised in the
presence of glycerol (75% w/v glycerol), suggest-
ing conformational gating^^ and a conformational
search for domain orientations that maximize
electronic coupling between the flavins is consis-
tent with the atomic structure of CPR (and related
diflavin enzymes), which are consistent with a
high degree of interdomain mobility^^' ^^' ^^ The
equilibrium distribution of enzyme species is
poised differently by reduction of CPR at the
three-electron level^^. Relaxation experiments
with three-electron reduced CPR give access to
the kinetics
(1/T
= 20 ± 0.2 s"^) of electron
transfer in the nonphysiological direction
(FAD,^/FMN,^ ^ FAD^^/FMN^^).
In the W676H mutant CPR, this rate is substan-
tially increased
(1/T
= 263 ± 3 s~^), but the rate
of electron transfer in the physiological direction
(FAD^qFMN^q ^ FAD^^FMNj^q) is decreased by
only a factor of ~2
(1/T
= 27 ± 1 s~^). This there-
fore points to another important role for Trp676 in
controlling electron transfer in CPR in favoring the
transfer of electrons in the physiological direction
(NADPH -^ FAD -^ FMN -^ heme).
Although the initial equilibrium is the same,
inter-flavin electron transfer rates for CPR
reduced by NADH
(1/T
= 18 ± 0.7 s"') are -3-
fold less than those for CPR reduced by NADPH
(1/T
= 55 ± 0.5 s~^). This cannot be attributed to
thermodynamic differences since the reduction
potentials of the reducing coenzymes are essen-
tially identical. This suggests a role for coenzyme
binding in modulating inter-flavin electron trans-
fgj.95,
97 jjjjg jg supported by the observation
that the rate of inter-flavin electron transfer in
dithionite-reduced CPR
(1/T
= 11 ± 0.5 s"^ is
clearly less than that for CPR reduced with
NADPH
(1/T
= 55 ±0.5s-^)^^ Furthermore,
addition of 2',5'-ADP to dithionite-reduced
enzyme effects a ~
3-fold
increase in the rate of
inter-flavin electron transfer
(1/T
= 35 ± 0.2 s~^),
and this is accompanied by an increase in the
amplitude of the absorption signal change^^. Thus,
the binding of the adenosine moiety of NADPH,
and in particular the 2'-phosphate group, is a
major factor in enhancing the rate of inter-flavin
electron transfer. Potentiometry measurements
have shown that the binding of 2',5'-ADP does not
perturb the midpoint reduction potentials of the
four flavin couples.
We
propose that ligand binding
in the coenzyme-binding pocket effects a confor-
mational change involving domain movement, the
rate of which controls inter-flavin electron transfer.
2.6.4. Interaction with and Electron
Transfer to P450
The microsomal P450 monooxygenase
complex is localized to the endoplasmic reticulum
membrane. Like P450, CPR contains an N-
terminal hydrophobic region that spans the lipid
membrane and anchors it to the surface. In yeast,

Electron Transfer Partners of Cytochrome P450 129
the soluble form of the enzyme can support P450
activity"^^, but soluble mammalian CPRs that have
had the hydrophobic anchoring peptide removed
are incapable of coupling with cytochrome P450.
The anchoring of these enzymes to the membrane
surface thus appears to be the principal factor
required for correct spatial orientation of the
redox centers for effective electron transfer.
Studying the architecture and topology of
membrane proteins on the phospholipid bilayer is
experimentally extremely challenging. However,
new developments are being applied to analyze
the topography of the monooxygenase complex on
a phospholipid bilayer. Sligar and coworkers have
used 10-nm scale phospholipid bilayer disk struc-
tures to orient CPR and cytochrome P450 2B4 on
a surface for visualization by AFM^^' ^^^. The
height of CYP2B4 protruding above the surface is
estimated at 3.5 nm, which is consistent with a
hydrophobic tip of the molecule being partially
inserted into the membrane. The exact spatial rela-
tionship with CPR is not yet clear, but it is thought
that CPR lies in an orientation such that both the
FMN and FAD/NADPH domains lie close to the
membrane surface, which would allow close
communication between the FMN and the P450
heme22'
ioo_
Transient monooxygenase complexes are
formed on the membrane surface as a result of
collisions between P450s and CPR as each
dif-
fuses laterally within the membrane of the endo-
plasmic reticulum. The role of the membrane in
mediating these interactions is poorly understood.
