Ortiz de Montellano Paul R.(Ed.) Cytochrome P450. Structure, Mechanism, and Biochemistry
Подождите немного. Документ загружается.


Computational Approaches to Cytochrome P450 Function
83
60.
Green, M.T. (1999). Evidence for sulfur-based
radicals in thiolate compound I intermediates.
J.Am.
Chem. Soc. 121, 7939-7940.
61.
Ohta, T., K. Matsuura, K. Yoshizawa, and I.
Morishima (2000). The electronic and vibrational
structures of iron-oxo porphyrin with a methoxide
or cysteinate axial ligand. J. Inorg. Biochem. 82,
141-152.
62.
Ogliaro, R, N. Harris, S. Cohen, M. Filatov, S.R De
Visser, and S. Shaik (2000). A model "rebound"
mechanism of hydroxylation by cytochrome P450:
Stepwise and effectively concerted pathways, and
their reactivity patterns. J. Am. Chem. Soc. 122,
8977-8989.
63.
Ogliaro, E, S. Cohen, S.P. De Visser, and S. Shaik
(2000).
Medium polarization and hydrogen bond-
ing effects on compound I of cytochrome P450:
What kind of a radical is it really? J. Am. Chem.
Soc. 122, 12892-12893.
64.
Rutter, R., L.R Hager, H. Dhonau, M. Hendrich,
M. Valentine, and P. Debrunner (1984). Chloro-
peroxidase compound I: Electron paramagnetic
resonance and Mssbauer studies. Biochemistry 23,
6809-6816.
65.
De Visser, S.P, F. Ogliaro, Z. Gross, and S. Shaik
(2001).
What is the difference between the man-
ganese porphyrin and corrole analogues of
cytochrome P450's compound I? Chem. Eur. J.
7,
4954^960.
66.
Ogliaro, K, S.R De Visser, J.T. Groves, and S. Shaik
(2001).
Chameleon states: High-valent metal-oxo
species of cytochrome P450 and its ruthenium
analogue. Angew. Chem. Int. Ed. 40, 2874-2878.
67.
Sharma, RK., S.R De Visser, F. Ogliaro, and S.
Shaik (2003). Is the ruthenium analog of compound
I of cytochrome P450 an efficient oxidant? A theo-
retical investigation of the methane hydroxylation
reaction. J ^m. Chem. Soc. 125, 2291-2300.
68.
Green, M.T. (2000). Imidazole-ligated compound I
intermediates: The effects of hydrogen bonding.
J.Am.
Chem. Soc. Ill, 9495-9499.
69.
Green, M.T. (2001). The structure and spin cou-
pling of catalase compound I: A study of noncova-
lent effects. J Am. Chem. Soc. 123, 9218-9219.
70.
Deeth, R.J. (1999). Saddle distortions of ferryl-
porphyrin models for peroxidase compound I:
A density functional study. J.Am. Chem. Soc. Ill,
6074-6075.
71.
Ogliaro, F. and S. Shaik (2003). Substituent effects
on structure and properties of compound I species.
Unpublished results.
72.
Wirstam, M., M.R.A. Blomberg, and RE.M.
Siegbahn (1999). Reaction mechanism of com-
pound I formation in heme peroxidases: A density
functional theory study. J. Am. Chem. Soc. Ill,
10178-10185.
73.
Lewis, D.FV (2001). Guide to Cytochromes P450.
Taylor and Francis, New York.
74.
Poulos, TL., B.C. Finzel, and A.J. Howard (1986).
Crystal structure of substrate-free Pseudomonas
putida Cytochrome P450. Biochemistry 25,
5314-5322.
75.
Mueller, E.J., RJ. Loida, and S.G. Sligar (1995).
Twenty-five years of P450cam research. In PR.
Ortiz de Montellano (ed.), Cytochrome P-450:
Structures, Mechanism and Biochemistry, 2nd edn.
pp.
83-124. Plenum Press, New York.
76.
Schoeboom, J.C, S. Cohen, H. Lin, S. Shaik, and
W Thiel (2004). Quantum mechanical/molecular
mechanical investigation of the mechanism of C-H
hydroxylation of camphor by cytochrome P450^gj^:
Theory supports a two-state rebound mechanism.
J Am. Chem. Soc. 126, 4017-4034.
77.
KeseriiG.M., I. Kolossv^, and B. Bertfe (1997).
Cytochrome P-450 catalyzed insecticide metabo-
lism. Prediction of regio- and stereoselectivity in
the primer metabolism of carbofuran: A theoretical
study, ^^m. Chem. Soc. 119, 5126-5131.
78.
KeserjiG.M., I. Kolossvfy, and I. Szfeely (1999).
Inhibitors of cj^ochrome P450 catalyzed insecticide
metabolism: A rational approach. Int. J. Quantum
Chem.
73, 123-135.
79.
Cavalli, A. and M. Recanatini (2002). Looking for
selectivity among cytochrome P450s inhibitors.
J Med Chem. 45,251-254.
80.
Lee, H., PR. Ortiz de Montellano, and
A.E. McDermott (1999). Deuterium magic angle
spinning studies of substrates bound to cytochrome
F450. Biochemistry 3S, 10808-10813.
81.
De Voss, J.J., O. Sibbesen, Z. Zhang, and PR. Ortiz
de Montellano (1997). Substrate docking algo-
rithms and prediction of the substrate specificity of
cytochrome P450cam and its L244A mutant.
J.
Am.
Chem.
Soc. 119, 5489-5498.
82.
Atkins, WM. and S.G. SHgar (1987). MetaboHc
switching in cytochrome P450cam: Deuterium
isotope effects on regiospecificity and the
monooxygenase/oxidase ratio. J. Am. Chem. Soc.
109,
3754-3760.
83.
Audergon, C, K.R. Iyer, J.R Jones, J.F, DarByshire,
and WE Trager (1999). Experimental and theoreti-
cal study of the effect of active-site constrained
substrate motion on the magnitude of the observed
intramolecular isotope effect for the P450 101 cat-
alyzed benzylic hydroxylation of isomeric xylenes
and 4,4'-dimethylbiphenyl. J. Am. Chem. Soc. Ill,
4\-41.
84.
Helms, V and R.C. Wade (1998). Hydration energy
landscape of the active site cavity in cytochrome
P450cam. Proteins 32, 381-396.
85.
Winn, P.J., S.K. Ldemann, R. Gauges, V Lounnas,
and R.C. Wade (2002). Comparison of the

84
Sason Shaik and Samuel P. De Visser
dynamics of substrate access channels in three
cytochrome P450s reveals different opening mecha-
nisms and a novel functional role for a buried argi-
nine.
Proc.
Natl
Acad.
Sci. USA. 99, 5361-5366.
86.
