Ortiz de Montellano Paul R.(Ed.) Cytochrome P450. Structure, Mechanism, and Biochemistry
Подождите немного. Документ загружается.


114 Thomas L. Poulos and Eric F. Johnson
active site of cytochrome P450cam as revealed by
the crystal structure on the modified protein.
J.
Am.
Chem.
Soc. 120, 46-52.
67.
Dmochowski, I.J., B.R. Crane, J.J. Wilker,
J.R. Winkler, and
H.B.
Gray (1999). Optical detection
of cytochrome P450 by sensitizer-linked substrates.
Proc. Natl.
Acad.
Sci. USA 9, 12987-12990.
68.
Dunn, A.R., A.M. Hays, D.B. Goodin, CD. Stout, R.
Chiu, J.R. Winkler et al. (2002). Fluorescent probes
for Cytochrome P450 structural characterization
and inhibitor screening. J. Am. Chem. Soc. 124,
10254-10255.
69.
Li, H. and
T.L.
Poulos (1995). Modeling protein sub-
strate interactions in the heme domain of cytochrome
P450BM-3.
Acta
Crystallogr.
D
51,
21-32.
70.
Paulsen, M.D. and R.L. Ornstein (1995). Dramatic
differences in the motions of the mouth of open and
closed cytochrome P450BM-3 by molecular
dynamics simulations. Proteins 21, 237-243.
71.
Park, S.Y., H. Shimizu, S. Adachi, A. Nakagawa,
I.
Tanaka, K. Nakahara et
al.
(1997), Crystal structure
of nitric oxide reductase from denitrifying fungus
Fusarium
oxysporum.
Nat. Struc. Biol. 4, 827-832.
72.
Zerbe, K., O. Pylypenko, F. Vitali, W Zhang,
S. Rouset, M. Heck
etal.
(2002). Crystal structure of
OxyB,
a cytochrome P450 implicated in an oxidative
phenol coupling reaction during vancomycin biosyn-
thesis.
J.
Biol. Chem. Ill, 47476-47485.
73.
Podust, L., Y. Kim, M. Arase, B. Neely, B. Beck,
H. Bach et al (2003). The 1.92 A structure of
Streptomyces coelicolor A3(2) Cypl54Cl: A new
monooxygenase that fiinctionalizes macrolide ring
systems. J. Biol. Chem. 278, 12214-12221.
74.
Podust, L.M., T.L. Poulos, and M.R. Waterman
(2001).
Crystal structure of cytochrome P450
14alpha-sterol demethylase (CYP51) from
Mycobacterium tuberculosis in complex with
azole inhibitors. Proc. Natl.
Acad.
Sci. USA 98,
3068-3073.
75.
Scott, E.E.,
Y.A.
He, M.R. Wester, M.A. White, C.C.
Chin, J.R. Halpert, E.F Johnson, and D. Stout
(2003).
An open confirmation of mammalian
cytochrome P450 2B4 at 1.6A resolution. Proc.
Natl.
Acad.
Sci. USA 100, 13196-13201.
76.
Winn, P.J., S.K. Ldemann, R. Gauges,
V.
Lounnas,
and R.C. Wade (2002). Comparison of the
dynamics of substrate access channels in three
cytochrome P450s reveals different opening
mechanisms and a novel functional role for a
buried arginine. Proc. Natl.
Acad.
Sci. USA 99,
5361-5366.
77.
Pochapsky, T, T.A. Lyons, S. Kazanis, T. Arakaki,
and G. Ratnaswamy (1996). A structure-based
model for cytochrome P450cam-putidaredoxin
interactions. Biochimie 78, 723-733.
4
Electron Transfer Partners of
Cytochrome P450
Mark J.l. Paine, Nigel S. Scrutton, Andrew W. Munro, Aldo Gutierrez,
Gordon C.K. Roberts, and C. Roland Wolf
1.
Introduction
Cytochromes P450 contain a heme center
where the activation of molecular oxygen occurs,
resulting in the insertion of a single atom of
oxygen into an organic substrate with the con-
comitant reduction of the other atom to water. The
monooxygenation reaction requires a coupled and
stepwise supply of electrons, which are derived
from NAD(P)H and supplied via a redox partner.
P450s are generally divided into two major classes
(Class I and Class II) according to the different
types of electron transfer systems they use. P450s
in the Class I family include bacterial and mito-
chondrial P450s, which use a two-component
shuttle system consisting of an iron-sulfur protein
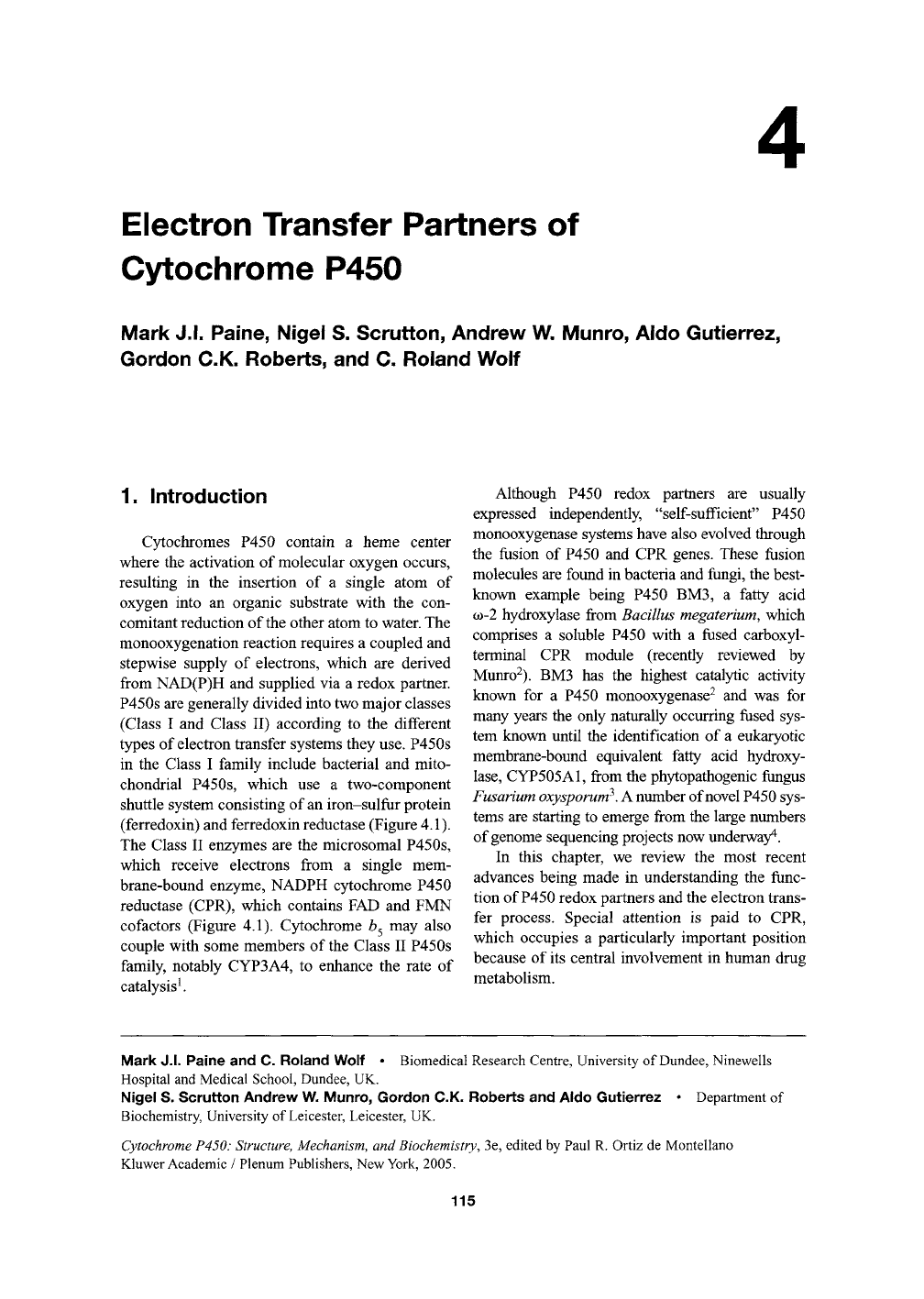
(ferredoxin) and ferredoxin reductase (Figure 4.1).
The Class II enzymes are the microsomal P450s,
which receive electrons from a single mem-
brane-bound enzyme, NADPH cytochrome P450
