Ortiz de Montellano Paul R.(Ed.) Cytochrome P450. Structure, Mechanism, and Biochemistry
Подождите немного. Документ загружается.


134
Mark J.I. Paine et al.
membrane-binding region^^^' ^^^. While the mem-
brane-bound form of
Z?5
participates in elongation
and desaturation of fatty acids^^'
^^^^ ^^^,
cholesterol
biosynthesis^^^, and drug metabohsm, soluble forms
of
Z?3
and
Z>3
reductase are also found in er5^hrocytes,
where they catalyze methemoglobin reduction^^^.
Mutations to either the b^ gene or the b^ reductase
gene can cause congenital methemoglobinemia^^^.
Soluble Z?5 has also been purified as a cytosolic
component in the NAD(P)H-reductive activation
pathway for porcine methionine synthase^^.
Like CPR, it is generally accepted that
charge-pair interactions drive the formation of
P450:Z?5 complexes^^^~^^^. This is supported by
site-directed mutagenesis of rabbit CYP2B4,
which has identified several positively charged
residues on the P450 surface (R122, R126, R133,
K139,
and K433) as being involved in binding
b^
(ref [58]). However, the precise mechanism by
which b^ supports P450 catalysis is not yet clear.
As the redox potentials of cytochrome b^
and ferric P450 are approximately +25 mV and
-300 mV^^^ respectively, a role in the first elec-
tron transfer step of the P450 catalytic cycle is
unlikely, suggesting that b^ may only be involved
in the donation of the second electron''^^'
•'*^.
Since
electrons may be supplied to cytochrome b^ by
either NADH cytochrome b^ reductase or CPR^^,
the pathway of electron movement is difficult to
establish. Furthermore, studies that show that
apocytochrome b^ can stimulate the catalytic
activity of CYP3A4^^4' ^^^ 2C9, 4A7, and 17^^^
question a formal role in electron transfer. Thus,
over the years several different mechanisms have
been suggested by which
b^
supports P450 cataly-
sis.
These include: electron transfer from NADPH
through CPR to cytochrome b^ and thence to
P450166,167. electron transfer from NADH through
b^ reductase to cytochrome b^ and thence to
P450'^^;
electron shuttling between P450 and b^
resulting in a tighter coupling between electron
flow and product formation^^^; reduction in the
uncoupled generation of superoxide or hydrogen
peroxide^''^; and an allosteric effect not dependent
on electron transfer^ ^'*' ^^^.
Much of the early data on cytochrome b^
interactions with P450 has come from in vitro
analysis using reconstituted systems. However, the
expression in
E.
coli^^^,
insect cells^^^ or yeast^^^
of a complete mammalian P450 monooxygenase
complex comprising cytochrome b^, CPR, and
P450 has provided a means of studying the actions
of the individual components in a more physio-
logical context. Using yeast systems, Perret and
Pompon^^^ have shown that the redox properties
of
Z?5
were strictly required to enhance CYP3A4
activity, with results of rapid kinetic analysis of Z73
reduction following NADPH addition suggesting
that
Z>3
was reduced by the 3A4 ferrous-dioxygen
complex and reoxidized by subsequent P450 oxy-
genated intermediates ^^^. In E. coli, cytochrome
b^ significantly enhances the testosterone and
nifedipine activity of CYP3A4 and results in
the stabilization of the P450 during substrate
turnover'^^. This is consistent with the proposal
that b^ interaction reduces uncoupling and super-
oxide anion release'^^' '^^.
With the increasing sophistication in the
genetic tools now available it is becoming possible
to investigate the complex interrelationships of
the monooxygenase system in the correct context
of the whole organism. For instance, the absence
of any detectable P450 activity in mice where
CPR has been knocked out of the liver provides
compelling evidence that the alternative cyto-
chrome b^/b^ reductase electron transfer pathway
does not play any significant role in vivo^^. We
now await the construction ofb^ knockout mice to
provide further clues as to the precise role of
b^
in the physiological function of P450s in higher
vertebrate organisms.
4. Iron-Sulfur Electron Donors:
Adrenodoxin, Putidaredoxin,
and their Reductases
4.1.
General
The Fe2S2-type ferredoxins can be arranged
into three distantly related classes based on amino
acid sequence homologies: bacterial-, plant-, and
vertebrate-type*^"*. Extensive information on the
function and mechanisms of this system has been
gained through work on the bacterial P450cam
system in Pseudomonas putida, which catalyzes
the conversion of (i-camphor to 5-exo-hydroxy-
camphor^^^. In
P.
putida, the iron-sulfur protein
is putidaredoxin (Pdx), a 106 amino acid resi-
due ferredoxin. For catalysis, two reducing equiv-
alents are sequentially transferred from NADH

Electron Transfer Partners of Cytochrome P450
135
to P450cam via an FAD-containing enzyme,
putidaredoxin reductase (Pdr) and Pd. The puti-
daredoxin: P450cam binary complex forms with
a K^ that is dependent on the oxidation states of
the proteins and on whether substrate is bound to
the P450^^^. In the first electron transfer, reduced
Pd interacts with ferric (i-camphor-bound
P450cam and reduces it to the ferrous form. In the
second electron transfer, reduced Pd forms a com-
plex with ferrous dioxygen and (i-camphor-bound
P450cam to transfer the second electron and
reductively cleave bound dioxygen, producing
hydroxycamphor and water*^^. While most struc-
tural and biophysical studies of P450/ferredoxin
interactions have focused on the Fe2S2 putidare-
doxin from
P.
putida (and its cognate P450cam),
other types of bacterial ferredoxins are recognized
to interact productively with P450s. For instance,
the Fe3S4 ferredoxin SoyB is a redox partner
for the Streptomyces griseus P450 Soy (SoyC)*^^
and the Fe^S^ ferredoxin from
B.
subtilis (fer) has
been shown to undergo redox reactions with P450
Biol from the same bacterium*^^.
In animals, ferredoxins are primarily involved
in electron transfer interactions involving mito-
chondrial P450s. This fact is in agreement with
the theory of the prokaryotic origin of this
organelle. Much work has been done with adreno-
doxin (Adx) and the transfer of electrons from
NADPH-dependent adrenodoxin reductase (AdR)
to CYPUAl, which is involved in cholesterol
side chain cleavage, and to CYPUB, involved in
the formation of Cortisol and aldosterone*^"*.
Bovine adrenodoxin mRNA is translated as a 186
amino acid precursor molecule, which undergoes
proteolytic processing upon import into the mito-
chondria to remove the N-terminal 58 residues *^^.
The mature Adx comprises 128 amino acid
residues and has a molecular weight of 14.4 kDa.
In plants, [2Fe-2S] ferredoxins are involved in
conjunction with ferredoxin-reductase in photo-
synthesis reactions and the transfer of electrons
from photosystem I to NADP^*^*. However, evi-
dence for involvement of ferredoxins in electron
transfer to plant P450s awaits a more detailed char-
acterization of the multiple P450 systems present
in the biotechnologically important plant species.
Ferredoxins receive their electrons from
ferredoxin reductase, which represents the first
component of the electron transfer system. These
enzymes contain FAD and belong to a large group
of electron transferases that includes bacterial-
type putidaredoxin reductase (PdR)*^^, animal-type
adrenodoxin reductase (AdR)*^^ *^^, and plant-
type ferredoxin-NADP^ reductases'^. An AdR-
type reductase system (FprA) has recently been
characterized in the pathogen M. tuberculosis^^
and the structure reveals extensive similarity with
the mammalian enzyme*^^. In humans, AdR is
most abundant in the steroid-producing cells of
the adrenal cortex, the ovary, and the testis*^'.