Das, B., V Helms, V Lounnas, and R.C. Wade
(2000).
Multicopy molecular dynamics simulations
suggest how to reconcile crystallographic and prod-
uct formation data for camphor enantiomers bound
to cytochrome P-450cam. J. Inorg. Biochem. 81,
121-131.
87.
Fruetel, J.A., J.R. Collins, D.L. Camper, G.H.
Loew, and P.R. Ortiz de Montellano (1992).
Calculated and experimental absolute stereochem-
istry of the styrene and p-methylstyrene epoxides
formed by cytochrome P450cam.
J.
Am. Chem. Soc.
114,
6987-6993.
88.
Harris, D.L. and G.H. Loew (1995). Prediction of
regiospecific hydroxylation of camphor analogs
by cytochrome P450cam. J. Am. Chem. Soc. 117,
2738-2746.
89.
Park, J.-Y. and D.L. Harris (2003). Construction
and assessment of models of
CYP2E1:
Predictions
of metabolism from docking, molecular dynamics
and density functional theoretical calculations.
J. Med. Chem. 46, 1645-1660.
90.
Vaz, A.D.N., D.F. McGinnity, and M.J. Coon
(1998).
Epoxidation of olefins by cytochrome
P450:
Evidence from site-specific mutagenesis for
hydroperoxo-iron as an electrophilic oxidant. Proc.
Natl. Acad Sci. USA. 95, 3555-3560.
91.
Newcomb, M. and PH. Toy (2000). Hypersensitive
radical probes and the mechanisms of cyto-
chrome P450-catalyzed hydroxylation reactions.
Ace. Chem. Res. 33, 449^55.
92.
Jin, S., T.M. Markis, T.A. Bryson, S.G. Sligar, and
J.H. Dawson (2003). Epoxidation of olefins by
hydroperoxo-ferric cytochrome
P450.
J.
Am. Chem.
Soc. 125, 3406-3407.
93.
Shaik, S., M. Filatov, D. Schrder, and H. Schwarz
(1998).
Electronic structure makes a difference:
Cytochrome P450 mediated hydroxylations of
hydrocarbons as a two-state reactivity paradigm.
Chem.
Eur.
J. 4, 193-199.
94.
Schrder, D., S. Shaik, and H. Schwarz (2000). Two-
state reactivity as a new concept in organometalHc
chemistry/ice. Chem. Res. 33, 139-145.
95.
Shaik, S., S.P De Visser, F Ogliaro, H. Schwarz,
and D. Schrder (2002). Two-state reactivity mech-
anisms of hydroxylation and epoxidation by
cytochrome P-450 revealed by theory. Curr. Opin.
Chem.
Biol. 6, 556-567.
96.
Sevin, A. and M. Fontecave (1986). Oxygen trans-
fer from iron oxo porphyrins to ethylene. A semi-
empirical MO/VB approach. J. Am. Chem. Soc.
108,
3266-3272.
97.
De Visser, S.P, F Ogliaro, and S. Shaik (2001).
Stereospecific oxidation by Compound I of
cytochrome P450 does not proceed in a concerted
synchronous manner. Chem. Comm. 2322-2323.
98.
Groves, J.T and G.A. McClusky (1976). Aliphatic
hydroxylation via oxygen rebound. Oxygen trans-
fer catalyzed by iron. J. Am. Chem. Soc. 98,
859-861.
99.
Ortiz de Montellano, PR. and R.A. Stearns (1987).
Timing of the radical recombination step in
cytochrome P450 catalysis with ring-strained
probes.
J:^W.
Chem. Soc. 109, 3415-3420.
100.
Newcomb, M., R. Shen, S.-Y. Choi, PH. Toy, PF
HoUenberg, A.D.N. Vaz et al. (2000). Cytochrome
P450-catalyzed hydroxylation of mechanistic
probes that distinguish between radicals and
cations. Evidence for cationic but not for radical
intermediates. J. Am. Chem. Soc. 122, 2677-2686.
101.
Filatov, M., N. Harris, and S. Shaik (1999). On the
"rebound" mechanism of alkane hydroxylation by
cytochrome P450: Electronic structure of the inter-
mediate and the electron transfer character in the
rebound
step.
Angew.
Chem.
Int. Ed. 38, 3510-3512.
102.
Harris, N., S. Cohen, M. Filatov, F Ogliaro, and S.
Shaik (2000). Two-state reactivity in the rebound
step of alkane hydroxylation by cytochrome P-450:
Origins of free radicals with finite lifetimes.
Angew. Chem. Int. Ed. 39, 2003-2007.
103.
Ogharo, F, M. Filatov, and S. Shaik (2000).
Alkane hydroxylation by cytochrome P450: Is
kinetic isotope effect a reliable probe of transition
state structure? Eur J. Inorg. Chem. 2455-2458.
104.
De Visser, S.P, F Ogliaro, PK. Sharma, and S.
Shaik (2002). Hydrogen bonding modulates the
selectivity of enzymatic oxidation by P450:
Chameleon oxidant behavior by Compound I.
Angew. Chem. Int. Ed 41, 1947-1951.
105.
De Visser, S.P, F Ogliaro, PK. Sharma, and
S. Shaik (2002). What factors affect the regiose-
lectivity of oxidation by cytochrome P450? A DFT
study of allylic hydroxylation and double bond
epoxidation in a model reaction.
J.
Am. Chem. Soc.
124,11809-11826.
106.
Cohen, S. and S. Shaik (2004). Quntum mechani-
cal/molecular mechanical study of the regioselec-
tivity of camphor and cyclohexene oxidations by
cytochrome P450cam. In preparation; part of the
Ph. D. thesis of
Mrs.
S. Cohen.
107.
Yoshizawa, K. (2002). Theoretical study on kinetic
isotope effects in the C-H bond activation of
alkanes by iron-oxo complexes.
Coord.
Chem. Rev.
226,251-259.
108.
Yoshizawa, K., T. Ohta, M. Eda, and T. Yamabe
(2000).
Two-step concerted mechanism for the
hydrocarbon hydroxylation by cytochrome P450.
Bull. Chem. Soc. Jpn. 73, 401^07.
109.
Yoshizawa, K., Y. Shiota, and Y Kagawa (2000).
Energetics for the oxygen rebound mechanism
of alkane hydroxylation by the iron-oxo species of

Computational Approaches to Cytochrome P450 Function
85
cytochrome P450. Bull. Chem. Soc. Jpn. 73,
2669-2673.
110.
Yoshizawa, K., Y. Kagawa, and Y. Shiota (2000).
Kinetic isotope effects in a C-H bond dissociation
by the iron-oxo species of cytochrome P450.
J. Phys. Chem. B 104, 12365-12370.
111.
Yoshizawa, K., T. Kamachi, and
Y.