reductase (CPR), which contains FAD and FMN
cofactors (Figure 4.1). Cytochrome b^ may also
couple with some members of the Class II P450s
family, notably CYP3A4, to enhance the rate of
catalysis ^
Although P450 redox partners are usually
expressed independently, "self-sufficient" P450
monooxygenase systems have also evolved through
the fusion of P450 and CPR genes. These fusion
molecules are found in bacteria and fungi, the best-
known example being P450 BM3, a fatty acid
(0-2 hydroxylase from Bacillus megaterium, which
comprises a soluble P450 with a fiised carboxyl-
terminal CPR module (recently reviewed by
Munro^). BM3 has the highest catalytic activity
known for a P450 monooxygenase^ and was for
many years the only naturally occurring ftised sys-
tem known until the identification of a eukaryotic
membrane-bound equivalent fatty acid hydroxy-
lase,
CYP505A1, from the phytopathogenic fungus
Fusarium
oxysporurrP.
A number of novel P450 sys-
tems are starting to emerge from the large numbers
of genome sequencing projects now underway^.
In this chapter, we review the most recent
advances being made in understanding the func-
tion of P450 redox partners and the electron trans-
fer process. Special attention is paid to CPR,
which occupies a particularly important position
because of its central involvement in human drug
metabolism.
Mark J.l. Paine and C. Roland Wolf
Hospital and Medical School, Dundee, UK.
Nigel S. Scrutton Andrew W. Munro, Gordon C.K. Roberts and Aldo Gutierrez
Biochemistry, University of
Leicester,
Leicester, UK.
Biomedical Research Centre, University of
Dundee,
Ninewells
Department of
Cytochrome
P450:
Structure,
Mechanism,
and
Biochemistry,
3e, edited by Paul R. Ortiz de Montellano
Kluwer
Academic
/ Plenum Publishers,
New
York,
2005.
115
116
Mark J.I. Paine
et
al.
Class I: Iron-sulfur partners
Adx/AdR Pdx/PdR
NAD(P)H NAD(P)*
NADPH NADP
i FADp^\mQ)
Mitochondrial membrane
Class II: Diflavin reductase partners
CPR CPR fusion
NADPH NADP
4
NADPH NADP
4
Novel Systems
Fe2S2 fusion
NAD*
NADH
Figure
4.1.
Electron transfer partners
of
cytochrome P450.
In
Class
I
systems, electrons
are
shuttled from
NAD(P)H through
an
FAD-containing ferredoxin reductase
and an
iron-sulfur containing ferredoxin
to
P450;
in
prokaryotes these
are
typified
by
putidoredoxin reductase (PdR)
and
putidoredoxin (Pdx),
and in
eukaryotes
by
mitochondrial membrane associated adrenodoxin reductase (AdR) and adrenodoxin (Adx). Class II systems are driven
by electrons delivered from NADPH through diflavin (FMN- and FAD-containing) reductases. In eukaryotes these are
bound to the endoplasmic reticulum, while fused systems such as P450 BM3 exist in bacteria and fungi. Novel systems
now include P450RhF, which contains
an
FMN-containing reductase fused with
a
ferredoxin-like center and
a
P450.
2.
NADPH-Cytochrome P450
Reductase and the Diflavin
Reductase Family
2.1.
Background
In view of the key role that cytochrome c
has recently been found to play in regulating
apoptosis and cellular homeostasis^' ^, it is inter-
esting that CPR was first isolated from yeast as an
FMN containing NADPH-dependent cytochrome
c reductase^. A mammalian equivalent was later
isolated from pig liver and reported to contain an
FAD cofactor^. By 1962, the enzyme was shown
to be localized at the endoplasmic reticulum^, and
the flavin cofactors to be involved in the reduction
Electron Transfer Partners of Cytochrome P450
117
of cytochrome c^^. The true physiological redox
partner was eventually discovered as P450 through
the reconstitution of laurate co-hydroxylase activ-
ity from a detergent solubilized preparation
of cytochrome P450, NADPH cytochrome c
reductase^
^' ^^,
and a heat stable component, which
was later identified as the phospholipid phos-
phatidylcholine^^.