However, early work has shown that the phospho-
lipid component of the membrane can affect
intermolecular interactions of the monooxygenase
complex^^^'
^^^
and influence substrate binding^^'
^^^
and may therefore be important in maintaining
efficient electron transfer from CPR to P450.
The structure and sequence of rat and human
CPR^^' ^^ show some interesting features that
might influence the orientation of the protein at
the membrane surface. One is a cluster of basic
residues R'^^KKK, which lies at the membrane
anchor: FMN domain junction. Their functional
significance is unclear, but it can be speculated
that the basic Arg and Lys side groups might
interact with anionic phospholipid head groups,
possibly to restrict the movement of CPR on the
membrane surface. The FMN-binding domain
has clusters of positive and negative charges at
opposite ends, leading to a strong electric dipole
moment (677 Debye)^^, which may influence the
orientation of the reductase at the membrane sur-
face.
The patch of positively charged residues
exposed at the surface of the human FMN-binding
domain (K72, K74, K75, R78, R97, KlOO, H103,
and R108) in particular could form an additional
membrane-binding site.
Protein-protein interactions are essential to
enable electron transfer from the reduced FMN
of the flavoprotein to the substrate-bound ferric
form of the P450. Since P450 is present in a
10-25-fold molar excess over CPR in the liver
microsome^^'^' ^^^, rapid association and dissocia-
tion of
P450:
CPR complexes is important for the
system to work effectively. A number of lines of
evidence point to electrostatic interactions being
the driving force behind the binding of P450 with
CPR. For instance, it has been demonstrated that
specific positively charged lysine and arginine
residues on the rat P450, CYPlAl, are involved
in forming an electron transfer complex with
CPR^^6.
It has also been reported that CYP2B1
and CYP2B4 interact with CPR through comple-
mentary charge interactions^^^' ^^^. In the case of
CYP2B4, site-directed mutagenesis has identified
a series of lysine and arginine residues on the
proximal surface near the heme ligand that inter-
act with CPR^^^. Neutralization of carboxylate
groups on CPR by chemical modification inhibits
both cytochrome c reductase activity and P450-
dependent monooxygenation"^^'
^^^.
Chemical cross-
linking studies indicated that a cluster of acidic
amino acids on CPR were involved in the interac-
tion with rat CYPlAl i^^.
The FMN domain may be assumed to provide a
major portion of the docking surface for P450s,
although as discussed above some reorientation
of this domain from its position in the crystal struc-
ture may be required. Electrostatic potential meas-
urement of the surface of the human CPR-
FMN-binding domain shows three distinct clusters
of acidic residues that could form ion-pair inter-
actions with the electron transfer partners^^' ^^^
Cluster
1
contains Asp207,Asp208,Asp209; cluster
2,
Glu213, Glu214, Asp215; and cluster 3, Glul42,
Asp
144,
Asp
147.
The first two clusters correspond
to the region of CPR that was cross-linked to a
lysine residue in cytochrome c^^^, and have been
investigated by site-directed mutagenesis in rat^^^
and human^^ CPR. The results of these experiments
130
Mark
J.I.
Paine
ef a/.
show that while cluster 1 mutations do not afifect
C3^ochrome c binding, removing the charge from
Asp208 alters the binding of cytochrome P450. In
contrast, cluster 2 mutations affected the binding of
cytochrome c, but not P450. Mutations of residues
in cluster 3 produce a modest decrease in P450
activity with no effect on cytochrome c reductase
activity^^. Thus, discrimination is apparent between
residues involved with the interaction of CPR with
cytochrome c and with P450.