4.2.
Interactions with P450
The structures and models of structures of
several bacterial, animal, and plant ferredoxins are
available (e.g., putidaredoxin^^^ and the Fe4S4
ferredoxin from Bacillus thermoproteolyticus^^^).
In particular, details of the electron transfer mecha-
nisms have developed through kinetic and muta-
tional analysis of the Pdx-P450cam system^'^.
These indicate that Pdx interacts with proximal sur-
face of P450cam through electrostatic interactions
involving the acidic Pdx residues Asp38 and Asp34
and the basic P450cam residue Arg 112^^^' ^^^
Additionally, Argl
12
appears to form the electron
transfer route in the P450cam-Pdx complex with
Cys39 and Asp38 of Pdx^^^'
^^^.
A role for the
C-terminal amino acid of Pdx (W106) in electron
transfer to P450cam was suggested on the basis of
diminished enzymatic activity in systems with
Pdx W106 mutants, but later studies of various
W106 mutants showed that this residue primarily
affects the K^ of Pdx for the P450i9^ Studies of
the interactions between cytochrome b^ and
P450cam show that b^ and Pdx compete for the
same binding site on the P450, suggesting a
common mode of charge-charge pairing that is
the major force driving interactions at the same
surface on the P450^^'^.
However, isothermal calorimetry experiments
show that the energetics of the P450cam/Pdx
association are most similar to those of binding
reactions of antibody to antigen complexes ^^^,
suggesting that van der Waals and hydrogen-bond-
ing interactions may predominate in the formation
of P450cam-Pdx complexes. This study also sug-
gested that binding between Pdr and Pdx might
be dominated by hydrophobic interactions. Not-
withstanding these data, a variety of mutagenesis
studies along with the fact that increased ionic
136 Mark J.I. Paine et al.
strength markedly increases the K^ of Pdx for the
P450,
highlight the important influence of electro-
statics on the docking process^^^' ^^^.
Catalytic interactions between Pdx and
P450cam are considered to follow a ping-pong
mechanism^^^. The flavin (FAD) of Pdr is reduced
directly to its hydroquinone by interaction with
the obligatory two-electron donor NADH, but
negligible formation of semiquinone is observed
during the successive single-electron transfer
reactions between Pdr and Pdx in steady-state
turnover of P450cam. This indicates destabiliza-
tion of the flavin semiquinone with respect to the
hydroquinone and oxidized forms ^^^. Rapid (laser
flash photolysis) kinetic studies indicate transient
formation of a blue semiquinone on the Pdr FAD,
which disproportionates rapidly ^^^.
Upon complex formation, Pdx induces confor-
mational changes in P450cam'^^ which convert
the high-spin state of ferric P450cam to low-spin
state and stretch the heme-axial ligand^^^.
NMR studies of the Pdx-P450cam complex also
show that Pdx binding perturbs several regions
involving the substrate access channel in
P450cam^^^ and induces a tilt in the heme plane,
leading to the movement of J-camphor and the
axial Cys to the heme-iron by 0.1-0.5 A and
facilitating heterolysis of the O-O bond^^^. It is
thus proposed that in addition to its redox
function, Pdx invokes structural changes that
facilitate the oxygen activation reaction^^^ Only
Pdx is capable of reducing the oxy form of
P450cam, in agreement with this hypothesis^^^.
The rate-limiting steps in the entire catalytic
cycle of P450cam are the electron transfers
between Pdx and the P450. The precise rate-
limiting step appears to change from the first
electron transfer to the second electron transfer as
the concentration of Pdx in the system is reduced
toward levels considered typical of the situation
in v/vo^^^.
With respect to the mammalian adrenodoxin/
adrenodoxin reductase system, crystal structures
of truncated and full-length oxidized bovine
Adx^^^'
^^"^
have been determined, along with
NMR structures of both oxidized and reduced
full-length forms of Adx^^^. Adrenodoxin, con-
tains a large hydrophobic core region that houses
a single Fe2S2 cluster, and an interaction domain
that contains acidic residues responsible for the
recognition of the redox partners, CYPllAl
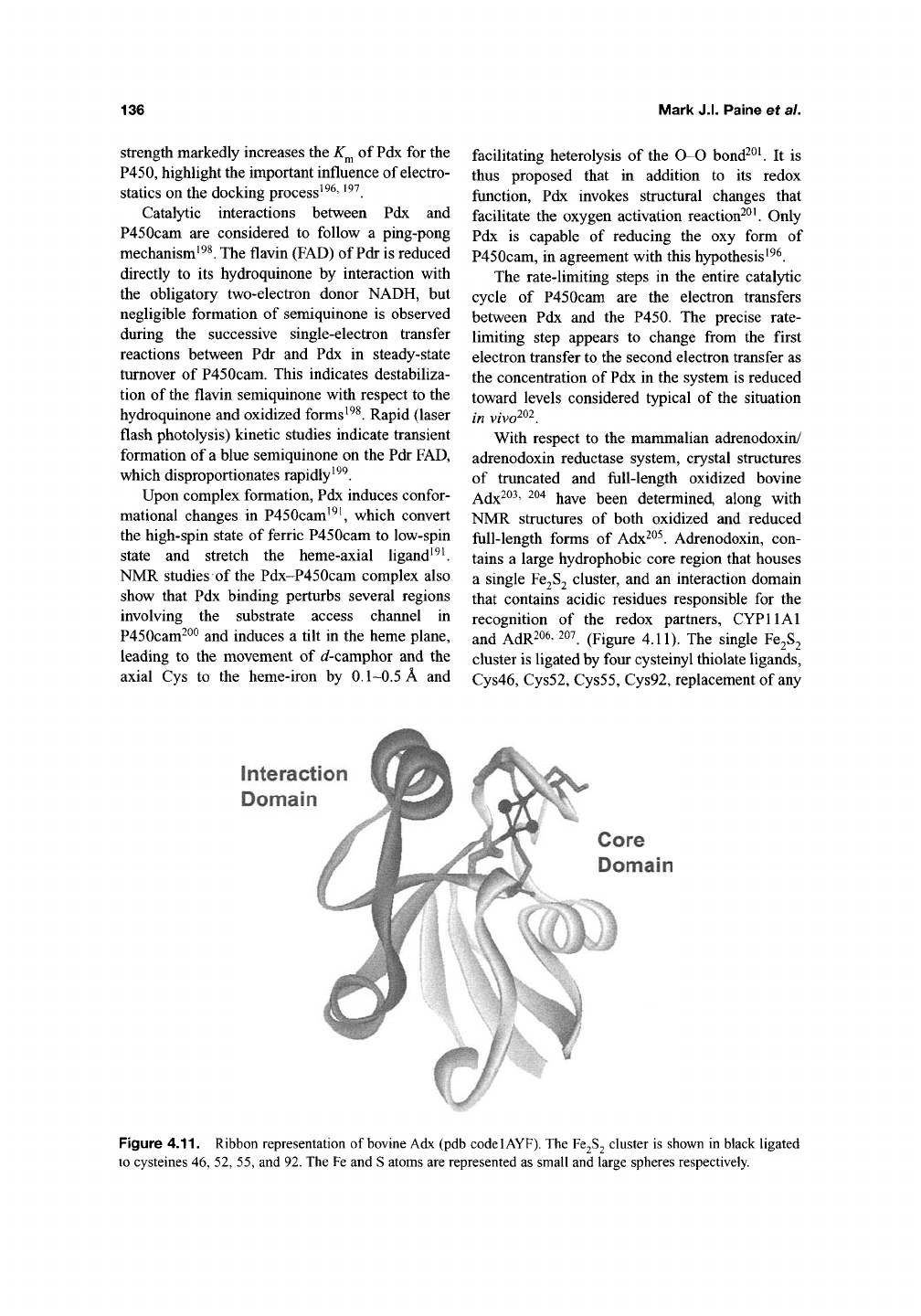
and AdR206' 207 (pigure 4.11). The single Fe2S2
cluster is ligated by four cysteinyl thiolate ligands,
Cys46,
Cys52, Cys55, Cys92, replacement of any
Interaction
Domain
Core
Domain
Figure 4.11. Ribbon representation of bovine Adx (pdb codelAYF). The Fe2S2 cluster is shown in black ligated
to cysteines 46, 52, 55, and 92. The Fe and S atoms are represented as small and large spheres respectively
Electron Transfer Partners of Cytochrome P450
137
of
which,
produces a nonfunctional apoprotein^^^.