The initial difficulties in identifying a physio-
logical role for CPR were due to the purification
methods used, which incorporated trypsin or lipase
treatment^' ^. These resulted in the cleavage of
the amino-terminal membrane anchoring domain,
which constitutes the first 60 or so amino acid
residues and is responsible for interactions with the
phospholipid bilayer and P450^'^. Thus, while
proteolytically cleaved CPR is fully functional and
capable of reducing c3^ochrome c and a range of
artificial electron accepting compounds, it is unable
to reconstitute with P450s. The intact form of the
reductase was eventually purified from liver micro-
somes using detergent solubilization procedures
and found to have a molecular weight of 76-80 kDa
and to support P450-dependent reactions^^' ^^.
In the early 1970s, the enzyme was shown to
contain one molecule each of FMN and
FAD^^'
^^.
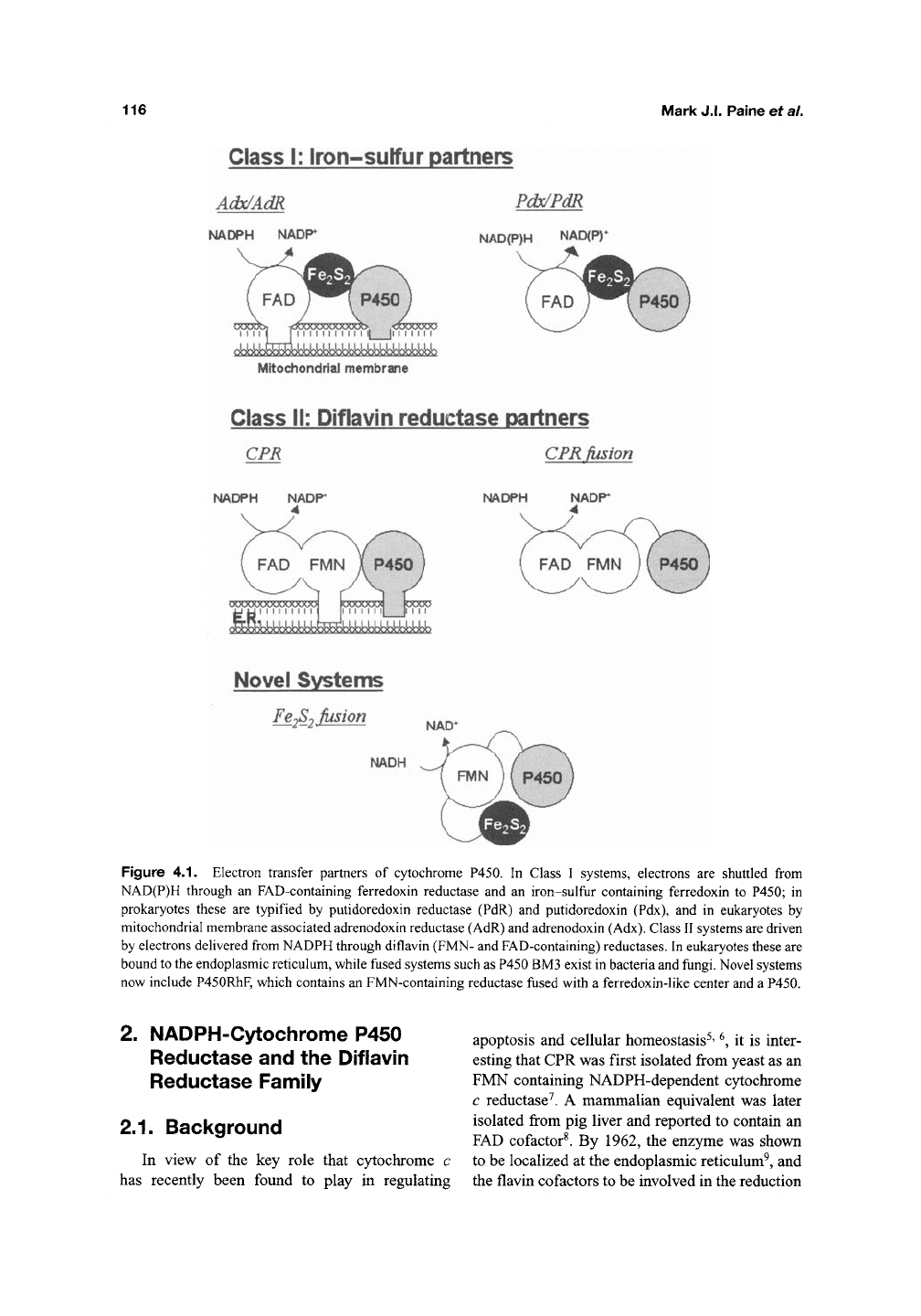
A useful feature of flavins is that their absorption
spectra are altered by changes in their reduction
state.
Thus, the reduction state can be examined
by measuring changes in the visible absorbance
range (Figure 4.2). This unique property stimu-
lated research into the redox properties'^' '^, of the
enzyme and the complex processes of hydride/
electron transfer from NADPH, across the flavins
and on to P450, discussed later in this chapter.
The cDNA and corresponding primary amino
acid sequences of several CPRs including rat'^,
rabbit^^, and human^' were obtained by the
mid-1980s, and the development of Escherichia
coli expression systems paved the way for detailed
molecular characterization of the polypeptide
through site-directed mutagenesis. The three-
dimensional structure of rat CPR was determined
by X-ray crystallography in 1997 by Kim and
coworkers^^, providing the structural prototype for
dual flavin oxidoreductases.
2.2.
The Diflavin Reductase
Family
CPR is the prototype for a small family of
diflavin reductases which are believed to have
300 400
500
600 700
Wavelength (nm)
Figure 4.2. Absorbance spectra of oxidized and reduced human CPR. The absorption maxima for the oxidized
enzyme are located at 380 nm and 454 nm. The direction of the arrow shows the absorption changes that occur upon
reducton of the flavins, in this case using a 2-fold and 20-fold excess of NADPH.
