Ortiz de Montellano Paul R.(Ed.) Cytochrome P450. Structure, Mechanism, and Biochemistry
Подождите немного. Документ загружается.

94
Thomas L. Poulos and Eric F. Johnson
Helix G*
Helix F'
Meander
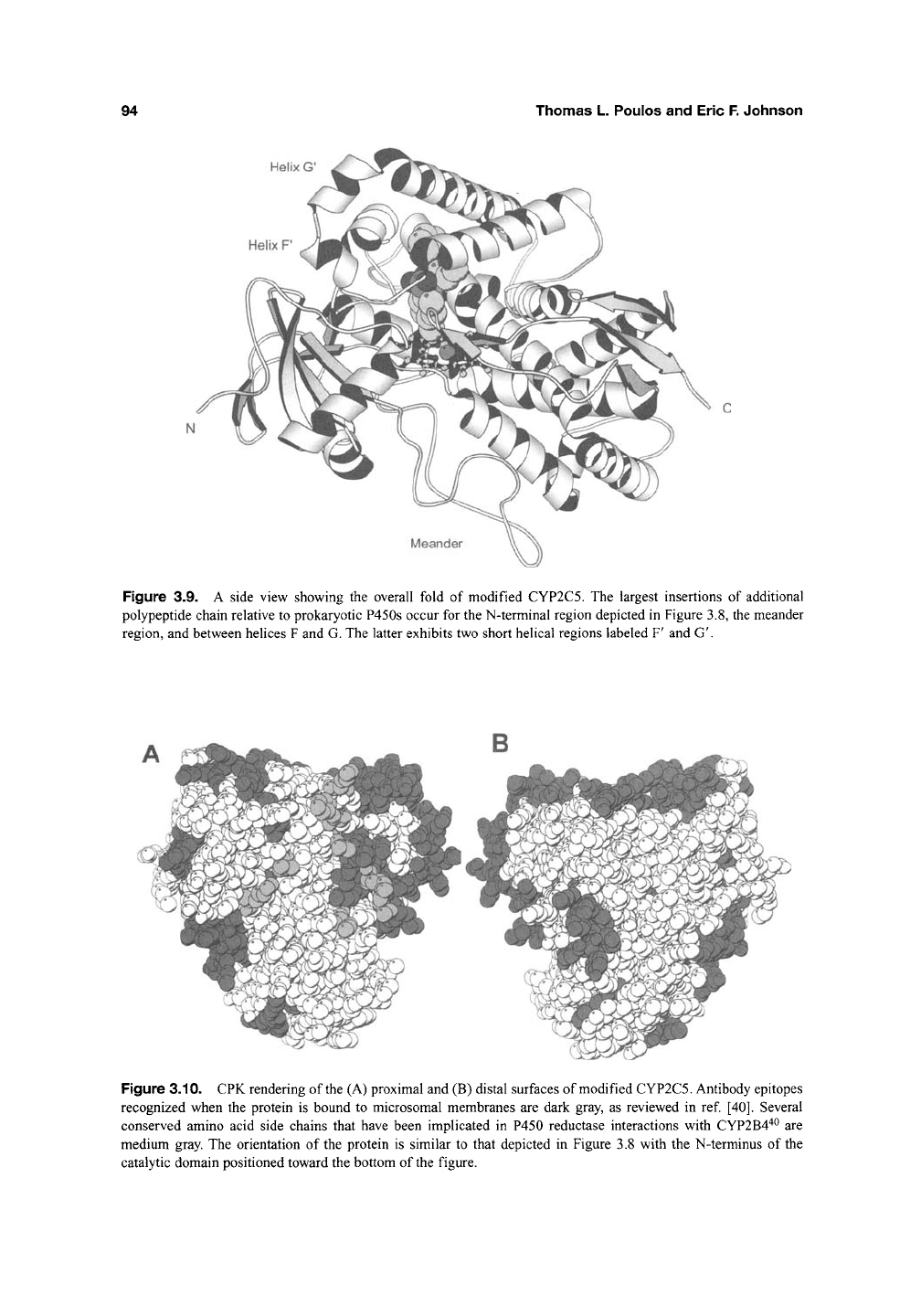
Figure 3.9. A side view showing the overall fold of modified CYP2C5. The largest insertions of additional
polypeptide chain relative to prokaryotic P450s occur for the N-terminal region depicted in Figure 3.8, the meander
region, and between helices F and G. The latter exhibits two short helical regions labeled F' and G'.
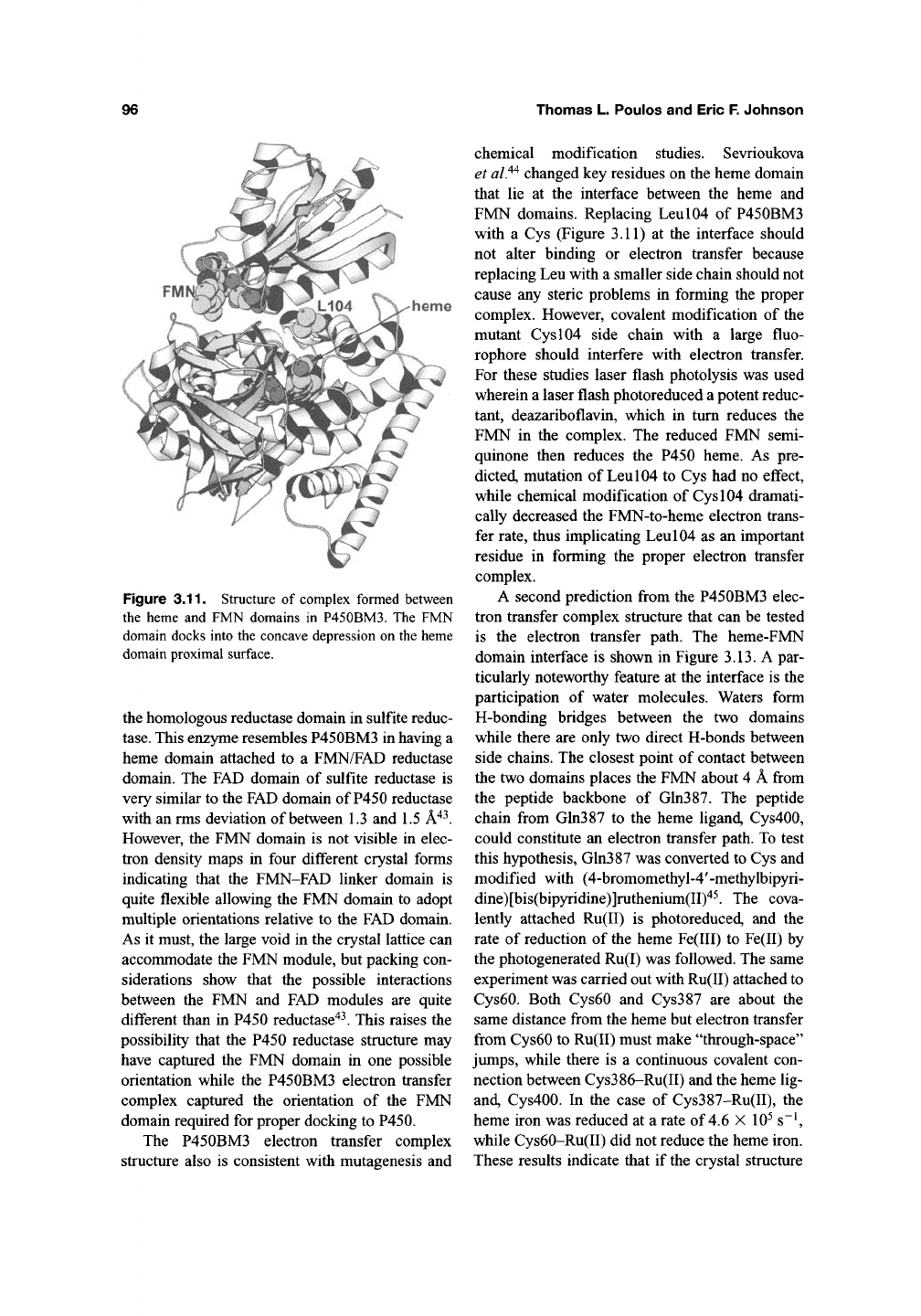
Figure 3.10. CPK rendering of
the
(A) proximal and (B) distal surfaces of modified CYP2C5. Antibody epitopes
recognized when the protein is bound to microsomal membranes are dark gray, as reviewed in ref [40]. Several
conserved amino acid side chains that have been implicated in P450 reductase interactions with CYP2B4'^^ are
medium gray. The orientation of the protein is similar to that depicted in Figure 3.8 with the N-terminus of the
catalytic domain positioned toward the bottom of the figure.

structures of Cytochrome P450 Enzymes
95
The results of these studies suggest that the angle
for the orientation of the heme relative to the
membrane surface is either
50°
or
70°
(See ref [36]).
The latter value is consistent with the orientation
seen in Figure 3.8 and leads to significant solvent
exposure for the surfaces of the catalytic domain.
Atomic force microscopy experiments estimate
that the height of P450 2B4 above a model phos-
pholipid membrane is roughly 35-45 nm^^. This
would require a portion of the protein to be buried
in the membrane, and this is likely to be the
hydrophobic region near the N-terminus of the
catalytic domain. Studies of the association of
P450 2B4 with Langmuir-Blodget, phospholipid
monolayers indicate that the protein displaces an
area that is larger than a single transmembrane
helix^^. This result would be consistent with the
penetration of the hydrophobic tip of the protein
into the lipid layer. A lower angle of the heme plane
would juxtapose a larger surface of the helix F-G
region with
the
membrane surface, which
is
hydrated
and largely formed by the zwitterionic phospholipid
head groups. A steeper angle would bury the
hydrophobic tip deeper into the membrane. The