Shiota (2001). A
theoretical study of
the
dynamic behavior of alkane
hydroxylation by a compound I model of cyto-
chrome P450. ^^m. Chem. Soc. 123, 9806-9816.
112.
Hata, M., Y. Hirano, T. Hoshino, and M. Tsuda
(2001).
Monooxygenation mechanism by cyto-
chrome P-450. ^^m. Chem. Soc. 123, 6410-6416.
113.
Shaik, S., S. Cohen, S.P de Visser, PK. Sharma,
D.
Kumar, S. Kozuchs et al. (2004). The
"rebound controversy": An overview and theoreti-
cal modeling of
the
rebound step in C-H hydroxy-
laion by cytochrome P450. Eur. J. Inorg. Chem.
207-226.
114.
Guallar, V, B.F. Gherman, W.H. Miller, S.J.
Lippard, and R.A. Friesner (2002). Dynamics of
alkane hydroxylation at the non-heme diiron center
in methane monooxygenase. J. Am. Chem. Soc.
124,
3377-3384.
115.
De Visser, S.P, F. Ogliaro, N. Harris, and S. Shaik
(2001).
Multi-state epoxidation of ethene by
cytochrome P450: A quantum chemical study.
J.
Am. Chem. Soc. 123, 3037-3047.
116.
De Visser, S.P, R Ogliaro, and S. Shaik (2001).
How does ethene inactivate cytochrome P450 en
route to its epoxidation? A density functional
study. Angew. Chem. Int. Ed. 40, 2871-2874.
117.
Groves, J.T., K.-H. Ahn, and R. Quinn (1988).
Cis-trans isomerization of epoxides catalyzed by
ruthenium(II) porphyrins. J. Am. Chem. Soc. 110,
4217^220.
118.
De Visser, S.P, D. Kumar, and S. Shaik (2004).
How do aldehyde side products occur during
alkene epoxidation by cytochrome P450? Theory
reveals a state-specific multi-state scenario where
the high-spin component leads to all side products.
J. Inorg. Biochem. in press.
119.
De Visser, S.P and S. Shaik (2003). A proton-shut-
tle mechanism mediated by the porphyrin in ben-
zene hydroxylation by cytochrome P450 enzymes.
J.Am.
Chem. Soc. 125, 7413-7424.
120.
Korzekwa, K.R., D.C. Swinney, and W.F Trager
(1989).
Isotopically labeled chlorobenzenes as
probes for the mechanism of cytochrome P450
catalyzed aromatic hydroxylation. Biochemistry
28,
9019-9027.
121.
Rietjens, I.M.C.M., A.E.M.E Soffers, C. Veeger,
and J. Vervoort (1993). Regioselectivity of
cytochrome P-450 catalyzed hydroxylation of flu-
orobenzenes predicted by calculated frontier
orbital substrate characteristics. Biochemistry 32,
4801-4812.
122.
Sharma, PK., S.P de Visser, and S. Shaik (2003).
Can a single oxidant with two-spin states masquer-
ade as two different oxidants? A study of the sul-
foxidation mechanism by cytochrome P450.
J.
Am.
Chem.
Soc. 125, 8698-8699.
123.
Groves, J.T., G.E. Avaria-Neisser, K.M. Fish, M.
Imachi, and R.L. Kuczkowski (1986). Hydrogen-
deuterium exchange during propylene epoxidation
by cytochrome P450. J. Am. Chem. Soc. 108,
3837-3838.
124.
Groves, J.T. and D.V Subramanian (1984).
Hydroxylation by cytochrome P450 and
metalloporphyrin models. Evidence for
allylic rearrangement. J. Am. Chem. Soc. 106,
2177-2181.
3
Structures of Cytochrome P450
Enzymes
Thomas L. Poulos and Eric R Johnson
1.
Introduction
Much of what
we
know about the molecular level
structure-function relationships
in P450s is
based on
studies with the camphor monooxygenase system
from Pseudomonasputida. Given that P450cam was
the first P450 to be purified in sufficient quantities
for structure-function relationships, it is not too sur-
prising that P450cam was the first P450 crystal
structure to be solved. The high-resolution structure
of P450cam was published in 1987^ and remained
the paradigm for P450 structure-function studies
until the P450BM3 structure was solved in 19931
Since then the rate at which new P450 structures are
being solved has increased dramatically and at pres-
ent there are structures for 20 unique P450s on
deposit in the Protein Data Bank with others waiting
in the wings. Some of the new advances that have
been made since the last edition of this book are the
structures of other redox components of P450
monooxygenase systems, of the first electron trans-
fer complex, of new substrate complexes, of the first
P450s from thermophilic organisms, and of the first
membrane-bound P450. hi the present chapter, we
summarize some of these more important recent
findings with full recognition that the P450 struc-
tural field is moving much more quickly than in the
time frame of previous editions of this book. As
a result, the current chapter must be considered
a snapshot in time on where we now stand in P450
crystallography.
2.
Overall Architecture
There are now a sufficient number of struc-
tures to safely state that the overall P450 fold is
quite conservative. Perhaps more surprising is that
the P450 fold is unique and despite the many new
structures that have been solved since P450cam,
no non-P450 structure has yet been found to share
the P450 fold. Thus, the P450 fold appears to be
uniquely adapted for the heme-thiolate chemistry
required for oxygen activation, the binding of
redox partners, and the stereochemical require-
ments of substrate recognition.