118
Mark J.I. Paine et al.
ferredoxin reductase |
flavodoxin I
FMN
CPR
IMS
BM3
iPSgf^:
NOS[
':^1^^''-
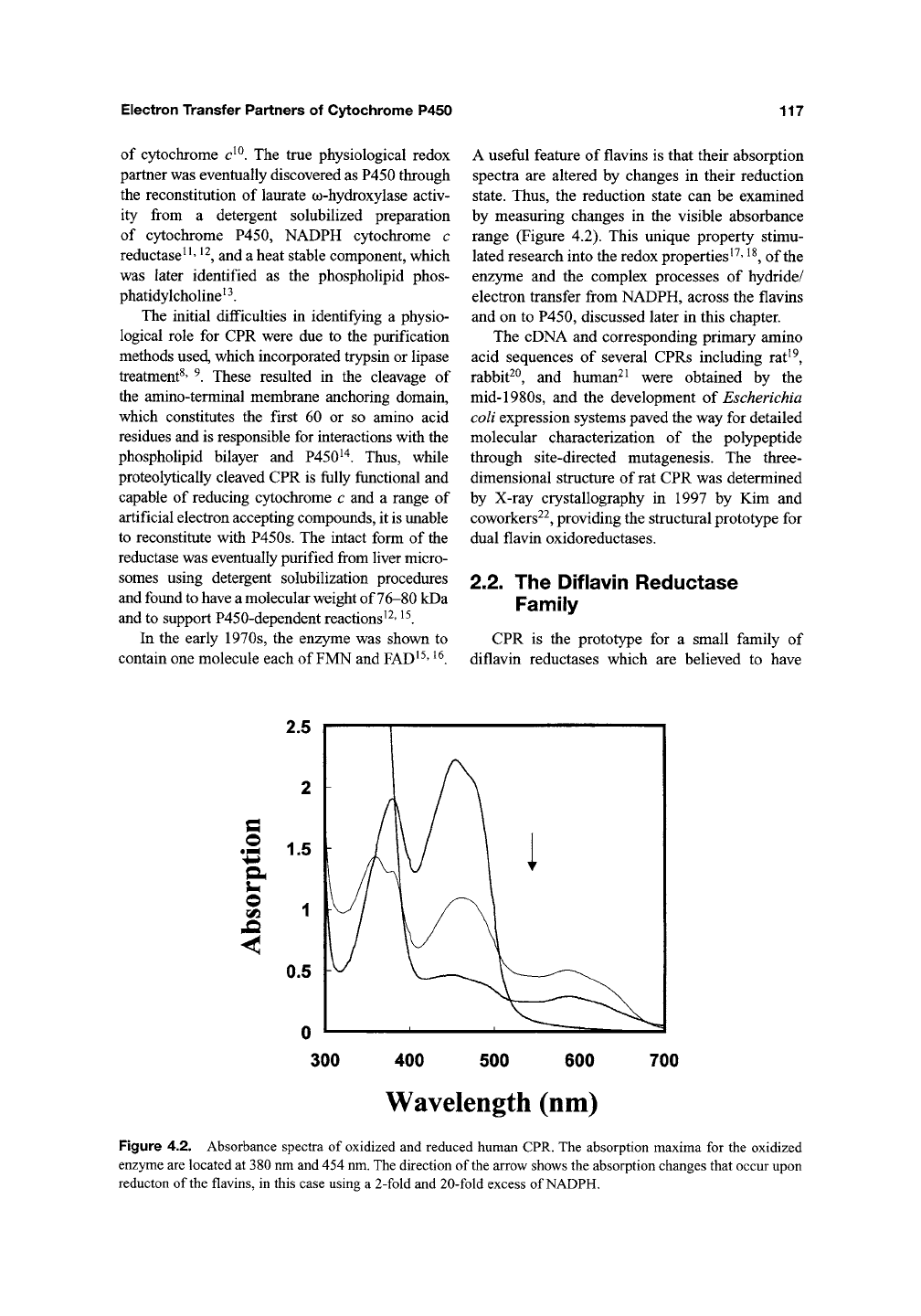
Figure 4.3. Schematic outline of the diflavin reductase family. Members contain an N-terminal FMN-binding
flavodoxin-like domain and
a
C-terminal FAD/NADPH-binding ferredoxin reductase-like domain, which contains an
additional linker
region.
Shown
are:
CPR, which has an amino-terminal membrane anchor region
(Anc);
NRl (Novel
reductase
1);
MSR (methionine synthase reductase), which contains an additional interdomain
sequence;
P450
BM3,
which is fused to a P450 domain; and NOS (nitric oxide synthases), which has linked to a heme-containing
oxygenase domain that is structurally distinct from the P450s.
evolved as a result of
a
gene fusion event between
ancestral FMN- and FAD-containing flavopro-
teins^^.
Indeed, CPR is one of only four mam-
malian enzymes that contain both FMN- and
FAD-binding domains, the other three being
methionine synthase reductase^"*, NRl^^, and the
nitric oxide synthases^^ (Figure 4.3). Bacterial
members of the family include the
B.
megaterium
cytochrome P450
BM3^^
and the reductase subunit
of sulfite reductase^^. While these are all struc-
turally related, there exist some key differences.
For instance, methionine synthase reductase and
NRl both contain the same domain organization as
P450 reductase but lack the membrane anchoring
sequence, and are thus located in the cytosol.
Methionine synthase also has a much larger inter-
domain linker than CPR, although the functional
significance of this is unclear. In nitric oxide syn-
thase, the reductase region is fused with a heme
domain, and has acquired an extra calmodulin-
binding domain to regulate electron transfer.
Experimental evidence for the gene fusion
concept comes from work of Smith et
al?"^,
who
dissected the FMN and FAD/NADH-binding
domains of human CPR and expressed them as
discrete functional units in E. colfl^. The individ-
ual domains not only folded correctly but also
could be reconstituted to form an active complex
capable of reducing cytochrome c and donating
electrons to the P450 monooxygenase complex,
albeit with greatly reduced efficiency^^. A num-
ber of other diflavin reductases have been dis-
sected into functional units including: rat CPR^^,
BM3^''
^^,
NRl^^, and methionine synthase reduc-
tase^"^.