Sites involved in redox partner binding are also
present in the core domain centered around an
acidic patch at position Asp59^^''.
Two different models have been described to
explain the electron delivery system mediated by
Adx: (a) an organized cluster model comprising
AdR: Adx: P450 ternary/quaternary complexes^^^
and (b) a shuttle system where Adx sequentially
transports one electron at a time from AdR to
P45Q210 Qf these^ t^g shuttle hypothesis is most
favorable since the formation of a ternary com-
plex formed by AdR, Adx, and P450 is unlikely
from a structural perspective due to overlapping
mteraction regions^
The sequential model
is also that favored for the well-characterized
P450cam system. Following the resolution of
the bovine AdR crystal structure it has become
possible to fully investigate the structural mecha-
nisms driving the protein complex formation^ ^^.
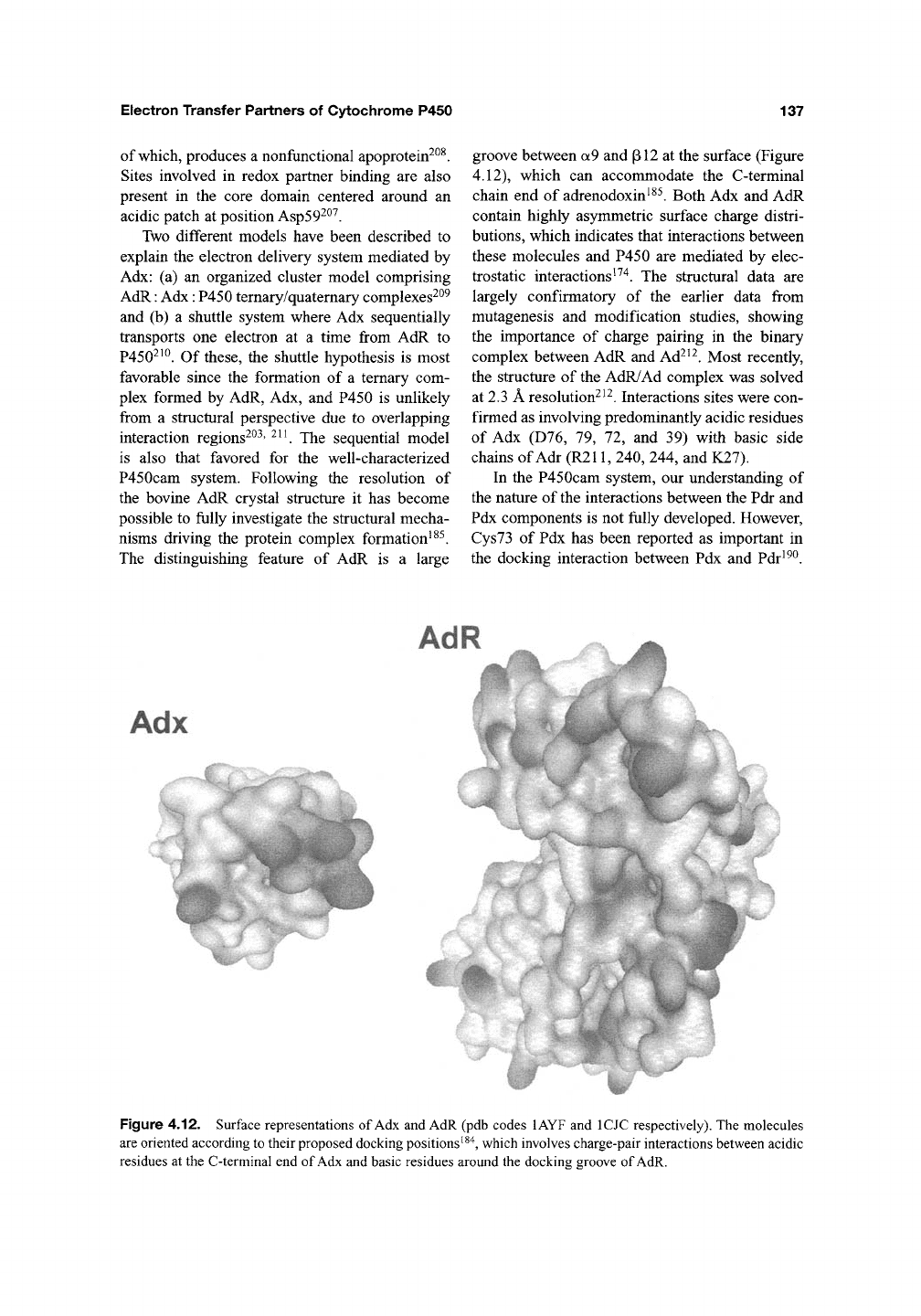
The distinguishing feature of AdR is a large
groove between a9 and pi2 at the surface (Figure
4.12), which can accommodate the C-terminal
chain end of adrenodoxin^^^. Both Adx and AdR
contain highly asymmetric surface charge distri-
butions, which indicates that interactions between
these molecules and P450 are mediated by elec-
trostatic interactions ^^'^. The structural data are
largely confirmatory of the earlier data from
mutagenesis and modification studies, showing
the importance of charge pairing in the binary
complex between AdR and Ad^^^. Most recently,
the structure of the AdR/Ad complex was solved
at 2.3 A resolution^^^. Interactions sites were con-
firmed as involving predominantly acidic residues
of Adx (D76, 79, 72, and 39) with basic side
chains of Adr
(R211,
240, 244, and K27).
In the P450cam system, our understanding of
the nature of the interactions between the Pdr and
Pdx components is not fully developed. However,
Cys73 of Pdx has been reported as important in
the docking interaction between Pdx and Pdr^^^.
AdR
Adx
,..
^^::,
Figure 4.12. Surface representations of Adx and AdR (pdb codes lAYF and ICJC respectively). The molecules
are oriented according to their proposed docking positions^^"^j which involves charge-pair interactions between acidic
residues at the C-terminal end of Adx and basic residues around the docking groove of AdR.

138
Mark J.I. Paine ef a/.