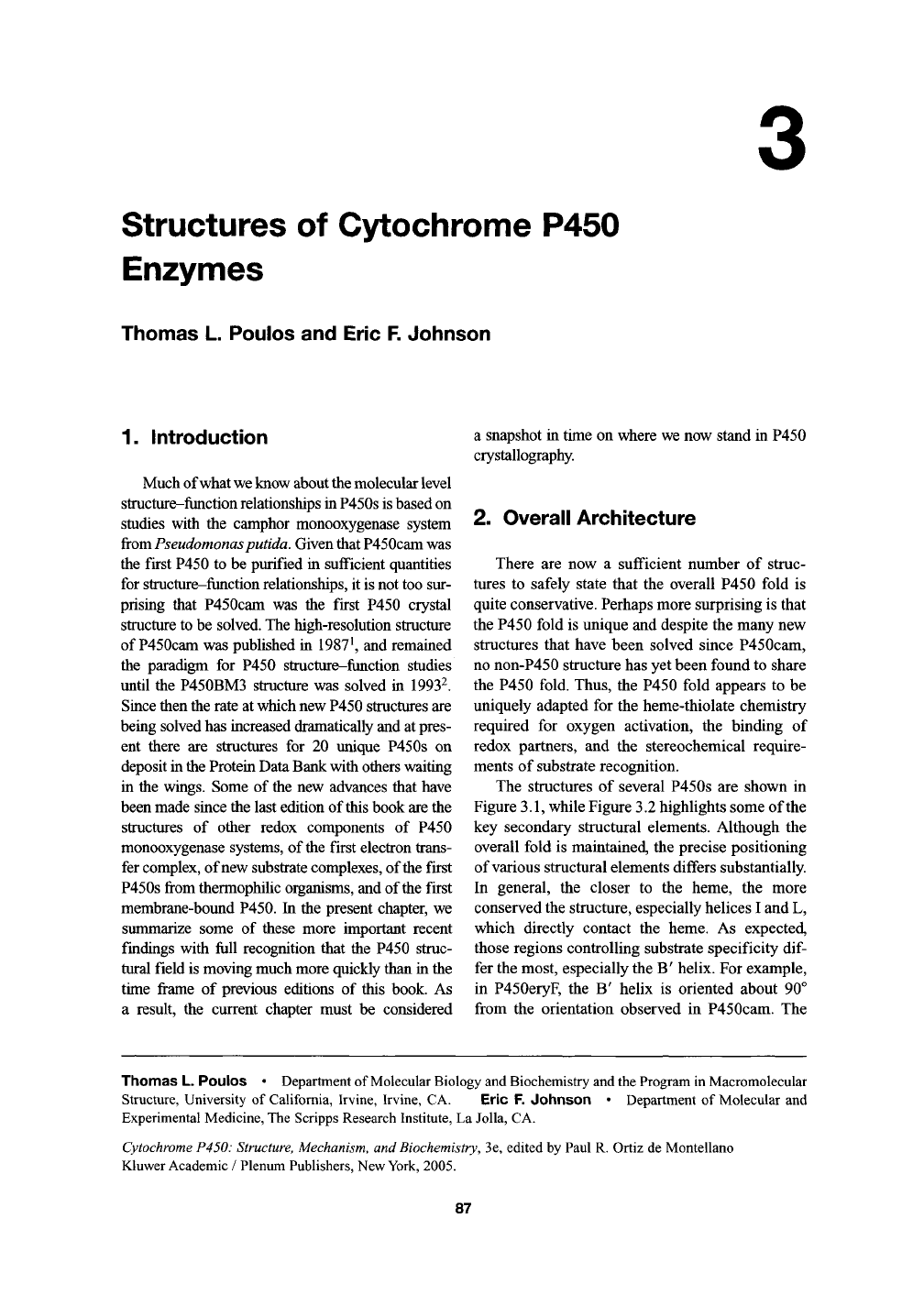
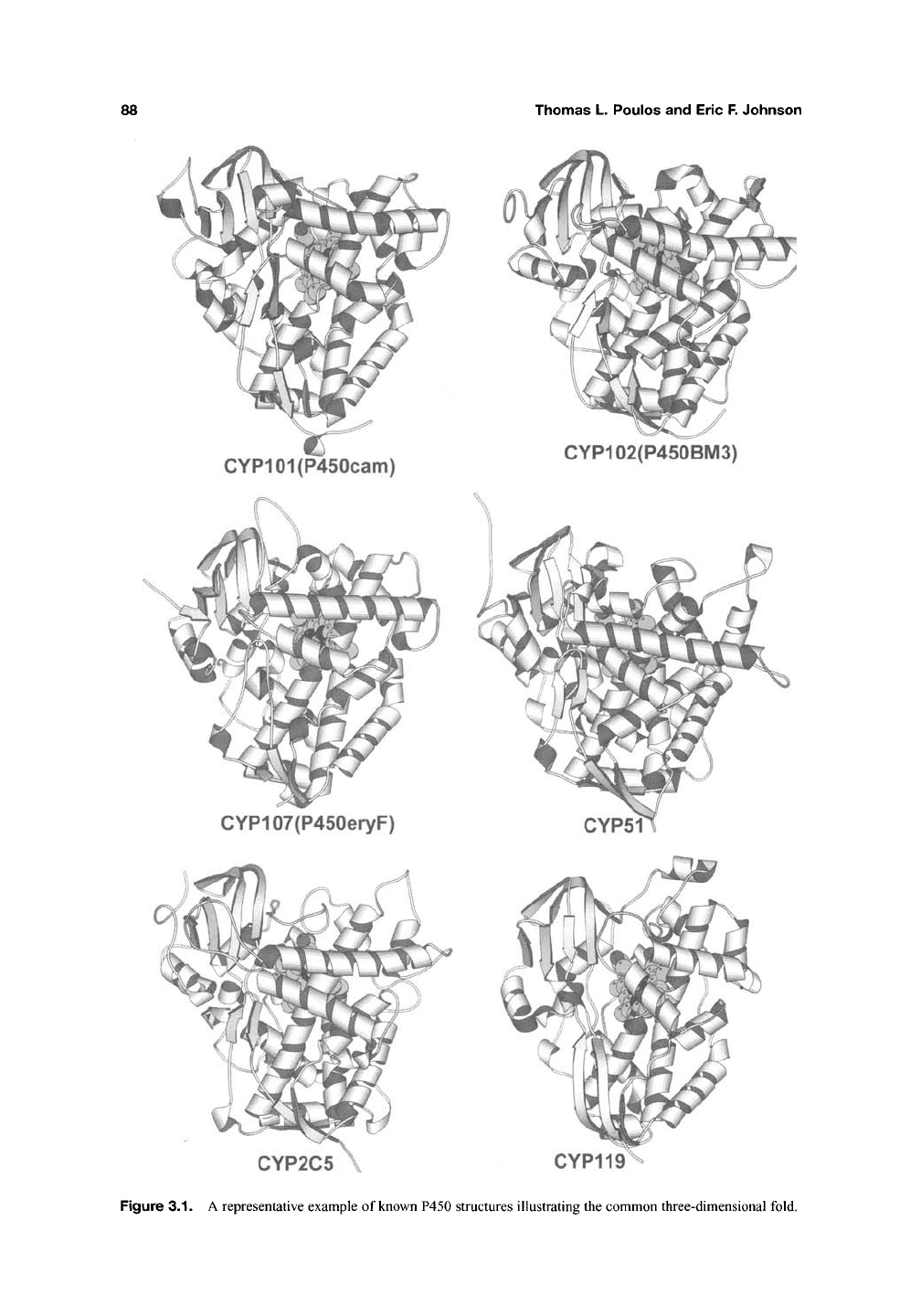
The structures of several P450s are shown in
Figure
3.1,
while Figure 3.2 highlights some of the
key secondary structural elements. Although the
overall fold is maintained, the precise positioning
of various structural elements differs substantially.