The ability to separate CPR and other
diflavin reductases into their component parts has
greatly facilitated structural and functional studies
of these enzymes.
2.3.
CPR Genes
Apart from plants, which contain multiple
CPR genes^^'^^, most organisms contain a single
CPR gene. At the time of writing, a relatively
modest total of 20 CPR genes have been identi-
fied, which will doubtlessly increase as a result of
the many genome sequencing projects currently
underway. New sequences can be monitored using
the website www.icgeb.trieste.it, which provides
sequence updates on P450s and related enzymes.
The rat gene was cloned in 1990^^ and comprises
16 exons, with the first exon being non-coding.
Amongst the coding exons there is a general
correspondence with the structural domains, with
exons 2 and 3 encoding the membrane anchor
region, exons 4-7 the FMN-binding domain, and
exons 8-16 the FAD/NADPH-binding region.

Electron Transfer Partners of Cytochrome P450
119
A similar intronic arrangement is found for the
mouse gene located on chromosome 6^^' ^^. The
human gene is located on chromosome 7^^
Although the regulation of expression of
individual P450s in response to exogenous and
endogenous factors has been studied extensively,
the factors controlling the expression of the
CPR gene are less well understood. Reductase
expression may be induced by a number of
cytochrome P450 inducers such as phenobarbi-
tal'^^
but the regulation of reductase gene expres-
sion is independent of that of cytochrome P450'^^.
The upstream regulatory region of the rat
CPR gene has a number of interesting features"^^.
Unlike many drug-metabolizing enzymes, the
promoter does not contain either a TATA or
CCAAT box, it is GC rich, contains multiple
Spl consensus sequences, and utilizes two major
transcription start sites.
The CPR gene is regulated by thyroid hor-
mone, which stimulates CPR expression via the
thyroid response element (TRE) in the upstream
promoter'*^'
^^.
Most recently, peroxisome prolifer-
ators,
which regulate a battery of rodent P450
genes,
have also been shown to regulate CPR
levels"^^,
possibly through a putative DNA bind-
ing site for PPARa that exists in the mouse
CPR promoter between -568 and -556 bp^^
Interestingly, following exposure to peroxisome
proliferators CPR gene transcript levels appear
to increase, while levels of CPR protein are
decreased"^^. Thus, opposing transcriptional and
translational or post-translational mechanisms
appear to play a role in the regulation of CPR by
peroxisome proliferators"^^.
2.4. Probing the Physiological
Role of CPR
The reductase gene has been knocked out in
mice^^'
^^;
this leads to embryonic lethality, thus
demonstrating that CPR is essential for normal
development in higher organisms, presumably
because it plays a key role in the biosynthesis of
signaling factors such as retinoic acid, sterols,
prostaglandins, and steroids that are dependent
on P450 catalysis. The precise role of CPR in
embryogenesis is still unclear and the effects of
gene deletion on the developing embryos are com-
plex. Embryos lacking CPR do not survive beyond
8-9 days and display a number of defects includ-
ing neural tube, cardiac, eye, and limb abnormali-
ties,
and generalized defects in cell adhesion"^^.
Kasper's group has examined the effects of
CPR gene disruption by removing the natural
translation initiation site and deleting the
membrane-binding domain of CPR"^^. However,
some homozygous null embryos (CPR~^~) were
found to produce a truncated 66 kDa c5^oplasmic
form of CPR, presumably through initiation at an
alternative start site. That these still produced the
spectrum of embryonic defects leading to mid-
gestational lethality provides strong evidence that
the microsomal location of CPR is essential for the
physiologically important functions of
the
P450s.
The problem of the lethality of a CPR knock-
out has been resolved by applying the regulatable
Cre/loxP system^^ to generate mice in which
CPR can be deleted post-natally in the liver^^. Such
"conditional knockout" mice, in which all the
hepatic P450s are essentially inactivated, provide
the means of evaluating P450 function in normal
homeostasis, drug pharmacology, and chemical
toxicity in vivo. Hepatic CPR-null mice exhibit
many intriguing phenotypes^^. Due to an inability
to produce bile acids, they no longer break down
cholesterol, and whereas hepatic lipid levels were
significantly increased, circulating levels of cho-
lesterol and triglycerides are severely reduced.
There are also profound changes in the in vivo
metabolism of pentobarbital and acetaminophen,
demonstrating the predominant role of the hepatic
P450s in the pharmacology and toxicology of
these compounds, and illustrating the power of
transgenic models in understanding P450 function.
One of the remarkable aspects of the hepatic
CPR deletion in mice was the fact that they live
and reproduce normally. This means that in adult
mice at least, the hepatic P450 system is not essen-
tial for life, and indicates most strongly a funda-
mental role in providing protection against toxic
environmental agents. The data from these trans-
genic models^^'
^^
also demonstrate that potential
alternative electron transfer pathways for P450s,
such as the cytochrome b^/b^ reductase systems
that are discussed later, play only a minor role, if
any, in vivo.
The earliest CPR gene deletions were of
course carried out with yeast, and the data offer
some interesting contrasts with mammalian
deletions"^^'
'^^.
The deletion of the CPR gene in

120 Mark J.I. Paine et al.
Saccharomyces cerevisae is not lethal but instead
produces a viable mutant with an increased sensi-
tivity (>200-fold) to ketoconazole, an azole
inhibitor of the ftingal P450
CYP51,
a lanosterol
14a-demethylase involved in the essential ergo-
sterol biosynthesis pathway"*^. Although this
hypersensitivity is suggestive of inefficient sterol
biosynthesis, it has been observed that the
cpr~ strains still accumulate significant amounts
of ergosterol, around 25% of those in the wild-
type parent"^^. This indicates that, unlike mice, an
alternative electron donor is effective in delivering
two electrons for P450 activity^^. In contrast to
Shen's observations with cpr-deleted mice"^^, a
cytosolic truncated version of native CPR with the
N-terminal membrane anchor removed effectively
reversed the phenotypes associated with cpr
deletion in yeast^^. Thus, there appear to be
fundamental differences in the mechanisms of P450
interactions between yeast and mammalian CPRs.