propensity of the helix F to helix G region to form
hehcal structures rich in aromatic residues would
enable this region to penetrate more deeply into the
membrane because the hydrogen bonding in the
helices would diminish the energetic cost of burying
the peptide bonds in a hydrophobic environment^^.
Membrane interactions with the catalytic
domain of microsomal P450s could promote
the transfer of hydrophobic substrates from the
membrane to the P450 and could also orient
the protein to facilitate interactions with P450
reductase as the two proteins diffuse along the sur-
face of the endoplasmic reticulum. Mutagenesis
experiments'^^ suggest that a group of relatively
conserved, positively charged amino acids on the
proximal surface of CYP2B4 interact with the
reductase (Figure 3.10). Significant insertions of
additional amino acid residues relative to most
prokaryotic P450 structures are also present on the
proximal surface of the protein, and these are also
in the structure of P450BM3. The additional
residues form the J' helix extend a portion of the
polypeptide chain termed the meander region
that precedes the beta-turn containing the cysteine
that provides the axial ligation for the heme. The
corresponding region of CYP2C5 is illustrated
in Figure 3.9. The functional importance of this
insertion is unclear, but it resides in close proxim-
ity to the probable site of P450 reductase binding
as discussed in the next section.
5. Electron Transfer Complexes
An important advance in understanding P450
monooxygenase electron transfer systems was the
solution of the P450 reductase structure"^^ With
the addition of the first mammalian P450 struc-
ture,
CYP2C5, the way is now open for under-
standing how these two microsomal proteins
interact and transfer electrons. Nevertheless, the
Bacillus megaterium fatty acid monooxygenase
system, P450BM3, has so far served as the best
model system for understanding reductase-P450
interactions. Although P450BM3 is a bacterial
enzyme, P450BM3 is more closely related in
sequence, structure, activity, and redox partner to
microsomal P450s than to other bacterial P450s.
The unique feature of P450BM3 is that the
diflavin P450 reductase is linked to the C-terminal
end of the heme domain thus giving a catalytically
self-sufficient enzyme.