In general, the closer to the heme, the more
conserved the structure, especially helices I and L,
which directly contact the heme. As expected,
those regions controlling substrate specificity
dif-
fer the most, especially the B' helix. For example,
in P450eryF, the B' helix is oriented about 90°
from the orientation observed in P450cam. The
Thomas L. Poulos • Department of Molecular Biology and Biochemistry and the Program in Macromolecular
Structure, University of California, Irvine, Irvine, CA. Eric F. Johnson • Department of Molecular and
Experimental Medicine, The Scripps Research Institute, La JoUa, CA.
Cytochrome P450: Structure, Mechanism, and Biochemistry, 3e, edited by Paul R. Ortiz de Montellano
Kluwer Academic / Plenum Publishers, New York, 2005.
87
88 Thomas L. Poulos and Eric F. Johnson
CYP101(P450cam)
CYP102(P450BM3)
CYP119
Figure 3.1. A representative example of known P450 structures illustrating the common three-dimensional fold.
structures of Cytochrome P450 Enzymes
89
Figure 3.2. The structure of P450cam with
key
hehcal
segments labeled.
effect is a substantial change in local environment
that is required for substrate selectivity.
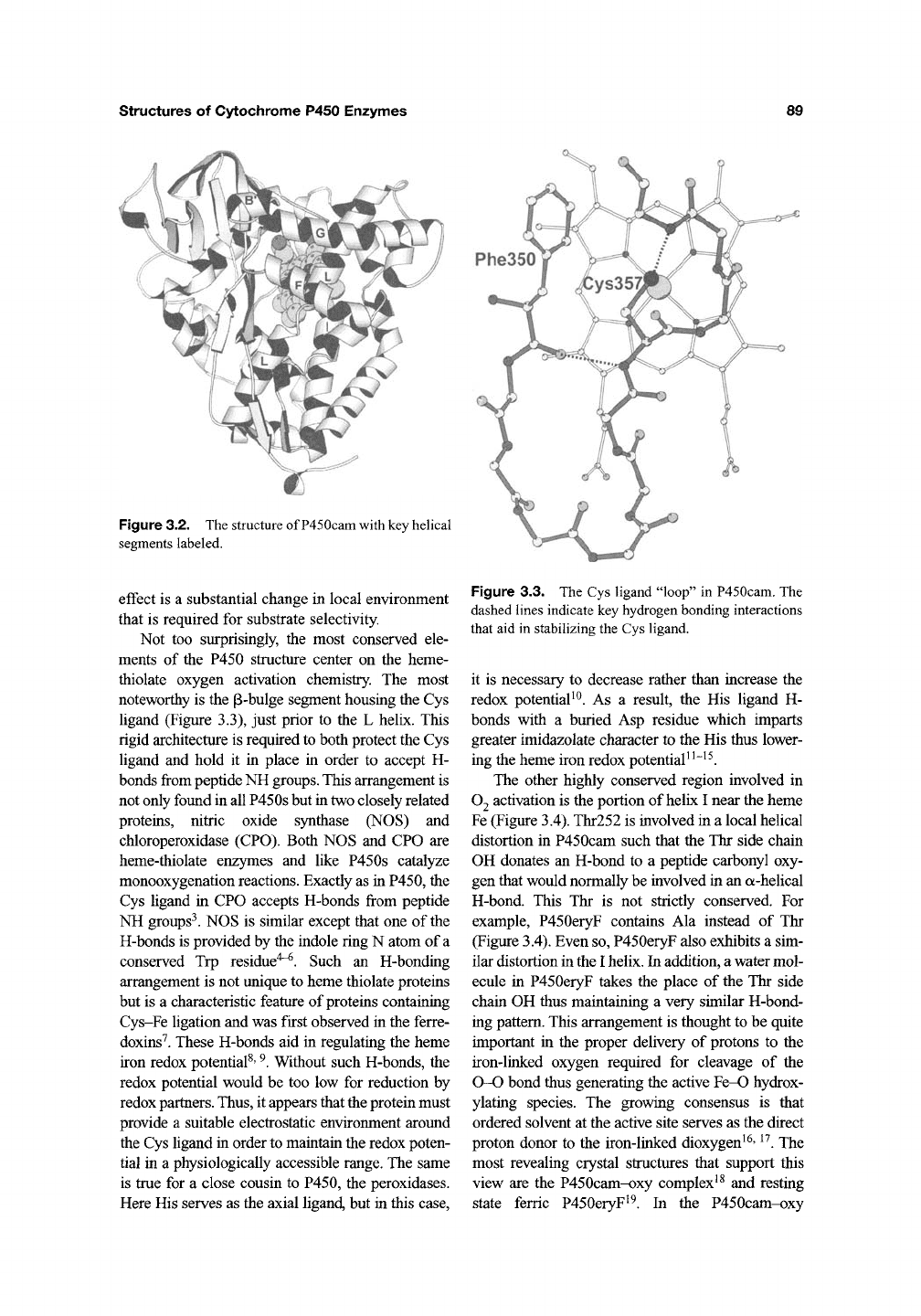
Not too surprisingly, the most conserved ele-
ments of the P450 structure center on the heme-
thiolate oxygen activation chemistry. The most
noteworthy is the p-bulge segment housing the Cys
ligand (Figure 3.3), just prior to the L helix. This
rigid architecture is required to both protect the Cys
ligand and hold it in place in order to accept H-
bonds from peptide NH
groups.
This arrangement is
not only found in all P450s but in
two
closely related
proteins, nitric oxide synthase (NOS) and
chloroperoxidase (CPO). Both NOS and CPO are
heme-thiolate enzymes and like P450s catalyze
monooxygenation
reactions.
Exactly as in P450, the
Cys ligand in CPO accepts H-bonds from peptide
NH groups^. NOS is similar except that one of the
H-bonds is provided by the indole ring N atom of a
conserved Trp residue^^. Such an H-bonding
arrangement is not unique to heme thiolate proteins
but is a characteristic feature of proteins containing
Cys-Fe ligation and was first observed in the ferre-
doxins^. These H-bonds aid in regulating the heme
iron redox potential^'
^.
Without such H-bonds, the
redox potential would be too low for reduction by
redox
partners.
Thus,
it appears that the protein must
provide a suitable electrostatic environment around
the Cys ligand in order to maintain the redox poten-
tial in a physiologically accessible range. The same
is true for a close cousin to P450, the peroxidases.
Here His serves as the axial ligand, but in this case.
Figure 3.3. The Cys ligand "loop" in P450cam. The
dashed lines indicate key hydrogen bonding interactions
that aid in stabilizing the Cys ligand.
it is necessary to decrease rather than increase the
redox potential^^. As a result, the His ligand H-
bonds with a buried Asp residue which imparts
greater imidazolate character to the His thus lower-
ing the heme iron redox potential^ ^~^^.