Mutations to the adjacent Pdx surface residue
Glu72 also affect electron transfer reactions in
the P450cam redox system^^^. The generation of
catalytically efficient putidaredoxin reductase-
putidaredoxin-P450cam triple fusion protein
demonstrates that a much more simple single
component camphor hydroxylase system can be
produced, with obvious biotechnological implica-
tions.
The addition of short peptide linkers
between the respective polypeptides was compati-
ble with strong expression of fusion proteins in
an E. coli system and efficient electron transfer
reactions between the redox centers^^'^. Although
much structure/function work remains to be done
on the Pdr component of the P450cam system, a
novel finding regarding its activity was made
recently with the demonstration that Pdr has
NAD(H)-dependent dithiol/disulfide oxidoreduc-
tase activity^^2. However, much structural and
kinetic work remains to be done to provide a clear
description of the nature of interactions between
the Pdr and Pdx components of the P450cam sys-
tem, and how these relate to those in the analogous
Adr/Adx mitochondrial system.
5. Novel Redox Systems
The identification of the P450 BM3 system led
to the realization that alternative types of P450
redox systems (beyond those typified by mam-
malian hepatic class and P450cam) exist in nature.
In recent years, a variety of alternative modes of
P450 reduction have been reported—including
P450s that require no electron transfer at all (i.e.,
catalyze molecular rearrangements without oxy-
gen chemistry) such as the dehydratase allene
oxide synthase^^^ and a P450 that appears to inter-
act directly with hydrogen peroxide to facilitate
fatty acid oxidation in
B.
subtilis^^^.
Various types
of ferredoxins have been shown to be competent
redox partners for selected P450 enzymes^'^
and fiavodoxins may also be physiologically
relevant mediators of electron transfer to P450s
in bacterial (and possibly plant) systems^^^' ^^^.
More recent studies suggest more exotic types
of P450 fusion enzymes may exist—including
fusions of P450s to ferredoxin and phthalate
dioxygenase reductase-like domains^^^'
•^^K
It
is highly likely that further insights into bio-
diversity of P450 redox systems will be gained as
ongoing genome sequencing projects reveal ever
more diverse means by which this intriguing
class of enzymes can operate and obtain reducing
power.
Acknowledgments
The authors gratefully acknowledge the sup-
port of the UK Medical Research Council, Cancer
Research UK, the Biotechnology and Biological
Sciences Research Council, the Wellcome Trust,
the Lister Institute of Preventative Medicine, and
the European Union, EPSRC (Engineering and
Physical Sciences Research Council) and the
Royal Society.
References
1.
Schenkman, J.B. and I. Jansson (1999). Interactions
between cytochrome P450 and cytochrome b5.
DrugMetab. Rev. 31, 351-364.
2.
Munro, A.W., D.G. Leys, K.J. McLean, K.R.
Marshall, TW.B. Ost, S. Daff et al. (2002). P450
BM3:
The very model of a modern flavocy-
tochrome.
Trends
Biochem. Sci. 21, 250-257.
3.
Nakayama, N., A. Takemae, and H. Shoun (1996).
Cytochrome P450foxy, a catalytically self-sufficient
fatty acid hydroxylase of the fungus Fusarium
oxysporum.
J.
Biochem. (Tokyo) 119, 435^40.
4.
De Mot, R. and A.H. Parret (2002). A novel class of
self-sufficient cytochrome P450 monooxygenases
in prokaryotes.
Trends
Microbiol. 10, 502-508.
5.
Kluck, R.M., E. Bossy-Wetzel, D.R. Green, and
D.D. Newmeyer (1997). The release of cytochrome
c from mitochondria: A primary site for Bcl-2
regulation of
apoptosis.
Science 275, 1132-1136.
6. Kluck, R.M., S.J. Martin, B.M. Hoffman, J.S. Zhou,
D.R. Green, and D.D. Newmeyer (1997). Cyto-
chrome c activation of CPP32-like proteolysis plays
a critical role in a Xenopus cell-free apoptosis
system. EMBO
J.
16, 4639-^649.
7.
Haas, E., B.L. Horecker, and T.T. Hogness (1940).
The enzymatic reduction of cytochrome c\ Cyto-
chrome c reductase.
J.
Biol. Chem. 136,141-714.
8. Horecker, B.L. (1950), Triphosphopyridine
nucleotide-cytochrome c reductase in liver. J. Biol.
Chem.
183, 593-605.
9. Williams, C.H., Jr. and H. Kamin (1962). Micro-
somal triphosphopyridine nucleotide—cytochrome
reductase of the liver. J. Biol. Chem. 237, 587-595.
10.
Phillips, A.H. and R.G. Langdon (1962).
Hepatic triphosphopyridine nucleotide cytochrome

Electron Transfer Partners of Cytochrome P450
139
c reductase: Isolation characterisation and kinetic
studies. J. Biol. Chem. 237, 2652-2660.
11.
Lu, A.Y. and M.J. Coon (1968). Role of hemopro-
tein P-450 in fatty acid omega-hydroxylation in a
soluble enzyme system from liver microsomes.
J. Biol. Chem. 243, 1331-1332.
12.
Lu, A.Y., K.W. Junk, and M.J. Coon (1969).
Resolution of the cytochrome P-450-containing
omega-hydroxylation system of liver microsomes
into three components. J. Biol. Chem. 244,
3714-3721.
13.
Strobel, H.W., A.Y. Lu, J. Heidema, and M.J. Coon
(1970).
Phosphatidylcholine requirement in the
enzymatic reduction of hemoprotein P-450 and in
fatty acid, hydrocarbon, and drug hydroxylation.
J. Biol. Chem. 245, 4851^854.
14.
Black, S.D., J.S. French, C.H. Williams, Jr., and
M.J. Coon (1979). Role of a hydrophobic poly-
peptide in the N-terminal region of NADPH-
cytochrome P-450 reductase in complex formation
with P-450LM. Biochem. Biophys. Res. Commun.
91,
1528-1535.
15.
Yasukochi, Y and B.S. Masters (1976). Some
properties of a detergent-solubilized NADPH-
cytochrome c (cytochrome P-450) reductase
purified by biospecific affinity chromatography.
J. Biol. Chem. 251, 5337-5344.
16.
lyanagi, T. and H.S. Mason (1973). Some proper-
ties of hepatic reduced nicotinamide adenine
dinucleotide phosphate-cytochrome c reductase.
Biochemistry 12, 2297-2308.
17.
lyanagi, T., N. Makino, and H.S. Mason (1974).
Redox properties of the reduced nicotinamide
adenine dinucleotide phosphate-cytochrome P-450
and reduced nicotinamide adenine dinucleotide-
cytochrome b5 reductases. Biochemistry 13,
1701-1710.
18.
Backes, W.L. (1993). NADPH-cytochrome P450
reductase: Function. In J.B. Schenkman and
H. Greim (eds) Handbook of Experimental
Pharmacology, \o\. 105. Springer-Verlag, Berlin.
19.
Porter, T.D. and C.B. Kasper (1985). Coding
nucleotide sequence of rat NADPH-cytochrome P-
450 oxidoreductase cDNA and identification of
flavin-binding domains. Proc. Natl.
Acad.
Sci. USA
82,
973-977.
20.
Katagiri, M., H. Murakami, Y Yabusaki,
T. Sugiyama, M. Okamoto,
T.
Yamano et al. (1986).
Molecular cloning and sequence analysis of full-
length cDNA for rabbit liver NADPH-cytochrome
P-450 reductase mRNA. J. Biochem. (Tokyo) 100,
945-954.
21.