A significant advance was made when
Sevrioukova et al.^^ succeeded in crystallizing a
P450BM3 construct that contains just the heme
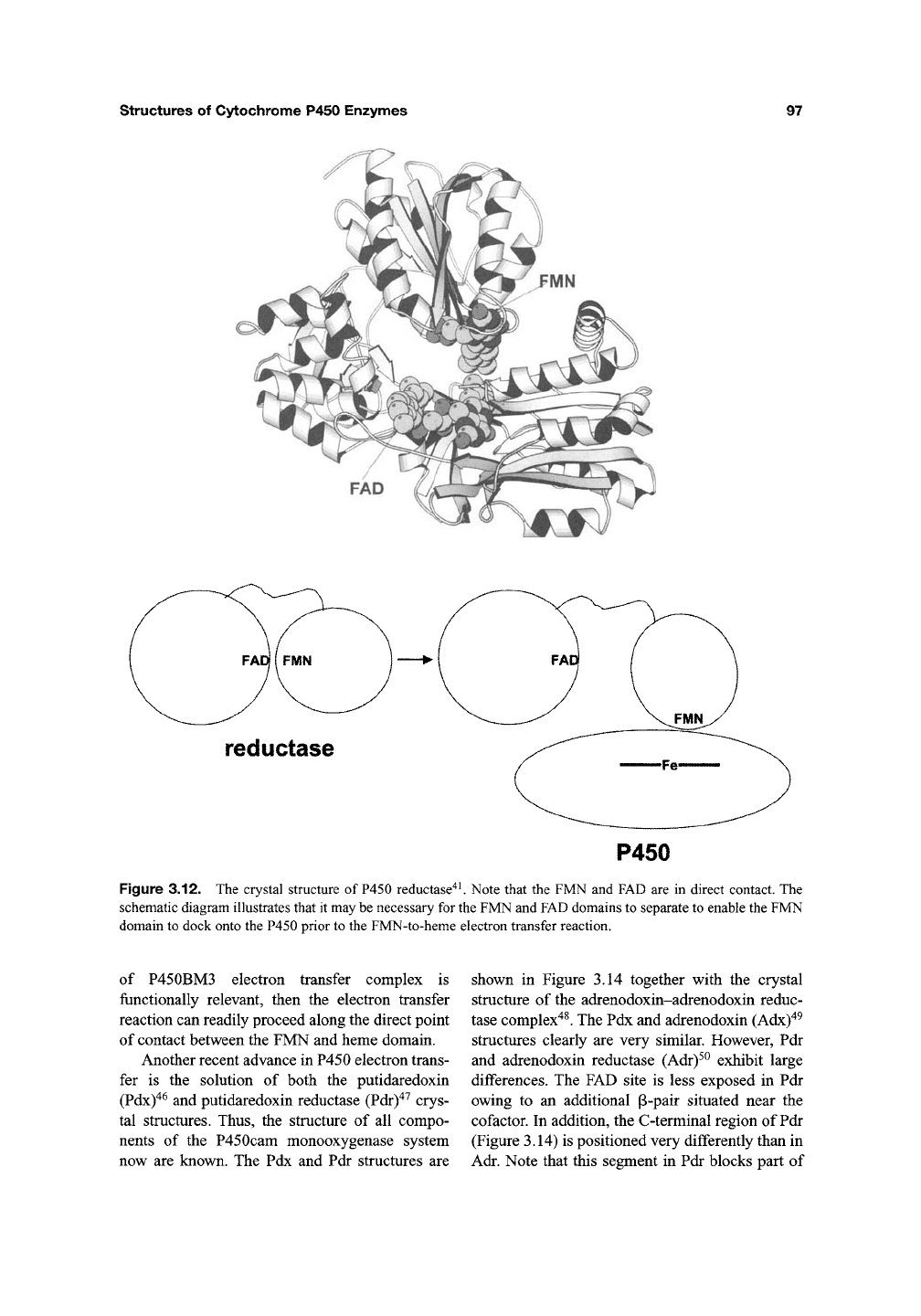
and FMN domains. The structure (Figure 3.11)
shows that the FMN domain docks on the proximal
surface of the P450, which was expected based on
complementary electrostatic surfaces and mutage-
nesis studies. Nevertheless, the heme-FMN con-
struct used to solve this structure was missing the
FAD domain and the linker connecting the heme
and FMN domains had been proteolyzed during
crystallization, thus raising the possibility that the
structure is an artifact of crystallization. In addi-
tion, the structure of the P450BM3 complex is not
compatible with the P450 reductase structure. As
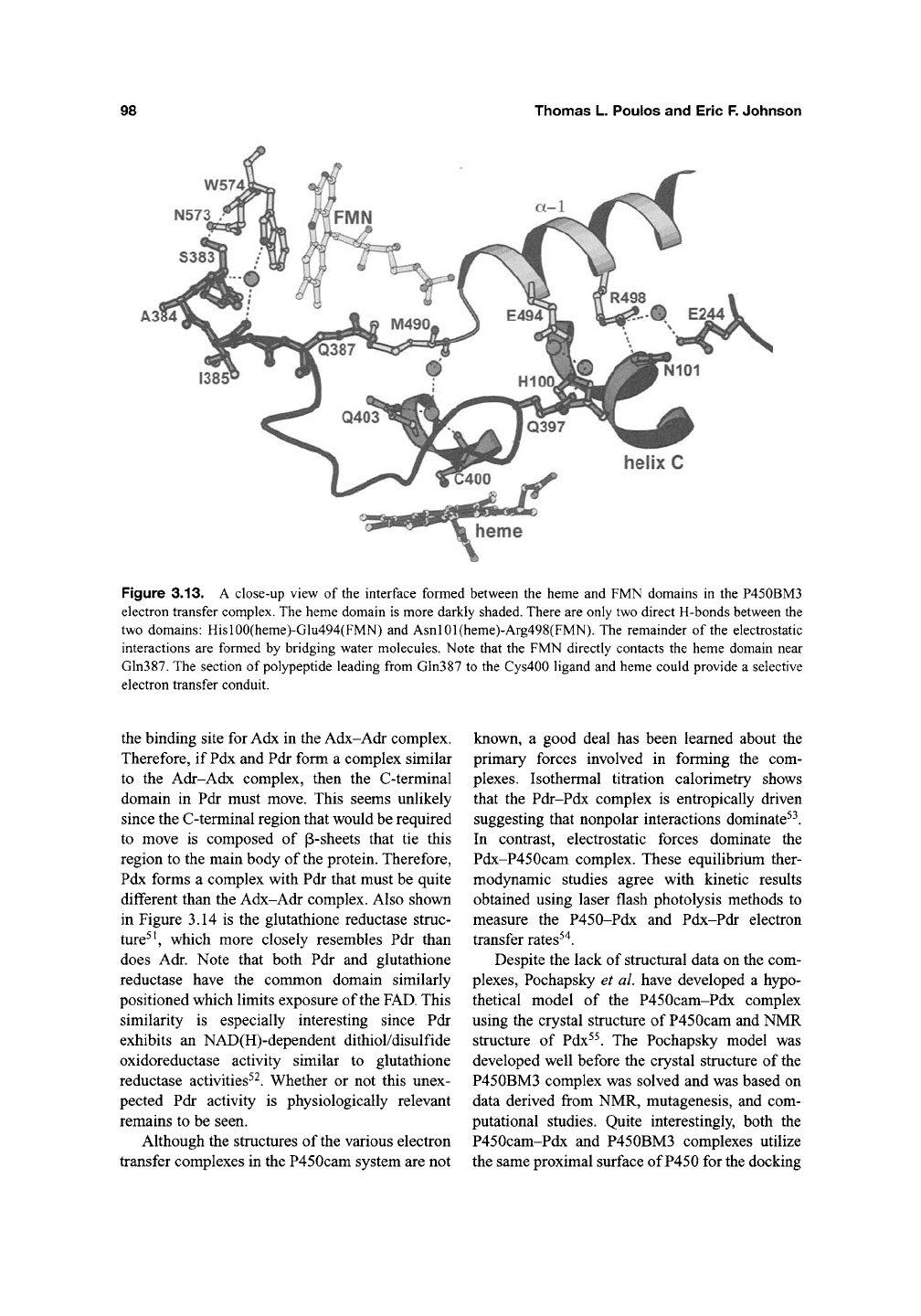
shown schematically in Figure 3.12, the FMN and
FAD in P450 reductase form a direct nonbonded
contact. In order for the FMN domain to dock onto
the proximal surface of
P450,
there must be a large
structural change which enables the FAD and
FMN domains to move apart (Figure 3.12). Indeed,
there are good indications that the linker connect-
ing the FAD and FMN domains is quite flexible.