The finding that mutation of residues in clus-
ters
1
and
3
on either side of the FMN-binding site
affect P450 activity, suggests that c3^ochromes
P450 bind at the tip of
the
FMN domain in such a
way as to cover the FMN cofactor^^. Examination
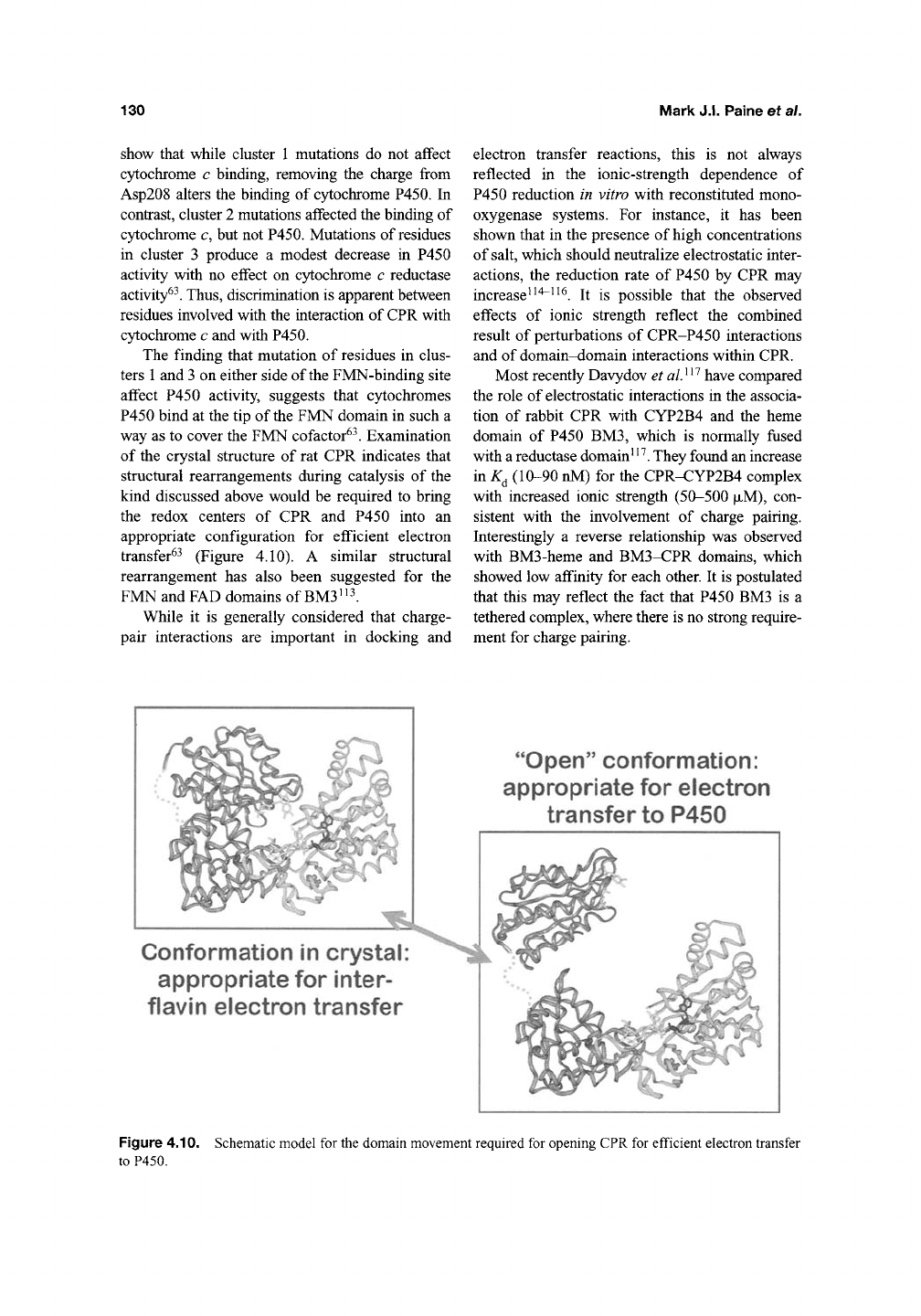
of the crystal structure of rat CPR indicates that
structural rearrangements during catalysis of the
kind discussed above would be required to bring
the redox centers of CPR and P450 into an
appropriate configuration for efficient electron
transfer^^ (Figure 4.10). A similar structural
rearrangement has also been suggested for the
FMN and FAD domains of
BM3'
^l
While it is generally considered that charge-
pair interactions are important in docking and
electron transfer reactions, this is not always
reflected in the ionic-strength dependence of
P450 reduction in vitro with reconstituted mono-
oxygenase systems. For instance, it has been
shown that in the presence of high concentrations
of
salt,
which should neutralize electrostatic inter-
actions, the reduction rate of P450 by CPR may
increase^
^'^~^^^.