Yamano, S., T. Aoyama, O.W. McBride,
J.P.
Hardwick, H.V Gelboin, and F.J. Gonzalez
(1989).
Human NADPH-P450 oxidoreductase:
Complementary DNA cloning, sequence and
vaccinia virus-mediated expression and localization
of the CYPOR gene to chromosome 7. Mol.
Pharmacol. 36, 83-88.
22.
Wang, M., D.L. Roberts, R. Paschke, T.M. Shea,
B.S.
Masters, and
J.J.
Kim (1997). Three-dimensional
structure of NADPH-cytochrome P450 reductase:
Prototype for FMN- and FAD-containing enzymes.
Proc. Natl.
Acad.
Sci. USA 94, 8411-8416.
23.
Porter, T.D. and C.B. Kasper (1986). NADPH-
cytochrome P-450 oxidoreductase: Flavin mononu-
cleotide and flavin adenine dinucleotide domains
evolved from different flavoproteins. Biochemistry
25,1682-1687.
24.
Leclerc, D., A. Wilson, R. Dumas, C. Gafuik,
D.
Song, D. Watkins et al. (1998). Cloning and
mapping of
a
cDNA for methionine synthase reduc-
tase,
a flavoprotein defective in patients with
homocystinuria. Proc. Natl.
Acad.
Sci. USA 95,
3059-3064.
25.
Paine, M.J., A.P Garner, D. Powell, J. Sibbald,
M. Sales, N. Pratt et al. (2000). Cloning and charac-
terization of a novel human dual flavin reductase.
J. Biol. Chem. 275, 1471-1478.
26.
McMillan, K., D.S. Bredt, D.J. Hirsch, S.H. Snyder,
J.E. Clark, and B.S. Masters (1992). Cloned,
expressed rat cerebellar nitric oxide synthase con-
tains stoichiometric amounts of heme, which binds
carbon monoxide. Proc. Natl.
Acad.
Sci. USA 89,
11141-11145.
27.
Narhi, L.O. and A.J. Fulco (1986). Characterization
of a catalytically self-sufficient 119,000-dalton
cytochrome P-450 monooxygenase induced by
barbiturates in Bacillus megaterium. J. Biol. Chem.
261,
7160-7169.
28.
Ostrowski, J., M.J. Barber, D.C. Rueger, B.E.
Miller, L.M. Siegel, and N.M. Kredich (1989).
Characterization of the flavoprotein moieties
of NADPH-sulfite reductase from Salmonella
typhimurium and Escherichia coli. Physicochemical
and catalytic properties, amino acid sequence
deduced from DNA sequence of
cysJ,
and compari-
son with NADPH-cytochrome P-450 reductase.
J. Biol. Chem. 264, 15796-15808.
29.
Smith, G.C.M., D.G. Tew, and C.R. Wolf (1994).
Dissection of NADPH-cytochrome P450 oxidore-
ductase into distinct functional domains. Proc. Natl.
Acad Sci. USA 91, 8710-8714.
30.
Hodgson, A.V and H.W Strobel (1996). Character-
ization of the FAD binding domain of cytochrome
P450 reductase. Arch. Biochem. Biophys. 325,
99-106.
31.
Sevrioukova, I., G. Truan, and J.A. Peterson (1996).
The flavoprotein domain of
P450BM-3:
Expression,
purification, and properties of the flavin adenine
dinucleotide- and flavin mononucleotide-binding
subdomains. Biochemistry 35, 7528-7535.

140
Mark J.I. Paine et al.
32.
Munro, A.W. (1993). Purification schemes for the
constituent domains of cytochrome P450 BM3 in
E. coli. Biochem. Soc.
Trans.
21, 316S.
33.
Finn, R.D., J. Basran, O. Roitel, C.R.
Wolf,
A.W.
Munro, M.J. Paine et al. (2003). Determination of
the redox potentials and electron transfer properties
of the FAD- and FMN-binding domains of the
human oxidoreductase NRl. Eur. J. Biochem. 270,
1164-1175.
34.
Wolthers, K.R., J. Basran, A.W. Munro, and N.S.
Scrutton (2003). Molecular dissection of human
methionine synthase reductase: Determination of
the flavin redox potentials in full-length enzyme and
isolated flavin-binding domains. Biochemistry 42,
3911-3920.
35.
Benveniste, I., A. Lesot, M.P. Hasenfratz, G. Kochs,
and F. Durst (1991). Multiple forms of NADPH-
cytochrome P450 reductase in higher plants.
Biochem. Biophys. Res. Commun. 177, 105-112.
36.
Koopmann, E. and K. Hahlbrock (1997).
Differentially regulated NADPH:cytochrome P450
oxidoreductases in parsley. Proc. Natl.
Acad.
Sci.
USA 94, 14954-14959.
37.
Urban, P., C. Mignotte, M. Kazmaier, F. Delorme,
and D. Pompon (1997). Cloning, yeast expression,
and characterization of the coupling of two distantly
related Arabidopsis thaliana NADPH-cytochrome
P450 reductases with P450 CYP73A5. J. Biol.
Chem.
212, 19176-19186.
38.
Porter, T.D., T.W Beck, and C.B. Kasper (1990).
NADPH-cytochrome P-450 oxidoreductase gene
organization correlates with structural domains of
the protein. Biochemistry 29, 9814-9818.
39.
Henderson, C.J., DM. Otto, D Carrie,
M.A. Magnuson, A.W. McLaren, I. Rosewell et al.
(2003).
Inactivation of the hepatic cytochrome P450
system by conditional deletion of hepatic cytochrome
P450 reductase.
J.
Biol. Chem. 278, 13480-13486.
40.
Shen, A.L., K.A. O'Leary, and C.B. Kasper (2002).
Association of multiple developmental defects and
embryonic lethality with loss of microsomal
NADPH-cytochrome P450 oxidoreductase. J. Biol.
Chem.
277,6536-6541.
41.
Gonzalez, FJ. and C.B. Kasper (1982). Cloning of
DNA complementary to rat liver NADPH-
cytochrome c (P-450) oxidoreductase and
cytochrome P-450b mRNAs. Evidence that pheno-
barbital augments transcription of specific genes.
J. Biol. Chem. 257, 5962-5968.
42.
Shen, A.L. and C.B. Casper (1993). Protein and
gene regulation of NADPH-cytochrome P450 oxi-
doreductase. In J.B. Shenkman and H. Grein (eds).
Handbook of
Experimental
Pharamacology,
Vol.
105.
Springer-Verlag, Berlin.
43.
O'Leary, K.A., P McQuiddy, and C.B. Kasper
(1996).
Transcriptional regulation of the TATA-less
NADPH cytochrome P-450 oxidoreductase gene.
Arch.
Biochem. Biophys. 330, 271-280.
44.
O'Leary, K.A., H.C. Li, PA. Ram, P McQuiddy,
D.J. Waxman, and C.B. Kasper (1997). Thyroid reg-
ulation of NADPH:cytochrome P450 oxidoreduc-
tase:
Identification of a thyroid-responsive element
in the 5'-flank of the oxidoreductase gene. Mol.
Pharmacol. 52,
46-53.
45.
Ram, PA. and D.J. Waxman (1992). Thyroid
hormone stimulation of NADPH P450 reductase
expression in liver and extrahepatic tissues.
Regulation by multiple mechanisms. J. Biol. Chem.
267,3294-3301.
46.