One of the more important studies related to the
question of domain flexibility is the structure of
96
Thomas L. Poulos and Eric F. Johnson
heme
Figure 3.11. Structure of complex formed between
the heme and FMN domains in P450BM3. The FMN
domain docks into the concave depression on the heme
domain proximal surface.
the homologous reductase domain in sulfite reduc-
tase.
This enzyme resembles P450BM3 in having a
heme domain attached to a FMN/FAD reductase
domain. The FAD domain of sulfite reductase is
very similar to the FAD domain of P450 reductase
with an rms deviation of between 1.3 and 1.5 A"^^.
However, the FMN domain is not visible in elec-
tron density maps in four different crystal forms
indicating that the FMN-FAD linker domain is
quite flexible allowing the FMN domain to adopt
multiple orientations relative to the FAD domain.
As it must, the large void in the crystal lattice can
accommodate the FMN module, but packing con-
siderations show that the possible interactions
between the FMN and FAD modules are quite
different than in P450 reductase"^^. This raises the
possibility that the P450 reductase structure may
have captured the FMN domain in one possible
orientation while the P450BM3 electron transfer
complex captured the orientation of the FMN
domain required for proper docking to P450.
The P450BM3 electron transfer complex
structure also is consistent with mutagenesis and
chemical modification studies. Sevrioukova
et
al.^^
changed key residues on the heme domain
that lie at the interface between the heme and
FMN domains. Replacing Leul04 of P450BM3
with a Cys (Figure 3.11) at the interface should
not alter binding or electron transfer because
replacing Leu with a smaller side chain should not
cause any steric problems in forming the proper
complex. However, covalent modification of the
mutant Cys 104 side chain with a large fluo-
rophore should interfere with electron transfer.
For these studies laser flash photolysis was used
wherein a laser flash photoreduced a potent reduc-
tant, deazariboflavin, which in turn reduces the
FMN in the complex. The reduced FMN semi-
quinone then reduces the P450 heme. As pre-
dicted, mutation of Leu 104 to Cys had no effect,
while chemical modification of Cys 104 dramati-
cally decreased the FMN-to-heme electron trans-
fer rate, thus implicating Leu 104 as an important
residue in forming the proper electron transfer
complex.
A second prediction from the P450BM3 elec-
tron transfer complex structure that can be tested
is the electron transfer path. The heme-FMN
domain interface is shown in Figure 3.13. A par-
ticularly noteworthy feature at the interface is the
participation of water molecules. Waters form
H-bonding bridges between the two domains
while there are only two direct H-bonds between
side chains. The closest point of contact between
the two domains places the FMN about 4 A from
the peptide backbone of Gln387. The peptide
chain from Gln387 to the heme ligand, Cys400,
could constitute an electron transfer path. To test
this hypothesis, Gln387 was converted to Cys and
modified with (4-bromomethyl-4'-methylbipyri-
dine)[bis(bipyridine)]ruthenium(II)'^^. The cova-
lently attached Ru(II) is photoreduced, and the
rate of reduction of the heme Fe(III) to Fe(II) by
the photogenerated Ru(I) was followed. The same
experiment was carried out with Ru(II) attached to
Cys60. Both Cys60 and Cys387 are about the
same distance from the heme but electron transfer
from Cys60 to Ru(II) must make "through-space"
jumps, while there is a continuous covalent con-
nection between Cys386-Ru(II) and the heme lig-
and, Cys400. In the case of Cys387-Ru(II), the
heme iron was reduced at a rate of 4.6 X 10^ s~^
while Cys60-Ru(II) did not reduce the heme iron.
These results indicate that if the crystal structure
structures of Cytochrome P450 Enzymes
97
reductase
P450
Figure 3.12. The crystal structure of P450 reductase^^ Note that the FMN and FAD are in direct contact. The
schematic diagram illustrates that it may be necessary for the FMN and FAD domains to separate to enable the FMN
domain to dock onto the P450 prior to the FMN-to-heme electron transfer reaction.
of P450BM3 electron transfer complex is
functionally relevant, then the electron transfer
reaction can readily proceed along the direct point
of contact between the FMN and heme domain.