It is possible that the observed
effects of ionic strength reflect the combined
result of perturbations of CPR-P450 interactions
and of domain-domain interactions within CPR.
Most recently Davydov et
al}^''
have compared
the role of electrostatic interactions in the associa-
tion of rabbit CPR with CYP2B4 and the heme
domain of P450 BM3, which is normally fused
with a reductase domain'
'^.
They found an increase
in K^ (10-90 nM) for the CPR-CYP2B4 complex
with increased ionic strength (50-500
JJLM),
con-
sistent with the involvement of charge pairing.
Interestingly a reverse relationship was observed
with BM3-heme and BM3-CPR domains, which
showed low affinity for each other. It is postulated
that this may reflect the fact that P450 BM3 is a
tethered complex, where there is no strong require-
ment for charge pairing.
Conformation in crystal
appropriate for inter-
flavin electron transfer
"Open"
conformation:
appropriate for electron
transfer to P450
Figure 4.10. Schematic model for the domain movement required for opening CPR for efficient electron transfer
to P450.

Electron Transfer Partners of Cytochrome P450
131
2.7.
Cytochrome P450 BM3
Since its discovery in the 1980s, flavocy-
tochrome P450 BM3 (CYP102A1) has undergone
intensive biochemical study and has established
itself as a model P450 system to rival P450cam.
The key aspect of
the
structure of P450 BM3 that
has led to this rise to prominence is the fact that it
was the first prokaryotic P450 enzyme recognized
to interact with a eukaryotic-like P450 reductase
redox partner (i.e., a FAD- and FMN-containing
NADPH-cytochrome P450 reductase) rather
than with an iron-sulfur ferredoxin and FAD-
containing ferredoxin reductase, long considered
to represent the "typical" bacterial P450 reductase
system^^l Thus P450 BM3, a soluble P450,
off-
ered great promise as a tool for understanding
redox partner interactions and electron transfer
in an experimentally more tractable system than
was the case for the membrane-bound eukaryotic
P450s and P450 reductase^. An even more attrac-
tive feature of P450 BM3 was that it was a natural
fusion protein—^with the reductase linked to the
P450 in a single 119 kDa pol5^eptide. Structural,
spectroscopic, and enzymological studies of P450
BM3 in the two decades since its discovery have
been extremely informative as regards P450 struc-
ture and mechanism, establishing P450 BM3
as a vitally important "model" system for the
P450 superfamily^.
P450 BM3 was discovered as a result of
studies by Armand Fulco's group on fatty acid
hydroxylase systems from the soil bacterium
B.
megaterium^^^.