While the major role of CPR is associated with
cytochrome P450 and the phase I metabolism of
xenobiotic compounds, the enzyme also has the
ability to reduce other electron acceptors such as
cytochrome c^, cytochrome
b^
(ref [50]), and heme
oxygenase^ ^ In addition, it has recently been impli-
cated, along with cytochrome
Z?^,
in the bioreduc-
tive activation of methionine synthase^^, thus
pointing to a possible role in the regulation of
methionine synthesis. CPR has also been shown to
be involved in the regulation of oxidative response
genes such as hypoxia-inducible factor
1
(ref [53]).
P450 reductase plays a role in the bioactivation
of therapeutic prodrugs through its ability to
reduce a range of one-electron acceptors such
as the quinone drugs doxorubicin^^ and mito-
mycin
c^^
and aromatic TV-oxides such as the novel
benzotriazine, tirapazamine^^. Evidence is also
emerging that other members of the CPR family
including nitric oxide synthases^^ and NRl^^ may
play a similarly important role in the metabolism
of chemotherapeutic drugs.
Overall, the biological relationships of CPR
with proteins other than P450 are not especially
well established. However, attention is starting
to shift toward understanding the role of CPR in
other redox pathways. In particular, data is accu-
mulating on CPR interactions with cytochrome
b^
(ref [58]) and heme oxygenase^^. Consistent with
the findings for cytochrome P450 enzymes,
molecular investigations on the binding of CPR to
human heme oxygenase-1 (hHO-1) establish that
ionic interactions contribute to the binding of these
proteins^^. Like P450, positively charged hHO-1
surface residues contribute to the binding of this
molecule to CPR, most likely via the negatively
charged residues in the FMN domain of CPR.
2-5.
Structure
of CPR
The crystal structure of rat CPR lacking the
N-terminal membrane-binding sequence^^ shows
that the catalytic region comprises three distinct
domains: an FMN-binding domain structurally
similar to flavodoxins; an NADPH/FAD-binding
domain similar to ferredoxin-NADP^ reductase;
and a "linker" domain, present as an insert in the
sequence of the NADPH/FAD-binding domain
(Figure 4.4). The "linker" sequence is the major
structural feature that distinguishes these mole-
cules from the single-domain ferredoxin reduc-
tases,
and is likely to play a structural role in
positioning the FMN- and FAD-binding domains
correctly for direct electron transfer. This is sup-
ported by mutagenesis studies in which compari-
son of wild-type and mutant structures shows
significant differences in the relative position of
the two flavin domains of rat CPR^^. Furthermore,
there is increasing evidence from structural stud-
ies with other dual flavin reductases that the inter-
play between NADPH, flavin cofactors, and heme
is directed by conformational changes. This is
exemplified by nNOS, where structural relaxation
appears to be an important part of the calmodulin-
dependent activation mechanism^ *.
2.5.1.
The FMN-Binding Domain
The FMN-binding domain interacts with
cytochrome P450 to transfer electrons during
catalysis. In addition to rat CPR, detailed struc-
tural studies of the isolated human FMN domain
have been carried out using NMR^^, and X-ray
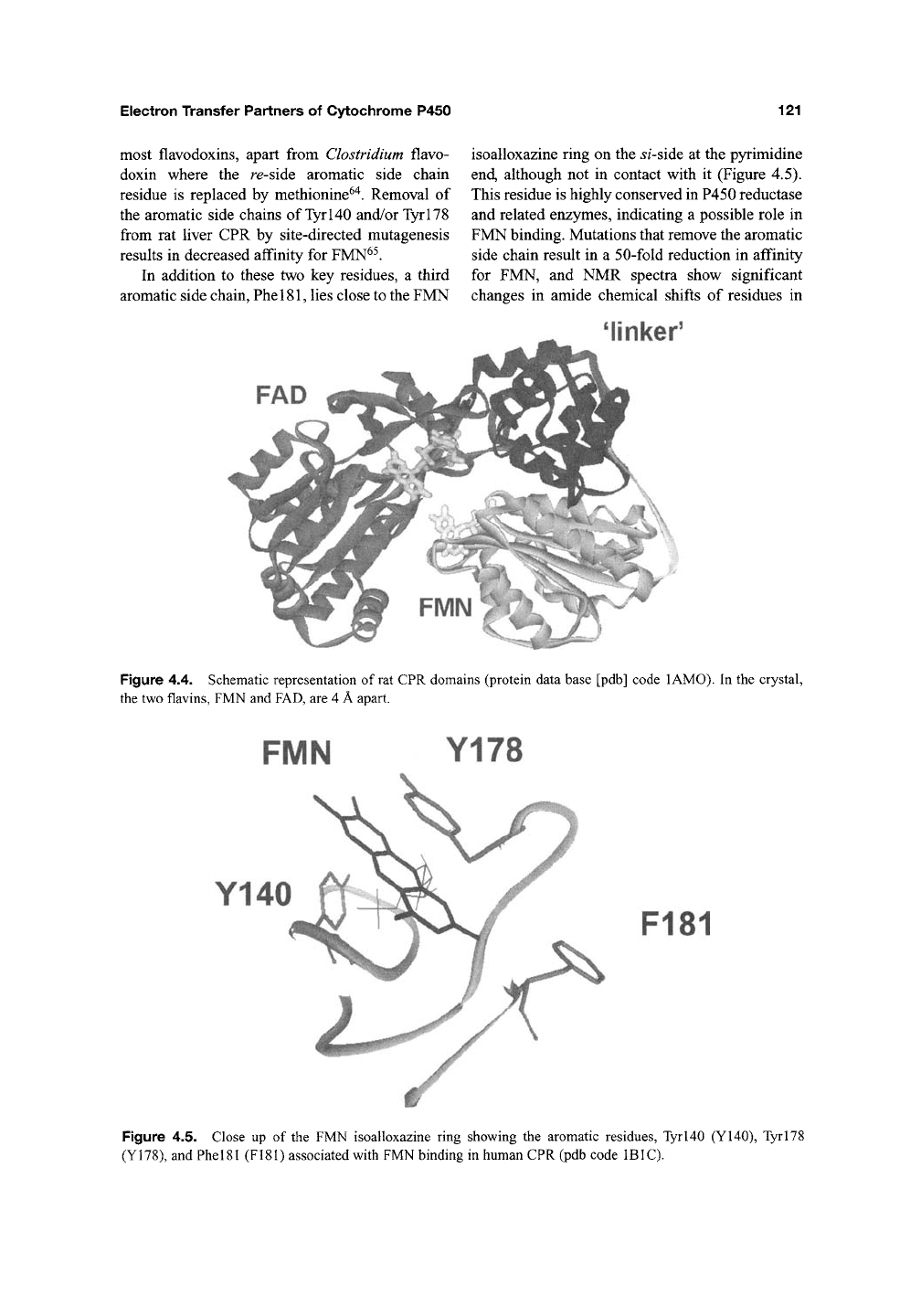
crystallography^^. The isoalloxazine ring of FMN
is sandwiched between two aromatic side chains,
Tyrl40 and TyrlTS^^'
62,63
jyj.173 ij^s parallel to
the 5/-side of the FMN ring, while the second
aromatic, Tyrl40, located on the re-side, lies at
an angle of ~40° to the isoalloxazine ring
(Figure 4.5). A similar arrangement is found in
the FMN-binding domain of P450 BM3 and in
Electron Transfer Partners of Cytochrome P450
121
most flavodoxins, apart from Clostridium flavo-
doxin where the r^-side aromatic side chain
residue is replaced by methionine^"^. Removal of
the aromatic side chains of Tyrl40 and/or Tyr 178
from rat liver CPR by site-directed mutagenesis
results in decreased affinity for FMN^^.