Another recent advance in P450 electron trans-
fer is the solution of both the putidaredoxin
(Pdx)"^^
and putidaredoxin reductase (Pdr)"^^ crys-
tal structures. Thus, the structure of all compo-
nents of the P450cam monooxygenase system
now are known. The Pdx and Pdr structures are
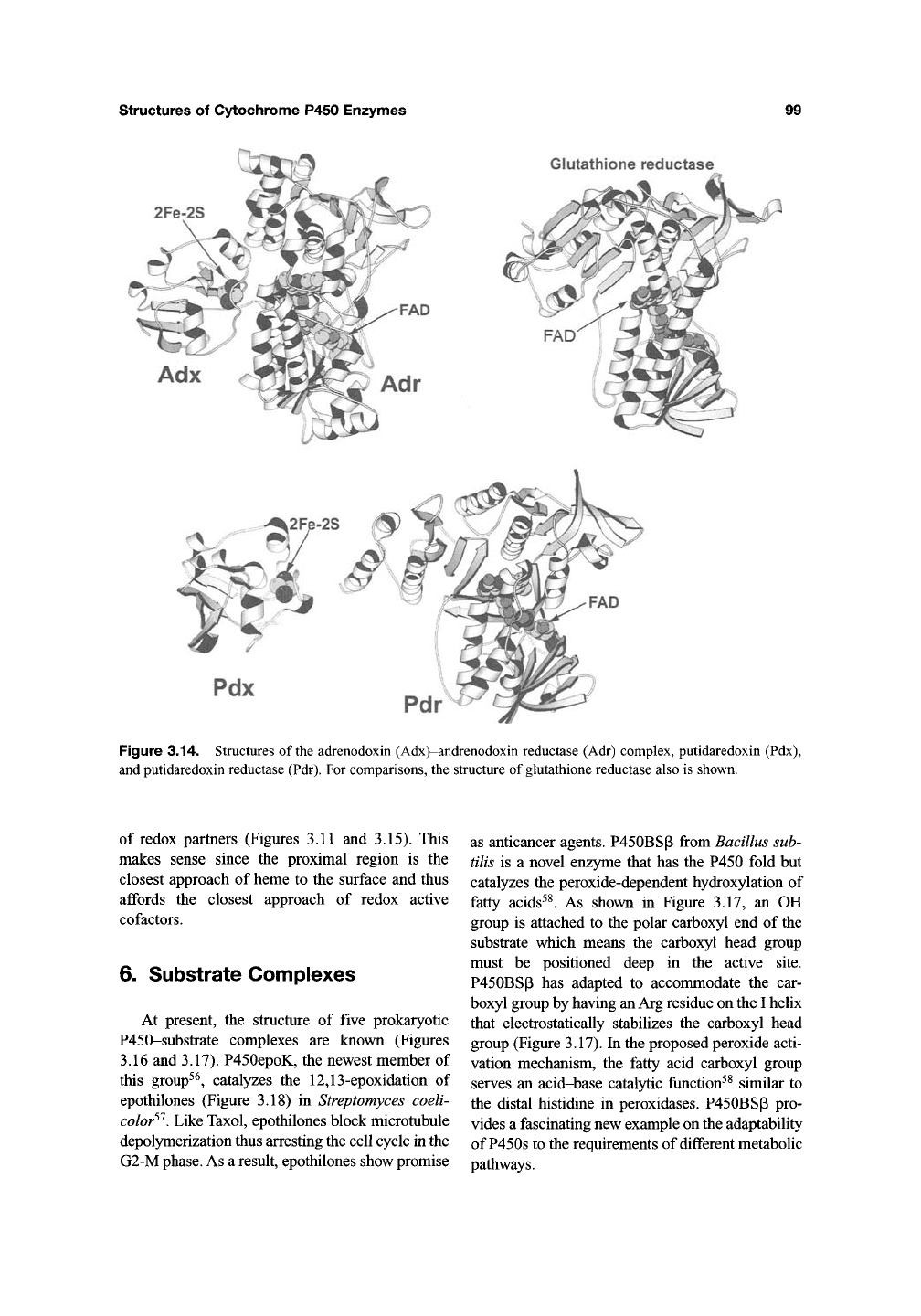
shown in Figure 3.14 together with the crystal
structure of the adrenodoxin-adrenodoxin reduc-
tase complex^^. The Pdx and adrenodoxin (Adx)"^^
structures clearly are very similar. However, Pdr
and adrenodoxin reductase (Adr)^^ exhibit large
differences. The FAD site is less exposed in Pdr
owing to an additional p-pair situated near the
cofactor. In addition, the C-terminal region of Pdr
(Figure 3.14) is positioned very differently than in
Adr. Note that this segment in Pdr blocks part of
98
Thomas L. Poulos and Eric F. Johnson
W574,
N573
Figure 3.13. A close-up view of the interface formed between the heme and FMN domains in the P450BM3
electron transfer complex. The heme domain is more darkly shaded. There are only two direct H-bonds between the
two domains: Hisl00(heme)-Glu494(FMN) and Asnl01(heme)-Arg498(FMN). The remainder of the electrostatic
interactions are formed by bridging water molecules. Note that the FMN directly contacts the heme domain near
Gln387. The section of polypeptide leading from Gln387 to the Cys400 ligand and heme could provide a selective
electron transfer conduit.
the binding site for Adx in the Adx-Adr complex.
Therefore, if Pdx and Pdr form a complex similar
to the Adr-Adx complex, then the C-terminal
domain in Pdr must move. This seems unlikely
since the C-terminal region that would be required
to move is composed of p-sheets that tie this
region to the main body of the protein. Therefore,
Pdx forms a complex with Pdr that must be quite
different than the Adx-Adr complex. Also shown
in Figure 3.14 is the glutathione reductase struc-
ture^',
which more closely resembles Pdr than
does Adr. Note that both Pdr and glutathione
reductase have the common domain similarly
positioned which limits exposure of the
FAD.
This
similarity is especially interesting since Pdr
exhibits an NAD(H)-dependent dithiol/disulfide
oxidoreductase activity similar to glutathione
reductase activities'^. Whether or not this unex-
pected Pdr activity is physiologically relevant
remains to be seen.
Although the structures of the various electron
transfer complexes in the P450cam system are not
known, a good deal has been learned about the
primary forces involved in forming the com-
plexes. Isothermal titration calorimetry shows
that the Pdr-Pdx complex is entropically driven
suggesting that nonpolar interactions dominate'^.
In contrast, electrostatic forces dominate the
Pdx-P450cam complex. These equilibrium ther-
modynamic studies agree with kinetic results
obtained using laser flash photolysis methods to
measure the P450-Pdx and Pdx-Pdr electron
transfer rates'"^.
Despite the lack of structural data on the com-
plexes, Pochapsky et al. have developed a hypo-
thetical model of the P450cam-Pdx complex
using the crystal structure of P450cam and NMR
structure of Pdx''. The Pochapsky model was
developed well before the crystal structure of the
P450BM3 complex was solved and was based on
data derived from NMR, mutagenesis, and com-
putational studies. Quite interestingly, both the
P450cam-Pdx and P450BM3 complexes utilize
the same proximal surface of P450 for the docking
structures of Cytochrome P450 Enzymes
99
Glutathione reductase
2Fe-2S
Figure 3.14. Structures of the adrenodoxin (Adx)-andrenodoxin reductase (Adr) complex, putidaredoxin (Pdx),
and putidaredoxin reductase (Pdr). For comparisons, the structure of glutathione reductase also is shown.
of redox partners (Figures 3.11 and 3.15). This
makes sense since the proximal region is the
closest approach of heme to the surface and thus
affords the closest approach of redox active
cofactors.