The other highly conserved region involved in
O2 activation is the portion of helix I near the heme
Fe (Figure 3.4). Thr252 is involved in a local helical
distortion in P450cam such that the Thr side chain
OH donates an H-bond to a peptide carbonyl oxy-
gen that would normally be involved in an a-helical
H-bond. This Thr is not strictly conserved. For
example, P450eryF contains Ala instead of Thr
(Figure 3.4). Even so, P450eryF also exhibits a sim-
ilar distortion in the I
helix.
In addition, a water mol-
ecule in P450eryF takes the place of the Thr side
chain OH thus maintaining a very similar H-bond-
ing pattern. This arrangement is thought to be quite
important in the proper delivery of protons to the
iron-linked oxygen required for cleavage of the
O-O bond thus generating the active Fe-0 hydrox-
ylating species. The growing consensus is that
ordered solvent at the active site serves as the direct
proton donor to the iron-linked dioxygen^^' ^^. The
most revealing crystal structures that support this
view are the P450cam-oxy complex^^ and resting
state ferric P450eryF^^. In the P450cam-oxy
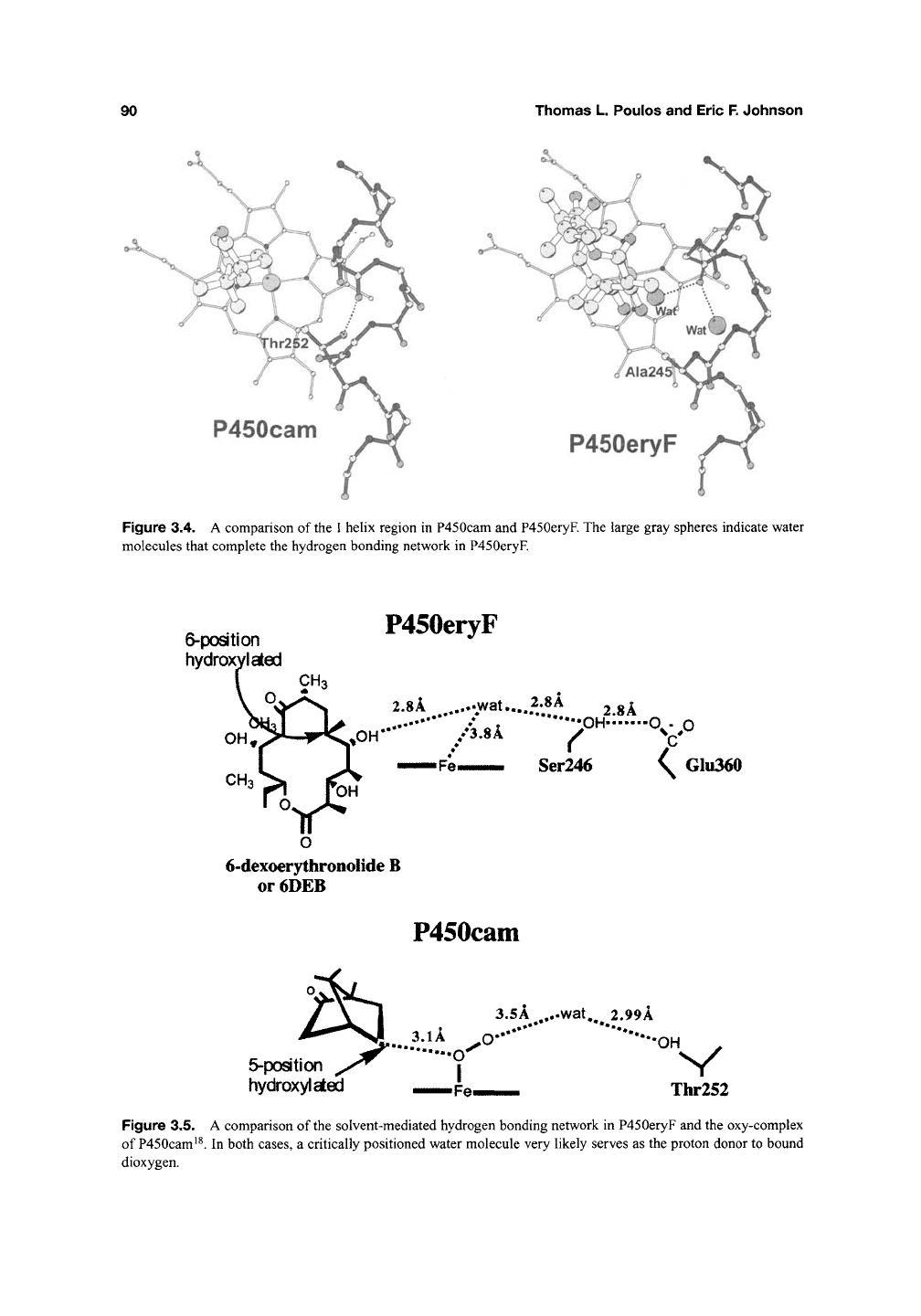
90
Thomas L. Poulos and Eric
F.
Johnson
P450cam
P450eryF
Figure 3.4. A comparison of the I helix region in P450cam and P450eryF. The large gray spheres indicate water
molecules that complete the hydrogen bonding network in P450eryF.
6-position
hydroxy I ated
P450eryF
2.8 A •wat....2;8A ^^
*: ^, -OH- o.
/3.8A /'^ ^y^
-Fe-
Ser246 ^ Glii360
6«dexoerythronolide B
or 6DEB
P450cam
&3
^. 3.lA
5-position y<^ |
hydroxylated
3.5A ...-wat., 2.^9A
.O-
••••OH
I
Fe-
V
Thr252
Figure 3.5. A comparison of
the
solvent-mediated hydrogen bonding network in P450eryF and the oxy-complex
of P450cam^^. In both cases, a critically positioned water molecule very likely serves as the proton donor to bound
dioxygen.
structures of Cytochrome P450 Enzymes
91
complex, two new waters are found in the active
site,
one of which is shown in Figure 3.5. This new
water is close to dioxygen and may participate in
relaying protons to dioxygen. P450eryF also has a
water similarly positioned (Figure 3.5). P450eryF,
however, uses a substrate-assisted mechanism since
a substrate OH anchors the key water
in
place via H-
bonding. While the details of the proton shuttle
machinery may differ from one P450 to the next, the
surrounding protein groups and, in at least one case,
the substrate, generally position solvent in the active
site for proton delivery to dioxygen resulting in
cleavage of the 0-0 bond.