Fan, L.Q., J. Coley, R.T Miller, R.C. Cattley, and J.C.
Corton (2003). Opposing mechanisms of NADPH-
cytochrome P450 oxidoreductase regulation by
peroxisome proliferators. Biochem. Pharmacol. 65,
949-959.
47.
Le,
Y.
and
B.
Sauer (2001). Conditional gene knock-
out using Cre recombinase. Mol. Biotechnol. 17,
269-275.
48.
Sutter, T.R. and J.C. Loper (1989). Disruption of the
Saccharomyces cerevisiae gene for NADPH-
cytochrome P450 reductase causes increased
sensitivity to ketoconazole. Biochem. Biophys. Res.
Commun. 160, 1257-1266.
49.
Venkateswarlu, K., DC. Lamb, D.E. Kelly, N.J.
Manning, and S.L. Kelly (1998). The N-terminal
membrane domain of yeast NADPH-cytochrome
P450 (CYP) oxidoreductase is not required for
catalytic activity in sterol biosynthesis or in recon-
stitution of CYP activity J. Biol. Chem. 273,
4492-4496.
50.
Enoch, H.G. and P Strittmatter (1979). Cytochrome
b5 reduction by NADPH-cytochrome P-450 reduc-
tase.
J 5/o/. Chem. 254, 8976-8981.
51.
Schacter, B.A., E.B. Nelson, H.S. Marver, and B.S.
Masters (1972). Immunochemical evidence for an
association of heme oxygenase with the microsomal
electron transport system. J. Biol. Chem. 247,
3601-3607.
52.
Chen, Z. and R. Banerjee (1998). Purification of
soluble cytochrome b5 as a component of the reduc-
tive activation of porcine methionine synthase.
J. Biol. Chem. 273, 26248-26255.
53.
Osada, M., S. Imaoka, T. Sugimoto, T Hiroi, and
Y Funae (2002). NADPH-cytochrome P-450 reduc-
tase in the plasma membrane modulates the activa-
tion of hypoxia-inducible factor 1. J. Biol. Chem.
277,
23367-23373.
54.
Bachur, N.R., S.L. Gordon, M.V. Gee, and H. Kon
(1979).
NADPH cytochrome P-450 reductase acti-
vation of quinone anticancer agents to free radicals.
Proc. Natl.
Acad.
Sci. USA 76, 954-957.
55.
Keyes, S.R., P.M. Fracasso, D.C. Heimbrook,
S. Rockwell, S.G. Sligar, and
A.C.
Sartorelh (1984).

Electron Transfer Partners of Cytochrome P450
141
Role of NADPH:cytochrome c reductase and DT-
diaphorase in the biotransformation of mitomycin
CI.
Cancer Res. 44, 5638-5643.
56.
Walton, M.I., C.R.
Wolf,
and R Workman (1992).
The role of cytochrome P450 and cytochrome P450
reductase in the reductive bioactivation of the novel
benzotriazine di-N-oxide hypoxic cytotoxin 3-amino-
l,2,4-benzotriazine-l,4-dioxide (SR
4233,
WIN
59075) by mouse liver. Biochem. Pharmacol. 44,
251-259.
57.
Vasquez-Vivar, J., P. Martasek, N. Hogg,
B.S.
Masters, K.A. Pritchard, Jr., and
B.
Kalyanaraman (1997). Endothelial nitric oxide
synthase-dependent superoxide generation from
adriamycin. Biochemistry 36, 11293-11297.
58.
Bridges, A., L. Gruenke, Y.T. Chang, I.A. Vakser,
G. Loew, and L. Waskell (1998). Identification of
the binding site on cytochrome P450 2B4 for
cytochrome b(5) and cytochrome P450 reductase.
J. Biol Chem. li:^, 17036-17049.
59.
Wang, J. and PR. Ortiz De Montellano (2003). The
binding sites on human heme oxygenase-1 for
cytochrome P450 reductase and biliverdin reduc-
tase.
J. Biol. Chem. 6, 6.
60.
Hubbard, PA., A.L. Shen, R. Paschke, C.B. Kasper,
and
J.J.
Kim (2001). NADPH-cytochrome P450 oxi-
doreductase. Structural basis for hydride and elec-
tron transfer. J. Biol. Chem. 276, 29163-29170.
61.
Craig, D.H., S.K. Chapman, and S. Daff (2002).
Calmodulin activates electron transfer through neu-
ronal nitric-oxide synthase reductase domain by
releasing an NADPH-dependent conformational
lock. J. Biol. Chem. Ill, 33987-33994.
62.
Barsukov, I., S. Modi, L.Y. Lian, K.H. Sze, M.J.
Paine, C.R. Wolfe/ al (1997). IH, 15N and 13C
NMR resonance assignment, secondary structure
and global fold of the FMN-binding domain of
human cytochrome P450 reductase.
J.
Biomol. NMR
10,
63-75.
63.
Zhao, Q., S. Modi, G. Smith, M. Paine, PD.
McDonagh, C.R. Wolfe/ al. (1999). Crystal struc-
ture of the FMN-binding domain of human
cytochrome P450 reductase at 1.93 A resolution.
Protein Sci. 8, 298-306.
64.
Ludwig, M.L., K.A. Pattridge, A.L. Metzger, M.M.
Dixon, M. Eren, Y. Feng et al. (1997). Control of
oxidation-reduction potentials in flavodoxin from
Clostridium beijerinckii: The role of conformation
changes. Biochemistry 36, 1259-1280.
65.
Shen, A.L., T.D. Porter, T.E. Wilson, and C.B.
Kasper (1989). Structural analysis of the FMN bind-
ing domain of NADPH-cytochrome P-450 oxidore-
ductase by site-directed mutagenesis. J. Biol. Chem.
264,
7584-7589.
66.
Paine, M.J.I., S. Ayivor, A. Munro, P. Tsan,
L.Y Lian,
G.C.K.
Roberts et al. (2001). Role
of the conserved phenylalanine 181 of NADPH-
cytochrome p450 oxidoreductase in FMN binding
and catalytic activity. Biochemistry 40,13439-13447.
67.
Shen, A.L. and C.B. Kasper (2000). Differential
contributions of NADPH-cytochrome P450 oxido-
reductase FAD binding site residues to flavin bind-
ing and catalysis. J. Biol. Chem. 275, 41087^1091.
68.
Sem, D.S. and C.B. Kasper (1992). Geometric rela-
tionship between the nicotinamide and isoalloxazine
rings in NADPH-cytochrome P-450 oxidoreduc-
tase:
Implications for the classification of evolu-
tionarily and functionally related flavoproteins.
Biochemistry 31, 3391-3398.
69.
Serre, L., F.M. Vellieux, M. Medina, C. Gomez-
Moreno, J.C. Fontecilla-Camps, and M. Frey,
(1996).
X-ray structure of the ferredoxin:NADP+
reductase from the cyanobacterium Anabaena PCC
7119 at 1.8 A resolution, and crystallographic stud-
ies of NADP+ binding at 2.25 A resolution. J. Mol.
Biol. 263, 20-39.
70.
Karplus, PA., M.J. Daniels, and J.R. Herriott
(1991).
Atomic structure of ferredoxin-NADP+
reductase: Protype for a structurally novel flavoen-
zyme family. Science 251, 60-66.
71.
Gruez, A., D. Pignol, M.