As implied by the name, BM3
was the third enzyme with fatty acid hydroxylase
activity identified in the bacterium. P450 BM3
became a major focus of Fulco's studies following
the finding that the gene was strongly induced by
addition of phenobarbital to the growth medium^^,
suggesting that the gene was under transcriptional
control akin to the types of regulation seen previ-
ously for mammalian drug-metabolizing P450
isoforms^^^. Subsequent studies have shown the
importance of
a
repressor protein (BM3R1) in the
control of CYP102A1 expression, with barbitu-
rate drugs and a range of other hydrophobic mol-
ecules being able to displace the repressor to
promote production of P450 BM312I' ^22. patty
acids are likely to perform the same role in vivo,
and have also been shown to facilitate expression
of a homolog of P450 BM3 in Bacillus subtilis.
The overproduction of P450 BM3 facilitated by
addition of phenobarbital to B. megaterium
growth medium simplified purification of the
enzyme, and cloning of the gene allowed its
expression and purification from E. coli^^^. It
quickly became apparent from analysis of both
gene and protein that P450 BM3 was an unusual
fusion protein comprised of a fatty acid-binding
P450 (homologous to the eukaryotic CYP4 fatty
acid hydroxylase family) fused to a CPR^^"^.
Analysis of the catalytic properties of the purified
enzyme showed it to have a very high catalytic
center activity relative to its eukaryotic fatty acid
hydroxylase relatives—with turnover numbers of
several thousand per minute with various long-
chain fatty acids^^' ^^^. This was rationalized as
being due to the efficiency of the electron transfer
system covalently associated with the P450, and
opened the door to mechanistic and kinetic studies
on the enzyme^^' ^^^' ^^^. In the BM3 system,
NADPH reduces the FAD flavin transiently to the
hydroquinone form. Electron transfer to the heme
is mediated by the FMN cofactor, which shuttles
electrons one at a time between the FAD and
heme^.
Genetic dissection provided further proof for
the multidomain nature of the enzyme-producing
first sub-genes encoding stable P450 (first —472
amino acids of P450 BM3) and CPR domain^^' ^^\
and then constructs encoding the individual
FAD/NADPH-binding and FMN-binding domains
of the CPR^^^. Domain dissection also enabled the
determination of the atomic structures of the heme
(P450) domain of the enzyme and also the struc-
ture of a nonstoichiometric complex of the heme
and FMN domains of the enzyme, arising from
proteolysis of a construct containing the heme
domain and the FMN-binding domain^^^.
The atomic structure of the heme domain has
been solved in substrate-bound and substrate-free
forms,
showing that a large structural change in
the protein accompanies the binding of substrate
(palmitoleic acid). Binding of fatty acid substrates
to the heme domain causes a shift in the heme iron
spin-state equilibrium toward the high-spin form,
which is accompanied by a large increase in
reduction potential for the ferric/ferrous transition
of the heme iron^^' ^^' ^^^. Binding of fatty acid
thus facilitates electron transfer to the heme iron
and triggers catalysis. In the absence of bound
substrate there is negligible electron transfer

132
Mark J.I. Paine et al.
between the FMN and heme cofactors. When
P450 BM3 binds its most efficient substrates, that
is those fatty acids, such as arachidonic acid, that
induce the greatest degrees of spin-state transition
and promote the fastest turnover^ ^^' ^^^, FMN-to-
heme electron transfer is rapid (c. 250 s^^ with
lauric acid) and catalysis occurs^^. The extent of
the increase in reduction potential accompanying
saturation with fatty acid is approximately 130 mV,
a change which elevates the heme reduction
potential above that of the ultimate reductant in
the system (NADPH: E^ = -320 mV cf -427
for BM3 heme in absence of substrate and -289
in presence of arachidonate) and within range of
that of the FMN cofactor (E^ = -203 mV for its
oxidized/hydroquinone couple)^^'
^^K
2.7.1.