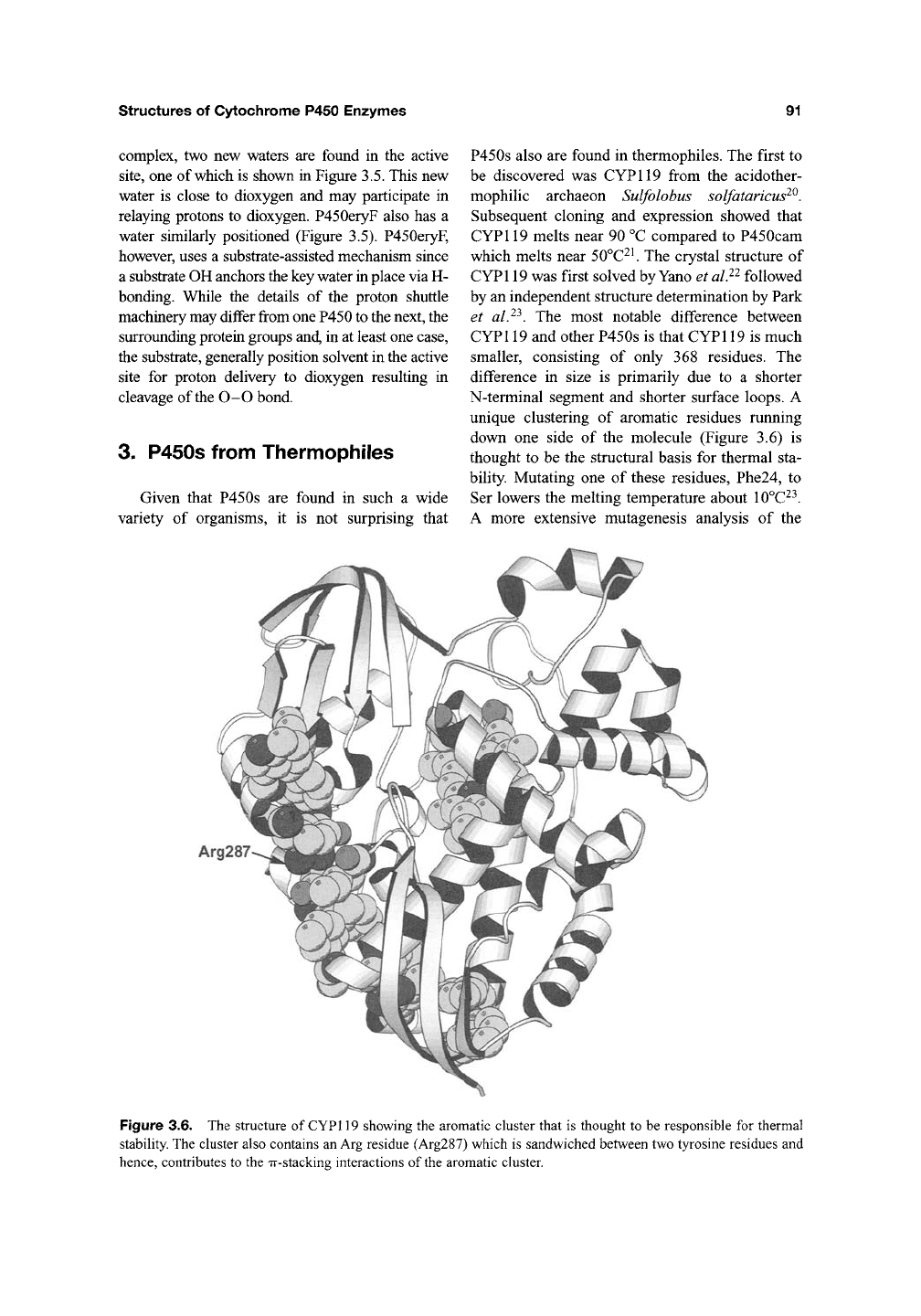
3. P450s from Thermophiles
Given that P450s are found in such a wide
variety of organisms, it is not surprising that
P450s also are found in thermophiles. The first to
be discovered was CYP119 from the acidother-
mophilic archaeon Sulfolobus solfataricus^^.
Subsequent cloning and expression showed that
CYPl 19 melts near 90 °C compared to P450cam
which melts near 50°C^^ The crystal structure of
CYPl 19 was first solved by Yano et alP followed
by an independent structure determination by Park
et alP. The most notable difference between
CYPl
19
and other P450s is that CYPl
19
is much
smaller, consisting of only 368 residues. The
difference in size is primarily due to a shorter
N-terminal segment and shorter surface loops. A
unique clustering of aromatic residues running
down one side of the molecule (Figure 3.6) is
thought to be the structural basis for thermal sta-
bility. Mutating one of these residues, Phe24, to
Ser lowers the melting temperature about 10°C^^.
A more extensive mutagenesis analysis of the
Arg287
Figure 3.6. The structure of CYPl
19
showing the aromatic cluster that is thought to be responsible for thermal
stability. The cluster also contains
an Arg
residue (Arg287) which is sandwiched between two tyrosine residues and
hence, contributes to the Tr-stacking interactions of
the
aromatic cluster.

92
Thomas L. Poulos and Eric F. Johnson
P450BM3
CYP175A1
Figure 3.7. A comparison between P450BM3 and the thermal stable P450, CYP175A1, showing the salt bridge
networks in both proteins.
aromatic cluster shows that the melting tempera-
ture can be lowered lO-lS^C^"^ further implicat-
ing aromatic clustering as a key factor in thermal
stability.
The second thermophilic P450 to be discovered
was CYP175A1 from Thermus thermophilus^^
which melts at 88°C. Like CYP119, CYP175A1
is also shorter than most other P450s and contains
378 residues. CYP175A1 most closely resem-
bles P450BM3 but with shorter surface loops.
CYP175A1 lacks the aromatic clustering observed
in CYPl
19
and hence, the structural basis for ther-
mal stability lies elsewhere. Yano et alP carried
out a detailed comparison of salt bridge networks
in various P450s and found that CYP175A1 con-
tains a larger fraction of salt bridges that contain
three or more residues. As shown in Figure 3.7,
CYP175A1 has 26 residues involved in 8 salt
bridge networks while the closest homologue to
CYP175A1,
P450BM3, has 14 residues participat-
ing in 4 salt bridge networks.
4. Membrane P450s
In contrast to prokaryotic P450s, eukaryotic
P450s are generally membrane-bound proteins.
Most eukaryotic P450s are incorporated into the
endoplasmic reticulum. However, several mam-
malian P450s that participate in the synthesis of
sterols, steroids, and bile acids are located on the
matrix side of
the
mitochondrial inner membrane.
A longer N-terminal polypeptide chain of roughly
30-50 amino acids precedes the catalytic domain
in eukaryotic P450s and mediates membrane tar-
geting. In the case of mitochondrial P450s, the tar-
geting sequences are cleaved during import of the
protein into mitochondria^^. In contrast, the leader
sequence of microsomal P450s is retained and is
co-translationally inserted into the endoplasmic
reticulum^^. The insertion process stops at the end
of
a
hydrophobic stretch of roughly 20 amino acid
residues, and the catalytic domain of microsomal
P450s resides on the cytoplasmic side of the endo-
plasmic reticulum. A short region that generally
contains positively charged residues links the cat-
alytic domain to a conserved proline rich motif at
the N-terminus of the structurally conserved P450
fold. The N-terminal transmembrane domain
extends across the membrane and is unlikely to be
closely associated with the catalytic domain
(Figure 3.8). This N-terminal domain is not
required for fiinction as illustrated by the
expression and successful reconstitution of several
P450 monooxygenases in which this region was
deleted28-32.