Zeghouf,
1 Coves,
M. Fontecave, J.L. Ferrer et al. (2000). Four crystal
structures of the 60 kDa flavoprotein monomer of
the sulfite reductase indicate a disordered flavo-
doxin-like module. J. Mol Biol 299, 199-212.
72.
Dohr, O., M.J. Paine, T. Friedberg, G.C. Roberts,
and C.R. Wolf (2001). Engineering of a functional
human NADH-dependent cytochrome P450 system.
Proc. Natl
Acad.
ScL USA 98, 81-86.
73.
Gutierrez, A., O. Doehr, M. Paine, C.R.
Wolf,
N.S.
Scrutton, and
G.C.K.
Roberts (2000). Trp-676
facilitates nicotinamide coenzyme exchange in the
reductive half-reaction of human cytochrome P450
reductase: Properties of the soluble W676H and
W676A mutant reductases. Biochemistry 39,
15990-15999.
74.
Piubelli, L., A. Aliverti, A.K. Arakaki, N. Carrillo,
E.A. Ceccarelli, PA. Karplus et al (2000).
Competition between C-terminal tyrosine and
nicotinamide modulates pyridine nucleotide affinity
and specificity in plant ferredoxin-NADP(+)
reductase. J 5/o/. Chem. 275, 10472-10476.
75.
Deng, Z., A. Aliverti, G. Zanetti, A.K. Arakaki,
J. Ottado, E.G. Orellano et al (1999). A productive
NADP+ binding mode of ferredoxin-NADP+
reductase revealed by protein engineering and crys-
tallographic studies. Nat. Struct. Biol 6, 847-853.
76.
Sem, D.S. and C.B. Kasper (1993). Interaction
with arginine 597 of NADPH-cytochrome P-450
oxidoreductase is a primary source of the uniform
binding energy used to discriminate between NADPH
and NADH. Biochemistry 32, 11548-11558.

142 Mark J.I. Paine ef al.
11.
Sem,
D.S.
and
C.B.
Kasper
(1993).
Enzyme-substrate
binding interactions of NADPH-cytochrome P-450
oxidoreductase characterized with pH and aher-
nate substrate/inhibitor studies. Biochemistry 32,
11539-11547.
78.
Elmore, C.L. and T.D. Porter (2002). Modification
of the nucleotide cofactor-binding site of cytochrome
P-450 reductase to enhance turnover with NADH
in vivo.
J.
Biol. Chem. 277, 48960-48964.
79.
Shen, A.L. and C.B. Kasper (1996). Role of Ser457
of NADPH-cytochrome P450 oxidoreductase in
catalysis and control of FAD oxidation-reduction
potential. Biochemistry 35, 9451-9459.
80.
Shen, A.L., D.S. Sem, and C.B. Kasper (1999).
Mechanistic studies on the reductive half-reaction
of NADPH-cytochrome P450 oxidoreductase.
J. Biol. Chem. 274, 5391-5398.
81.
Shen, A.L., D.S. Sem, and C.B. Kasper (1999).
Mechanistic studies on the reductive half-reaction
of NADPH-cytochrome P450 oxidoreductase.
J. Biol. Chem. 274, 5391-5398.
82.
Vermilion, J.L. and M.J. Coon (1978). Identification
of the high and low potential flavins of liver micro-
somal NADPH-C3^ochrome P-450 reductase.
J. Biol. Chem. 253, 8812-8819.
83.
Vermilion, J.L. and M.J. Coon (1978). Purified liver
microsomal NADPH-cytochrome P-450 reductase.
Spectral characterization of oxidation-reduction
states.
J. Biol. Chem. 253, 2694-2704.
84.
Yasukochi, Y., J.A. Peterson, and B.S. Masters
(1979).
NADPH-cytochrome c (P-450) reductase.
Spectrophotometric and stopped flow kinetic
studies on the formation of reduced flavoprotein
intermediates. ^ 5/o/. Chem. 254, 7097-7104.
85.
Griffith, O.W. and D.J. Stuehr (1995). Ann. Rev.
Physiol. 57, 707-736.
86.
Miles, J.S., A.W. Munro, B.N. Rospendowski,
W.E. Smith, J. McKnight, and
A.J.
Thomson (1992).
Domains of the catalytically self-sufficient
cytochrome P-450 BM-3. Genetic construction,
overexpression, purification and spectroscopic char-
acterization. Biochem. J. 288, 503-509.
87.
Gutierrez, A., L.Y. Lian, C.R.
Wolf,
N.S. Scrutton,
and G.C.K. Roberts (2001). Stopped-flow kinetic
studies of flavin reduction in human cytochrome
P450 reductase and its component domains.
Biochemistry
^{^,
1964-1975.
88.
Munro, A.W., M.A. Noble, L. Robledo, S.N.
Daff,
and S.K. Chapman (2001). Determination of the
redox properties of human NADPH-cytochrome
P450 reductase. Biochemistry 40, 1956-1963.
89.
Daff,
S.N., S.K. Chapman, K.L. Turner, R.A. Holt,
S. Govindaraj, T.L. Poulos et al. (1997). Redox con-
trol of the catalytic cycle of flavocytochrome P450
BM3.
Biochemistry 36, 13816-13823.
90.
Munro, A.W., S.
Daff,
J.R. Coggins, J.G. Lindsay, and
S.K. Chapman (1996). Probing electron transfer in
flavocytochrome P-450 BM3 and its component
domains. Eur J. Biochem. 239, 403^09.
91.
Noble, M.A., A.W. Munro, S.L. Rivers, L.
Robledo, S.N.
Daff,
L.J. Yellowlees et al. (1999).
Potentiometric analysis of the flavin cofactors of
neuronal nitric oxide synthase. Biochemistry 38,
16413-16418.
92.
Knight, K. and N.S. Scrutton (2002). Stopped-
flow kinetic studies of electron transfer in the reduc-
tase domain of neuronal nitric oxide synthase:
Reevaluation of the kinetic mechanism reveals new
enzyme intermediates and variation with cyto-
chrome P450 reductase. Biochem. J. 367, 19-30.
93.
McLean, K.J., N.S. Scrutton, and A.W. Munro
(2003).
Kinetic, spectroscopic and thermodynamic
characterization of the Mycobacterium tuberculo-
sis adrenodoxin reductase homologue FprA.
Biochem. J.
:^12,?>\1-?>21.
94.
Noble, M.A., C.S. Miles, S.K. Chapman,
D.A. Lysek, A.C. Mackay, G.A. Reid et
al.
(1999).
Roles of key active site residues in flavocy-
tochrome P450 BM3. Biochem. J. 339, 371-379.
95.
Gutierrez, A., A.W. Munro, A. Grunau, C.R.
Wolf,
N.S.
Scrutton, and G.C.K. Roberts (2003).
Interflavin electron transfer in human cytochrome
P450 reductase is enhanced by coenzyme binding.
Eur. J. Biochem. 210, 2612-2621.
96.
Bhattacharyya, A.K., J.J. Lipka, L. Waskell, and G.
Tollin (1991). Laser flash photolysis studies of the
reduction kinetics of NADPH.cytochrome P-450
reductase. Biochemistry 30, 759-765.
97.
Gutierrez, A., M. Paine, C.R.
Wolf,
N.S. Scrutton,
and G.C.K. Roberts (2002). Relaxation kinetics
of cytochrome P450 reductase: Internal electron
transfer is limited by conformational change and
regulated by coenzyme binding. Biochemistry 41,
4626-4637.
98.
Page, C.C, C.C. Moser, X. Chen, and PL. Dutton
(1999).