Electron Transfer Properties of
BM3 Reductase
Interestingly, the BM3 reductase is the only
member of the diflavin reductase class of enzymes
which does not stabilize a neutral, blue semi-
quinone on its FMN. Formation of a blue semi-
quinone during reductive titration is a feature
typical of flavodoxins, and retained in other mem-
bers of the diflavin reductase family (e.g., nitric
oxide synthase, methionine synthase reductase,
and other eukaryotic CPRs). The fact that the
semiquinone is destabilized with respect to the
hydroquinone in BM3's CPR and FMN domains
has been rationalized on the basis of the structure
of the FMN domain. The presence of basic
residues (particularly Lys572 and Lys580) may
stabilize the anionic hydroquinone form and
destabilize the semiquinone^'^. The redox state of
the flavins is of great importance to the catalytic
properties of BM3, since early studies on the
enzyme showed that there was strong inhibition of
fatty acid hydroxylase activity following preincu-
bation of the enzyme with NADPH^^. It appears
that an unfavorable three-electron (and possibly
four-electron) reduced form of the enzyme accu-
mulates within a few minutes of incubation of the
enzyme with NADPH, and that electron transfer
in this species is diminished and results in a cat-
alytic rate of fatty acid hydroxylation less than
20%
of the maximal rate. Analysis of the redox
state of the flavins during turnover suggests that
both may exist predominantly in a semiquinone
state as catalysis progresses (a neutral semi-
quinone in the case of the FAD and a red, anionic
semiquinone in the case of the FMN), suggesting
that the CPR domain of the enzyme cycles favor-
ably between 0-2-1-0 electron-reduced states
during catalysis'^^. The situation appears similar
for the housefly CPR, but contrasts with that
reported for many eukaryotic CPRs, where the
cycle appears to be
1-3-2-1
'^^.
However, the pre-
cise reason for the apparent inactivation of BM3
in an "over-reduced" form remains obscure. Since
electron transfer to exogenous electron acceptors
via the flavins remains largely unaflected'^^, it
appears to be the case that the catalytic step per-
turbed is that of first or second electron transfer to
the heme iron. This may indicate that the reduc-
tion state and/or coenzyme-binding affects the
conformational status of the reductase domain,
in particular the movement of the FMN-binding
domain relative to the FAD-binding domain
discussed above.
Stopped-flow absorption spectroscopy has
provided information on some of the internal elec-
tron transfer processes in P450
BM3^^.
In particu-
lar, the rate of electron transfer between NADPH
and the FAD (in itself a composite of rates for
NADPH association, orientation and the actual
hydride transfer event) is very fast (500 s~' at
5°C) relative to those measured for mammalian
CPRs,
nitric oxide synthase, methionine synthase
reductase, and novel reductase 1 (NRl)^^' ^'^' ^^.
The rate of internal (inter-flavin) electron transfer
remains to be determined, but should be greater
than 250 s~' (the turnover rate with arachidonate).
The rapid electron transfer from NADPH to
flavin is diminished considerably for the NADH-
dependent reaction, indicating the importance of
the 2' phosphate group of NADPH in both recog-
nition and binding of
the
pyridine nucleotide, and
in the actual electron transfer event. Comparison
of the thermodynamic properties of the BM3
reductase with those of the other members of the
diflavin reductase family (Figure 4.8) indicates
that increased driving force does not underlie the
enhanced rate of NADPH-to-FAD electron trans-
fer observed for this isoform^^. For instance, in
human CPR there is a more favorable thermody-
namic balance for electron transfer between
NADPH (E^' = -320 mV) and the FAD cofactor
(E^
= -328 mV for the two-electron reduction to
the hydroquinone form) and an apparent rate of

Electron Transfer Partners of Cytochrome P450
133
electron transfer of ~3 s~^ at 25°C with excess
NADPH^^' ^l For BM3, the midpoint reduction
potential for the FAD cofactor is virtually identi-
cal to that for human CPR (-332 mV), and yet the
rate of electron transfer is apparently —200-300-
fold that of human CPR under similar conditions.