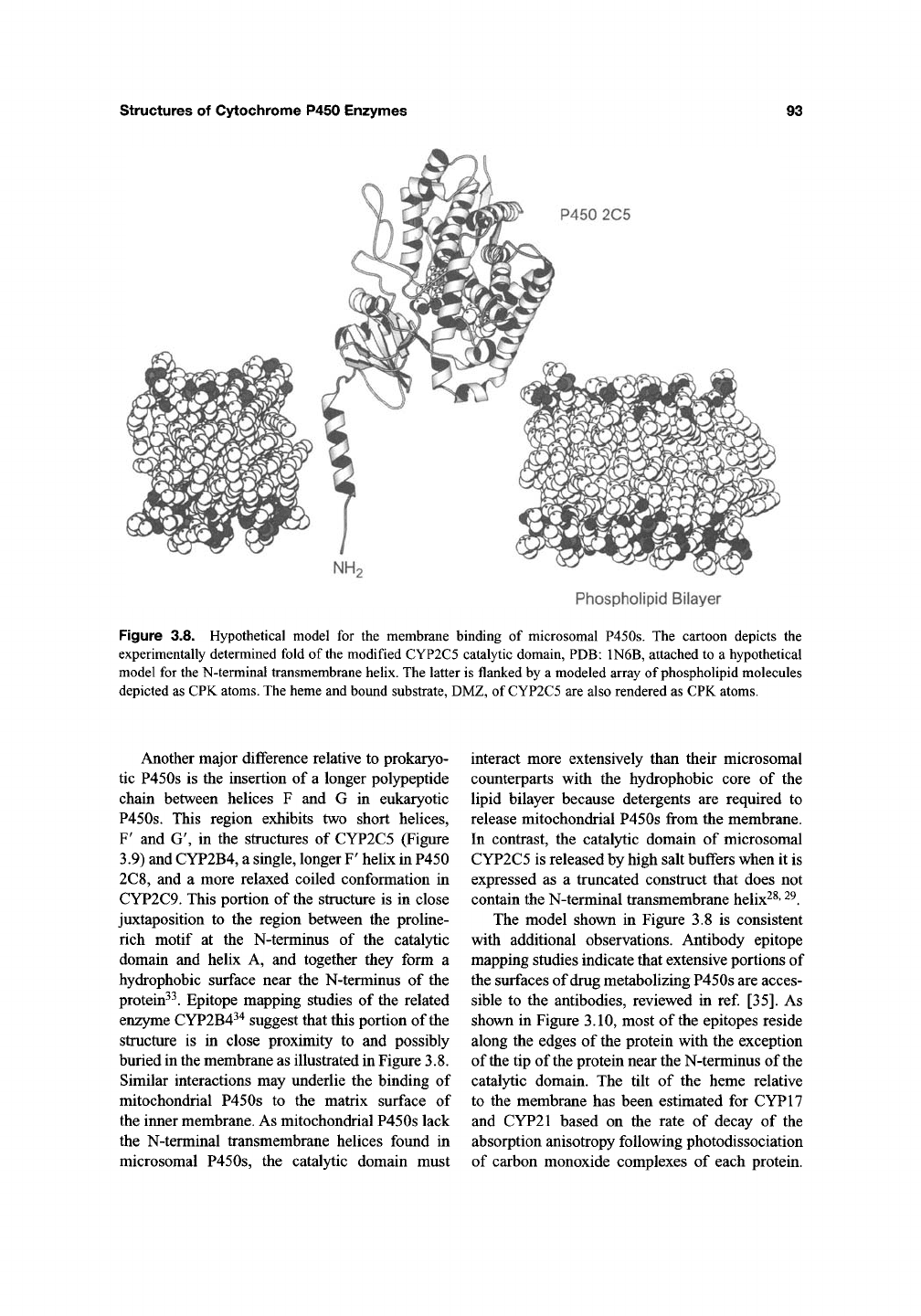
structures of Cytochrome P450 Enzymes
93
P450 2C5
Phospholipid Bilayer
Figure 3.8. Hypothetical model for the membrane binding of microsomal P450s. The cartoon depicts the
experimentally determined fold of the modified CYP2C5 catalytic domain,
PDB:
1N6B, attached to a hypothetical
model for the N-terminal transmembrane helix. The latter is flanked by a modeled array of phospholipid molecules
depicted as CPK
atoms.
The heme and bound substrate, DMZ, of
CYP2C5
are also rendered as CPK atoms.
Another major difference relative to prokaryo-
tic P450s is the insertion of a longer polypeptide
chain between helices F and G in eukaryotic
P450s. This region exhibits two short helices,
F'
and G', in the structures of CYP2C5 (Figure
3.9) and CYP2B4, a single, longer F' helix in P450
2C8,
and a more relaxed coiled conformation in
CYP2C9. This portion of the structure is in close
juxtaposition to the region between the proline-
rich motif at the N-terminus of the catalytic
domain and helix A, and together they form a
hydrophobic surface near the N-terminus of the
protein^^. Epitope mapping studies of the related
enzyme CYP2B4^'^ suggest that this portion of the
structure is in close proximity to and possibly
buried in the membrane as illustrated in Figure 3.8.
Similar interactions may underlie the binding of
mitochondrial P450s to the matrix surface of
the inner membrane. As mitochondrial P450s lack
the N-terminal transmembrane helices found in
microsomal P450s, the catalj^ic domain must
interact more extensively than their microsomal
counterparts with the hydrophobic core of the
lipid bilayer because detergents are required to
release mitochondrial P450s from the membrane.
In contrast, the catalytic domain of microsomal
CYP2C5 is released by high salt buffers when it is
expressed as a truncated construct that does not
contain the N-terminal transmembrane helix^^'
^^.
The model shown in Figure 3.8 is consistent
with additional observations. Antibody epitope
mapping studies indicate that extensive portions of
the surfaces of drug metabolizing P450s are acces-
sible to the antibodies, reviewed in ref [35]. As
shown in Figure 3.10, most of the epitopes reside
along the edges of the protein with the exception
of the tip of the protein near the N-terminus of the
catalytic domain. The tilt of the heme relative
to the membrane has been estimated for CYP17
and CYP21 based on the rate of decay of the
absorption anisotropy following photodissociation
of carbon monoxide complexes of each protein.