6. Substrate Complexes
At present, the structure of five prokaryotic
P450-substrate complexes are known (Figures
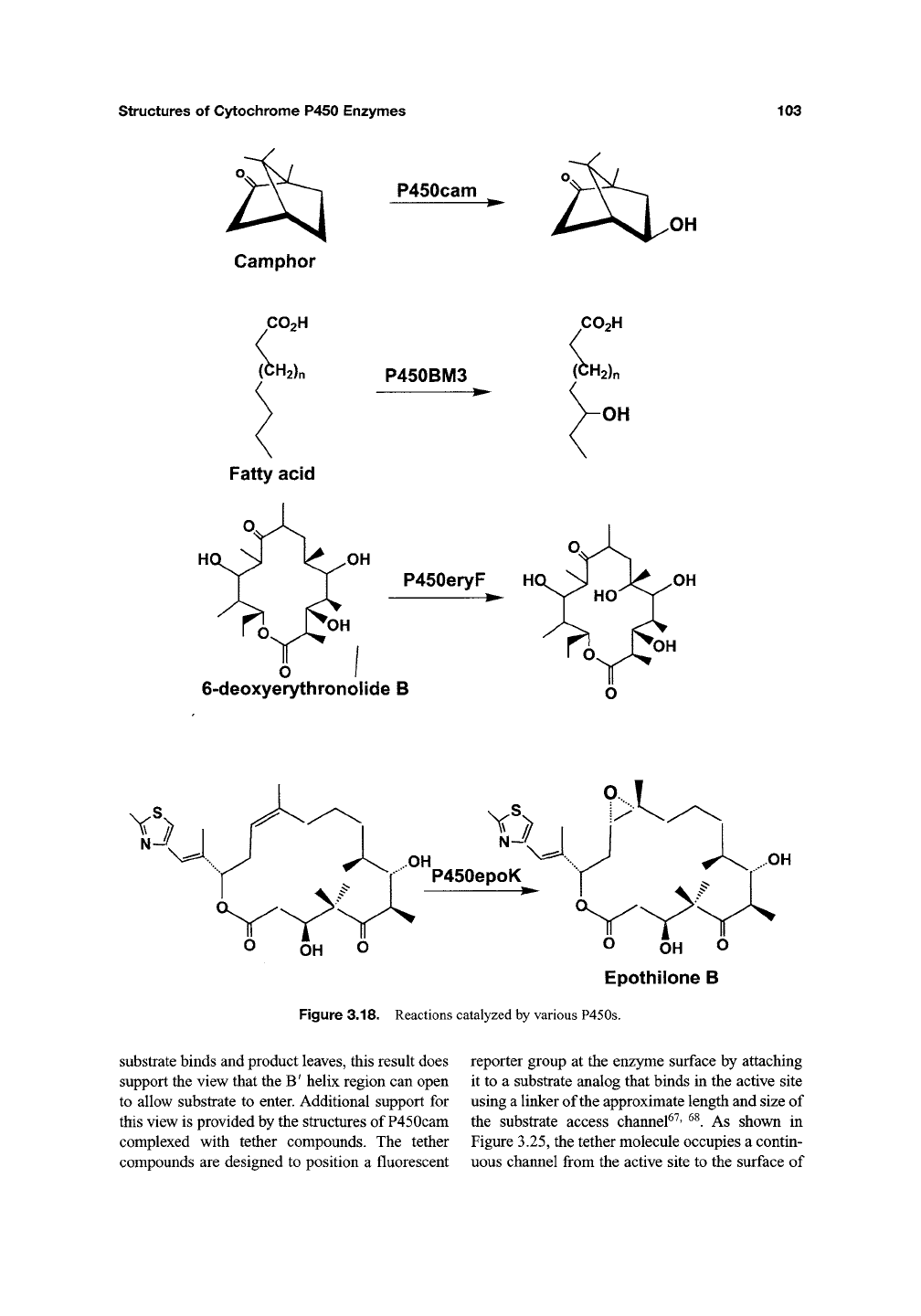
3.16 and 3.17). P450epoK, the newest member of
this group^^, catalyzes the 12,13-epoxidation of
epothilones (Figure 3.18) in Streptomyces coeli-
color^^.
Like Taxol, epothilones block microtubule
depolymerization thus arresting the cell cycle in the
G2-M
phase.
As a result, epothilones show promise
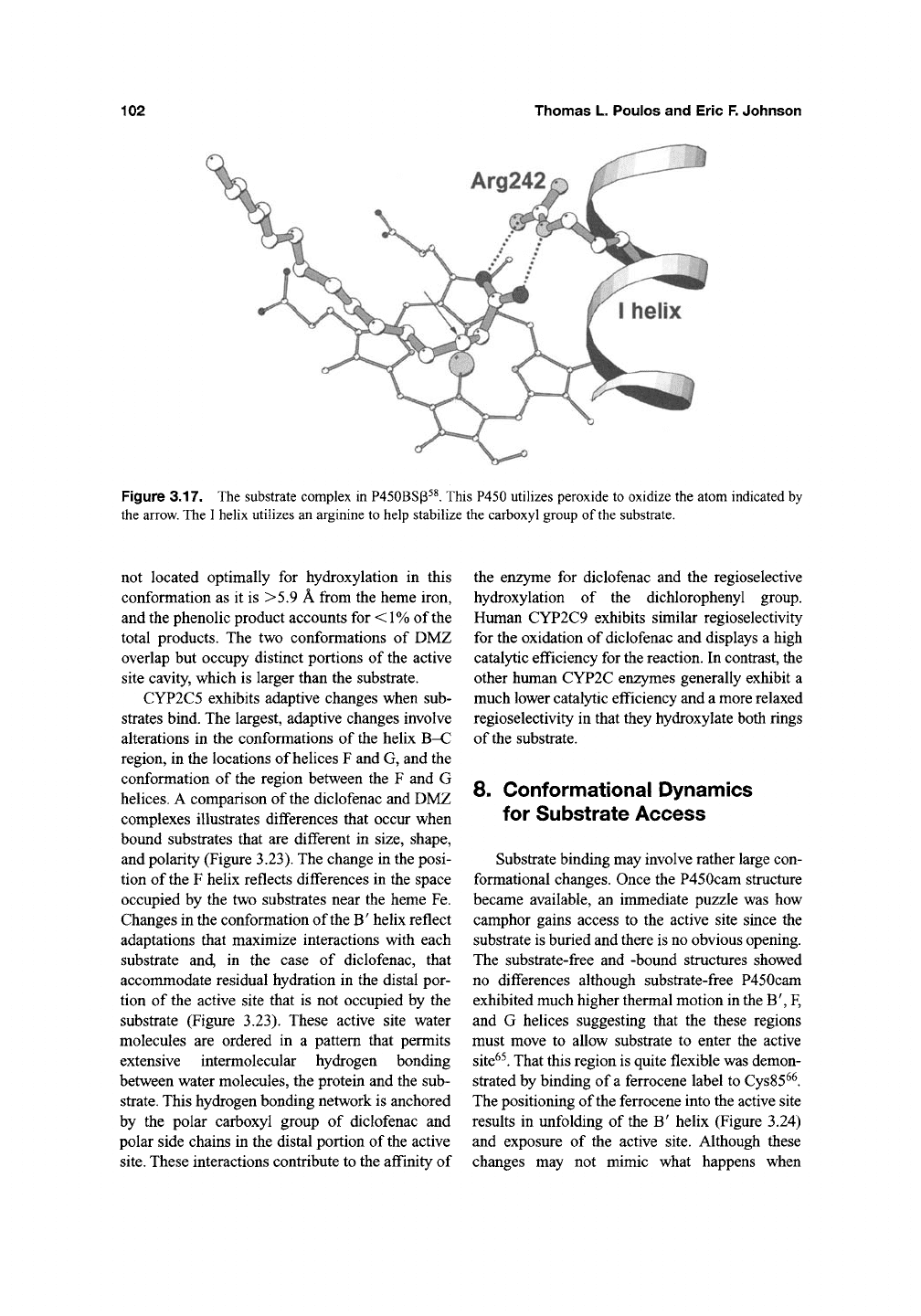
as anticancer agents. P450BSP from Bacillus sub-
tilis is a novel enzyme that has the P450 fold but
catalyzes the peroxide-dependent hydroxylation of
fatty acids^^. As shown in Figure 3.17, an OH
group is attached to the polar carboxyl end of the
substrate which means the carboxyl head group