In addition to these two key residues, a third
aromatic side chain, PhelSl, lies close to the FMN
isoalloxazine ring on the ^/-side at the pyrimidine
end, although not in contact with it (Figure 4.5).
This residue is highly conserved in P450 reductase
and related enzymes, indicating a possible role in
FMN binding. Mutations that remove the aromatic
side chain result in a 50-fold reduction in affinity
for FMN, and NMR spectra show significant
changes in amide chemical shifts of residues in
_ 'linker'
Figure 4.4. Schematic representation of rat CPR domains (protein data base [pdb] code lAMO). In the crystal,
the two flavins, FMN and FAD, are 4 A apart.
FMN
Y178
Y140
F181
Figure 4.5. Close up of the FMN isoalloxazine ring showing the aromatic residues, Tyrl40 (Y140), Tyrl78
(Y178), and PhelSl (F181) associated with FMN binding in human CPR (pdb code IBIC).

122
Mark J.I. Paine et aL
the p4-a6 loop. This loop includes PhelSl, the
key FMN-binding residue Tyrl78, and three
other residues that form hydrogen bonds to the
isoalloxazine ring, as well as residues in the adja-
cent p5-a7 loop^^. These are consistent with local
changes in structure on substitution of PhelSl,
and with the idea that the packing of this con-
served residue plays a significant role maintaining
the orientation of residues which interact with the
isoalloxazine ring and hence in the formation of
the FMN-binding site.
2.5.2.
FAD/NADPH-Binding Domain
Like FNR, FAD is bound in an extended con-
formation in rat CPR^^. Key residues that serve to
regulate flavin binding and reactivity are Arg454,
Tyr456, Cys472, Gly488,
Thr491,
and Trp67722.
Site-directed mutagenesis of these residues has
been carried out to examine their role in FAD
binding and catalysis^^. Trp677 is stacked against
the re-face of
FAD,
while Tyr456 lies at its 5/-face.
Deleting Trp677 reduces catalytic activity 50-fold
but has little effect on FAD content. However,
mutations to Tyr456, which also hydrogen bonds
to the ribityl 4'-hydroxyl, decreases affinity for
FAD by around 8,000-fold. Side chains from
Thr491 and Arg454 stabilize the pyrophosphate
group of FAD. Substitution of Thr491 has
a relatively modest effect on FAD binding (~
100-
fold decrease) compared with mutation of Arg454,
which decreases affinity 25,000-fold.
NMR studies show that the pro-R-hydrogen
attached to the C-4 atom of NADPH is transferred
directly to FAD as a hydride ion^^. However, an
understanding of NADPH binding and the factors
involved in hydride transfer have been hampered by
the spatial disorder in this region of the molecule
in the crystal structure. While the electron density
of the adenosine moiety of the coenzyme is well
defined in the structure, that from the nicotinamide
ring is not, and is consistent with at least two
dif-
ferent positions for this ring, neither of which is in
contact with the isoalloxazine ring of FAD^^.