Natural engineering principles of elec-
tron tunnelling in biological oxidation-reduction.
Nature 402, 47-52.
99.
Bayburt, T.H., J.W Carlson, and S.G. Sligar
(1998).
Reconstitution and imaging of a membrane
protein in a nanometer-size phospholipid bilayer.
J. Struct. Biol. 123, 37^4.
100.
Bayburt, TH. and S.G. Sligar (2002). Single-
molecule height measurements on microsomal
cytochrome P450 in nanometer-scale phospholipid
bilayer disks. Proc. Natl.
Acad.
Sci. USA 99,
6725-6730.
101.
Ingelman-Sundberg, M., T Haaparanta, and
J. Rydstrom (1981). Membrane charge as effector
of cytochrome P-450LM2 catalyzed reactions
in reconstituted liposomes. Biochemistry 20,
4100^106.
102.
Ingelman-Sundberg, M., J. Blanck, G. Smettan, and
K. Ruckpaul (1983). Reduction of cyto-chrome

Electron Transfer Partners of Cytochrome P450
143
P-450 LM2 by NADPH in reconstituted phospho-
lipid vesicles is dependent on membrane charge.
Eur.
J. Biochem. 134, 157-162.
103.
Coon, M.J., A.P. Autor, and H.W. Strobel (1971).
Role of phospholipid in electron transfer in a
reconstituted liver microsomal enzyme system
containing cytochrome P-450. Chem. Biol.
Interact. 3, 248-250.
104.
Estabrook, R.W., A.G. Hildebrandt, J. Baron,
K.J. Netter, and K. Leibman (1971). A new spec-
tral intermediate associated with cytochrome P450
function in liver microsomes. Biochem. Biophys.
Res.
Commun. 42, 132-139.
105.
Peterson, J.A., R.E. Ebel, D.H. O'Keeffe,
T. Matsubara, and R.W. Estabrook (1976).
Temperature dependence of cytochrome P-450
reduction. A model for NADPH-cytochrome
P-450 reductase cytochrome P-450 interaction.
J. Biol. Chem. 251, 4010^016.
106.
Shimizu, T., T. Tateishi, M. Hatano, and
Y. Fujiikuriyama (1991). Probing the role of
lysines and arginines in the catalytic function of
cytochrome-P450d by site-directed mutagenesis—
interaction with NADPH-cytochrome-P450 reduc-
tase.
J. Biol. Chem. 266, 3372-3375.
107.
Shen, S. and H.W. Strobel (1993). Role of lysine
and arginine residues of cytochrome P450 in the
interaction between cytochrome P4502B1 and
NADPH-cytochrome P450 reductase. Arch.
Biochem. Biophys. 304, 257-265.
108.
Bridges, A., L. Gruenke, Y.T. Chang, LA. Vakser,
G. Loew, and L. Waskell (1998). Identification of
the binding site on cytochrome P450 2B4 for
cytochrome b{5) and cj^ochrome P450 reductase.
J. Biol. Chem. 213, 17036-17049.
109.
Strobel, H.W., S.G. Nadler, and D.R. Nelson (1989).
Cytochrome P-450: Cytochrome P450 reductase
interactions. DrugMetab. Rev. 20, 519-533.
110.
Nadler, S.G. and H.W. Strobel (1991). Iden-
tification and characterization of an NADPH-
cytochrome P450 reductase derived peptide
involved in binding to cytochrome P450. Arch.
Biochem. Biophys. 290, 277-284.
111.
Shen, S. and
H.W.
Strobel (1994). Probing the puta-
tive cytochrome P450- and cytochrome c-binding
sites on NADPH-cytochrome P450 reductase
by anti-peptide antibodies. Biochemistry 33,
8807-8812.
112.
Nisimoto,
Y.
(1986). Localization of cytochrome c-
binding domain on NADPH-cytochrome P-450
reductase. J. Biol. Chem. 261, 14232-14239.
113.
Sevrioukova, I.F., H. Li, H. Zhang, J.A. Peterson,
andT.L. Poulos (1999). Structure of a cytochrome
P450-redox partner electron-transfer complex.
Proc. Natl.
Acad.
Sci. USA 96, 1863-1868.
114.
Voznesensky, A.I. and J.B. Schenkman (1994).
Quantitative analyses of electrostatic interactions
between NADPH-cytochrome P450 reductase and
cytochrome P450 enzymes. J. Biol. Chem. 269,
15724-15731.
115.
Voznesensky, A.I. and J.B. Schenkman (1992). The
cytochrome P450 2B4-NADPH cytochrome P450
reductase electron transfer complex is not formed by
charge-pairing. J. Biol. Chem. 267, 14669-14676.
116.
Schenkman, J.B., A.I. Voznesensky, and I. Jansson
(1994).
Influence of ionic strength on the P450
monooxygenase reaction and role of cytochrome
b5 in the process. Arch. Biochem. Biophys. 314,
234-241.
117.
Davydov, D.R., A.A. Kariakin, N.A. Petushkova,
and J.A. Peterson (2000). Association of cyto-
chromes P450 with their reductases: Opposite sign
of the electrostatic interactions in P450BM-3 as
compared with the microsomal 2B4 system.
Biochemistry 39, 6489-6497.
118.
Munro, A.W and J.G. Lindsay (1996). Bacterial
cytochromes P-450.
Mol.
Microbiol.
20,1115-1125.
119.
Matson, R.S., R.S. Hare, and A.J. Fulco (1977).
Characteristics of a cytochrome P-450-dependent
fatty acid omega-2 hydroxylase from Bacillus
megaterium. Biochim. • Biophys. Acta. 487,
487^94.
120.
Sueyoshi,
T.
and M. Negishi (2001). Phenobarbital
response elements of cytochrome P450 genes and
nuclear receptors. Ann. Rev. Pharmacol. Toxicol.
41,
123-143.
121.
Shaw, G.C. and A.J. Fulco (1993). Inhibition by
barbiturates of the binding of Bm3Rl repressor
to its operator site on the barbiturate-inducible
cytochrome P450BM-3 gene of Bacillus mega-
terium.
J.Biol. Chem. 268, 2997-3004.
122.
Palmer, C.N., E. Axen, V Hughes, and C.R. Wolf
(1998).
The repressor protein, Bm3Rl, mediates
an adaptive response to toxic fatty acids in Bacillus
megaterium. J. Biol. Chem. 273, 18109-18116.
123.
Fulco, A.J. (1991). P450BM-3 and other inducible
bacterial P450 cytochromes: Biochemistry and
regulation. Ann. Rev. Pharmacol. Toxicol. 31,
177-203.
124.
Narhi, L.O. and A.J. Fulco (1987). Identification
and characterization of two functional domains
in cytochrome P-450BM-3, a catalytically
self-
sufficient monooxygenase induced by barbiturates
in Bacillus megaterium. J. Biol. Chem. 262,
6683-6690.
125.
Boddupalli, S.S., B.C. Pramanik, C.A. Slaughter,
R.W. Estabrook, and J.A. Peterson (1992). Fatty
acid monooxygenation by P450BM-3: Product
identification and proposed mechanisms for the
sequential hydroxylation reactions. Arch. Biochem.
Biophys. 292, 20-28.
126.
Peterson, J.A. and S.S. Boddupalli (1992).
P450BM-3:
Reduction by NADPH and sodium
dithionite. Arch. Biochem. Biophys. 294,
654-661.