must be positioned deep in the active site.
P450BSP has adapted to accommodate the car-
boxyl group by having an
Arg
residue on the I helix
that electrostatically stabilizes the carboxyl head
group (Figure 3.17). In the proposed peroxide acti-
vation mechanism, the fatty acid carboxyl group
serves an acid-base catal3^ic function^^ similar to
the distal histidine in peroxidases. P450BSp pro-
vides a fascinating new example on the adaptability
of P450s to the requirements of different metabolic
pathways.
100
Thomas L. Poulos and Eric F. Johnson
heme
P450cam
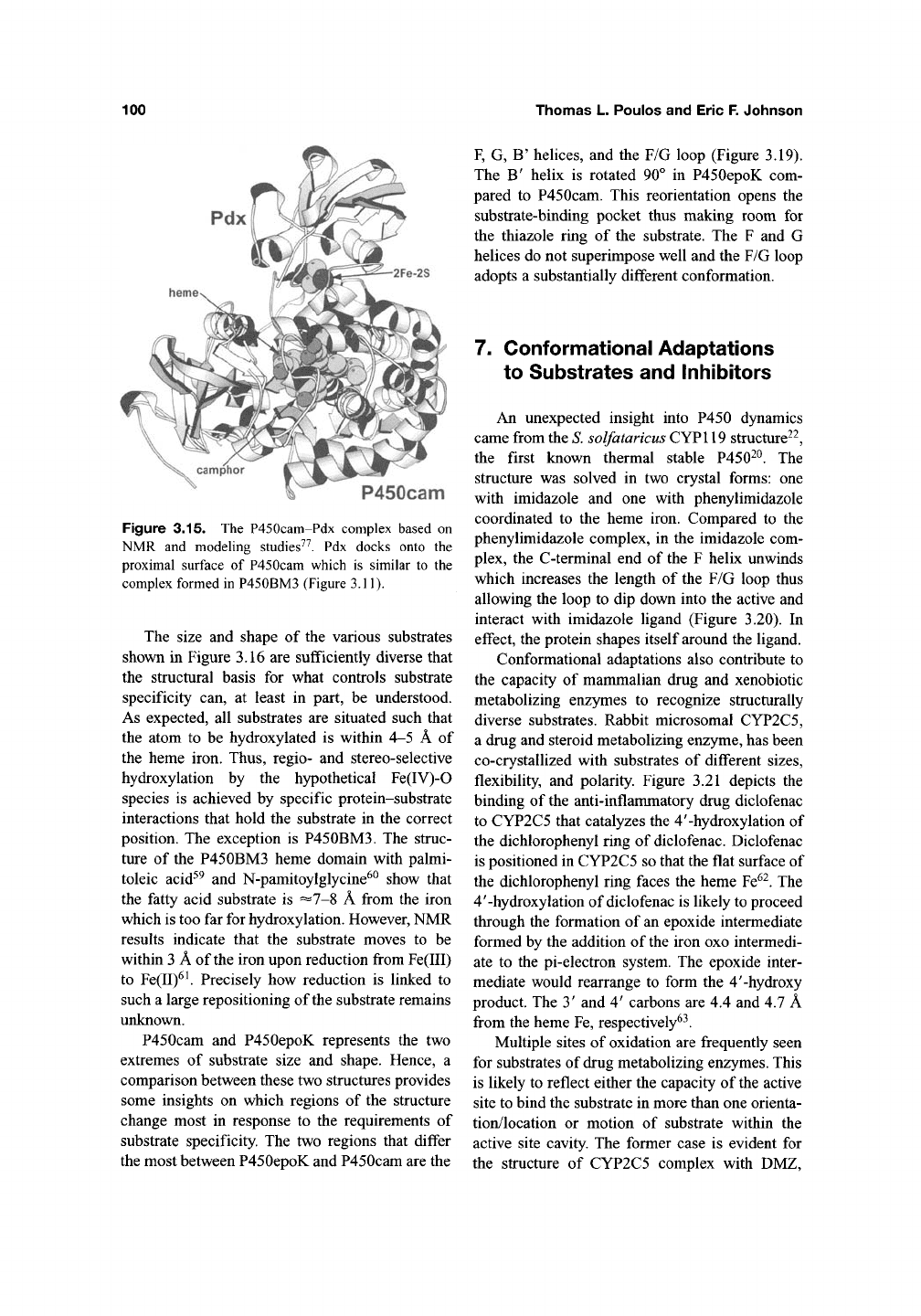
Figure 3.15. The P450cam-Pdx complex based on
NMR and modeling studies^^. Pdx docks onto the
proximal surface of P450cam which is similar to the
complex formed in P450BM3 (Figure 3.11).