Similar observations have been made in enzymes
of
the
FNR family which are structurally similar to
the FAD/NADPH-binding domain of CPR^^-^i. An
aromatic amino acid residue is located at the car-
boxyl-terminus in all members of
the
FNR family
In rat and human CPR, this position is occupied by
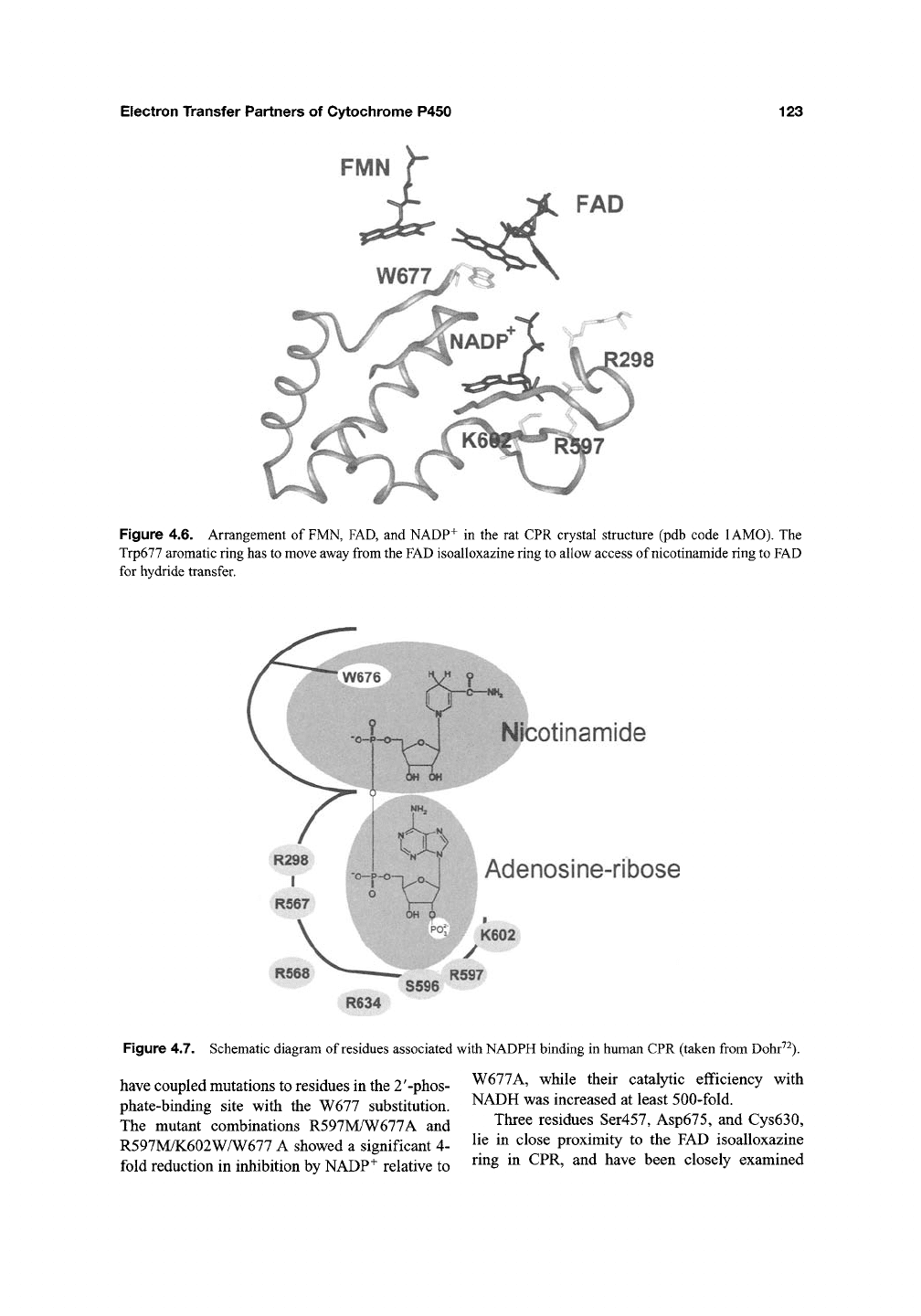
Trp677 and Trp676, respectively. The planar side
chain of
the
tryptophan stacks against the isoallox-
azine ring of FAD, and structural rearrangements
are thought to be essential to enable access of the
nicotinamide ring to the isoalloxazine ring of
FAD
for hydride transfer (Figure 4.6). The proposed
mechanism for binding involves the tryptophan
residue flipping away from FAD to be replaced by
the nicotinamide region of
NADPtf^.
There has been extensive site-directed mutage-
nesis of amino acid residues in the vicinity of the
NADPH-binding region to examine their role in
cofactor binding and hydride transfer (Figure 4.7).
Removal of the aromatic side chain from Trp676
shows that it plays an essential role in the
discrimination between NADPH and NADH as
cofactors for CPR^^' ^^, notwithstanding the fact
that it does not interact with the 2'-phosphate
which is the sole difference between the two
cofactors. Investigation of the steady-state kinet-
ics shows that substitution of Trp676 with Ala
decreases the K^ for NADH from 48 to 0.3 mM,
and increases the
k^^^
to ~4,000 min~^ while the
^^NADPH ^Qps tQ 0.2
|ULM
and
k^^^
to lOmin^^
The Trp676Ala mutant therefore binds both
coenzyme molecules more tightly, the change in
cofactor specificity (expressed as a k^JK^ ratio)
being predominantly a reflection of changes in
k^^^.
A similar change in cofactor specificity also
occurs with pea FNR with the analogous mutation
Tyr308Ser^4' ^^ Both in pea FNR^^ and in CPR^^
substitution of the C-terminal aromatic residue
allows the nicotinamide ring of the cofactor to
bind close to the isoalloxazine ring, the thermody-
namically unfavorable flipping no longer being
required. Hence, removal of this physical rate-lim-
iting step produces enzymes that have lower
apparent K^ values for nicotinamide cofactors.
Further kinetic evidence for a critical role for this
Trp residue in regulating NADPH binding and
hydride transfer is detailed in Section
2.6.1.
Several conserved amino acids including ser-
ine 596, arginine 597, and lysine 602 have been
proposed to be involved in binding the adenosine-
ribose moiety of NADPH (Figure 4.7), and
specific charge-charge interactions between these
and the 2'-phosphate of NADPH are thought
to be responsible for discrimination against
NADtf^'
^^-^l Working on the hypothesis that
mutations to these residues might reduce the
affinity of the enzyme for NADP(H) and thus pre-
vent inhibition by NADP^, Elmore and Porter^^
Electron Transfer Partners of Cytochrome P450
123
298
Figure 4.6. Arrangement of
FMN,
FAD, and NADP+ in the rat CPR crystal structure (pdb code lAMO). The
Trp677 aromatic ring has
to move
away from the
FAD
isoalloxazine ring
to
allow access of nicotinamide ring
to
FAD
for hydride transfer.
-oJ.,,-^J Nicotinamide
Adenosine-ribose
RS^
S596
y
R597
R634
Figure 4.7. Schematic diagram of residues associated with NADPH binding in human CPR (taken from Dohr^^).
have coupled mutations to residues in the 2'-phos-
phate-binding site with the W677 substitution.
The mutant combinations R597MAV677A and
R597M/K602WAV677 A showed a significant 4-
fold reduction in inhibition by NADP^ relative to
W677A, while their catalytic efficiency with
NADH was increased at least 500-fold.
Three residues Ser457, Asp675, and Cys630,
lie in close proximity to the FAD isoalloxazine
ring in CPR, and have been closely examined