Obviously, structural differences in BM3 reduc-
tase underlie its capacity to drive electron transfer
so efficiently with respect to other diflavin reduc-
tases.
However, these differences await clarifica-
tion through structural elucidation of the BM3
reductase and/or its FAD/NADPH domain. In
view of the proximity of the redox couples of
NADPH and the FAD flavin in BM3 (and other
diflavin reductases), reversibility of the electron
transfer process (i.e., hydride transfer from FAD
hydroquinone to NAD(P)^) might be expected.
This has been shown to occur for both mammalian
CPR and for BM3 reductase^^'
^^'
1^4
Many aspects of the electron transfer reactions
in P450 BM3 are now reasonably well understood,
and the first mutagenesis experiments on the
enzyme ruled out the involvement of
a
tryptophan
residue adjacent to the heme in the flavin-to-heme
electron transport process^^^. However, even after
two decades of study of this enzyme, several fun-
damental issues remain unresolved. Key among
these are the reasons why the electron transfer
reactions in the reductase domain of the enzyme
(and between FMN and heme) are so efficient by
comparison with the eukaryotic P450 and CPR
systems. Thus, further detailed rapid kinetic and
structural studies are critical to gain a complete
understanding of this efficient electron transfer
system.
2.8.
Artificial CPR-P450 Fusion
Constructs
Many attempts have been made to increase
the catalytic efficiency of mammalian P450s to the
level of BM3 through artificial fusions with
CPR. The first such artificial fusion between rat
P450 CYPlAl and rat CPR was constructed by
Murakami et alP^. Since then, several fusions
with rat CPR have been constructed including
bovine CYP17a (ref [137]), human CYP3A4
(ref [138]), human CYPlAl (ref [139]), CYP4A1
(ref [140]), and rat CYP2C11 (ref [141]). In
addition, yeast CPR has been fused to rat CYPlAl
(ref [142]), and human CYP3A4 (ref [143]).
Most recently, a fusion comprising entirely human
genes was constructed between CYP2D6 and
human CPR^44
As yet none of these artificial systems
approach P450 BM3 in terms of its high catalytic
activity. Indeed, most artificial mammalian
fusions achieve no greater turnover levels than
their physiological counterparts in which the
reductase and P450 form noncovalent complexes.
This may indicate that the exceptional catalytic
rate exhibited by P450 BM3 arises from the
details of its mechanism rather than simply
from the fact that the reductase and P450 are
covalently linked. Nevertheless, such catalytically
self-sufficient P450-CPR fusion enzymes are
extremely useful for probing the biochemical
mechanisms involved in catalysis and electron
transfer between P450 and CPR. They also have
important practical applications in the develop-
ment of efficient P450-based biocatalysts and
high throughput screening systems.
3. Electron Transfer to P450s
from Cytochrome
b^
Cytochrome b^ was originally discovered in
silkworm larvae
^"^^
and has since been most exten-
sively studied in mammals^'
^^^.
Like cytochromes
P450 and CPR, cytochrome
b^
is an integral mem-
brane protein located on the C5^osolic side of the
endoplasmic reticulum where it is principally
involved in lipid biosynthesis. It functions along
with cytochrome b^ reductase as the electron
donor to microsomal desaturases that synthe-
size unsaturated fatty acids, plasmalogens, and
sterols^'^^. Indications of the involvement of
cytochrome b^ in P450 reactions date from early
studies that showed that NADH stimulates
NADPH-supported metabolism of drugs in micro-
somes^"^^' ^^^. Since then, evidence has accumu-
lated that c)^ochrome b^ has a significant role in a
number of P450 systems^^^, most significantly
CYP3A4 (ref. [151]), which is involved in the
majority of human drug oxidations.
Cytochrome b^ is a small polypeptide
(~17kDa) containing 134 residues, which are
divided into an amino-terminal hydrophilic heme
domain and a carboxyl-terminal hydrophobic