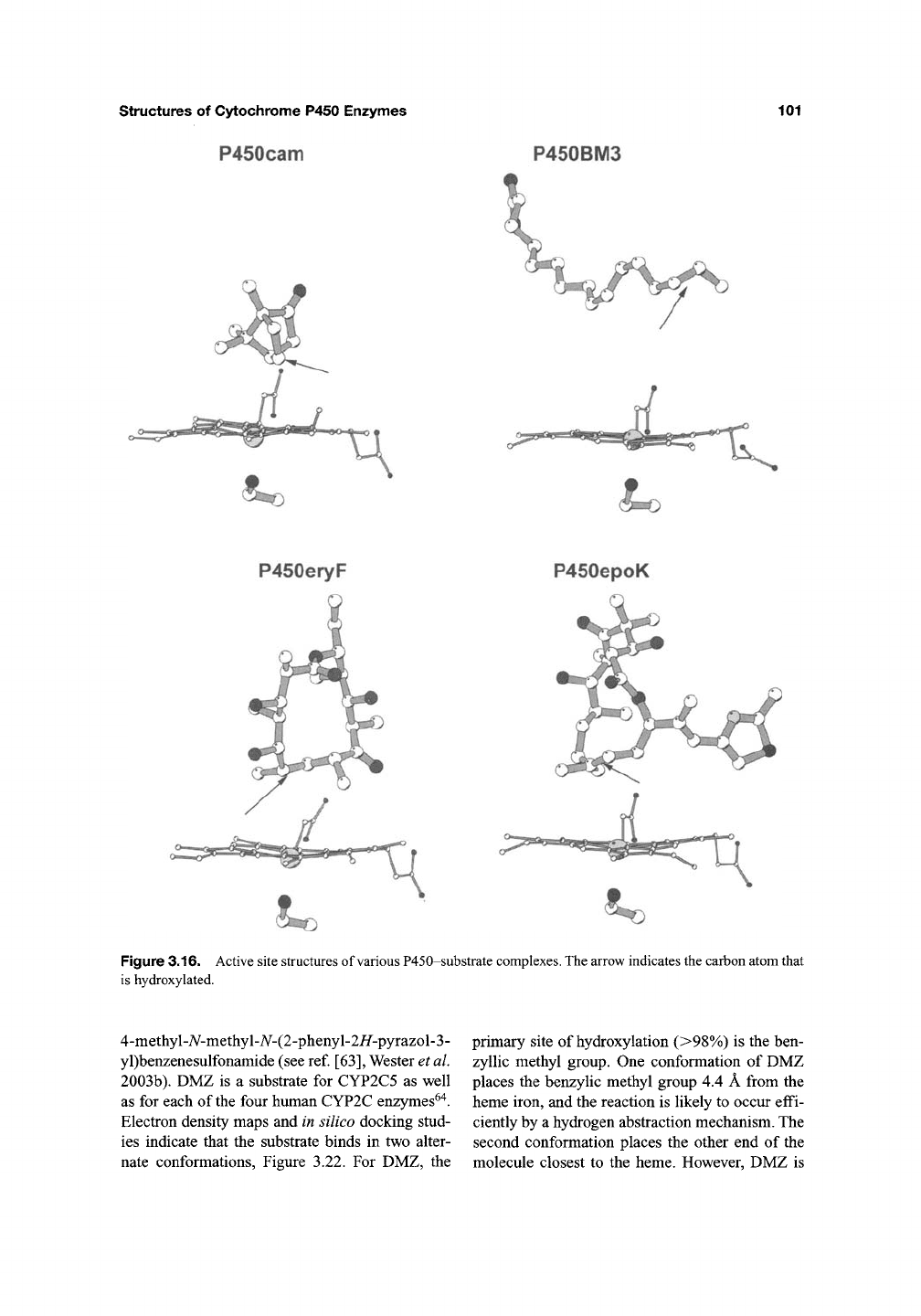
The size and shape of the various substrates
shown in Figure 3.16 are sufficiently diverse that
the structural basis for what controls substrate
specificity can, at least in part, be understood.
As expected, all substrates are situated such that
the atom to be hydroxylated is within 4-5 A of
the heme iron. Thus, regio- and stereo-selective
hydroxylation by the hypothetical Fe(IV)-0
species is achieved by specific protein-substrate
interactions that hold the substrate in the correct
position. The exception is P450BM3. The struc-
ture of the P450BM3 heme domain with palmi-
toleic acid^^ and N-pamitoylglycine^^ show that
the fatty acid substrate is ===7-8 A from the iron
which is too far for hydroxylation. However, NMR
results indicate that the substrate moves to be
within 3 A of the iron upon reduction from Fe(III)
to Fe(II)^^ Precisely how reduction is linked to
such a large repositioning of the substrate remains
unknown.
P450cam and P450epoK represents the two
extremes of substrate size and shape. Hence, a
comparison between these two structures provides
some insights on which regions of the structure
change most in response to the requirements of
substrate specificity. The two regions that differ
the most between P450epoK and P450cam are the
F,
G, B' helices, and the F/G loop (Figure 3.19).
The B' helix is rotated 90° in P450epoK com-
pared to P450cam. This reorientation opens the
substrate-binding pocket thus making room for
the thiazole ring of the substrate. The F and G
helices do not superimpose well and the F/G loop
adopts a substantially different conformation.
7. Conformational Adaptations
to Substrates and Inhibitors
An unexpected insight into P450 dynamics
came from the
S.
solfataricus CYPl
19
structure^^,
the first known thermal stable P450^^. The
structure was solved in two crystal forms: one
with imidazole and one with phenylimidazole
coordinated to the heme iron. Compared to the
phenylimidazole complex, in the imidazole com-
plex, the C-terminal end of the F helix unwinds
which increases the length of the F/G loop thus
allowing the loop to dip down into the active and
interact with imidazole ligand (Figure 3.20). In
effect, the protein shapes itself around the ligand.
Conformational adaptations also contribute to
the capacity of mammalian drug and xenobiotic
metabolizing enzymes to recognize structurally
diverse substrates. Rabbit microsomal CYP2C5,
a drug and steroid metabolizing enzyme, has been
co-crystallized with substrates of different sizes,
flexibility, and polarity. Figure 3.21 depicts the
binding of the anti-inflammatory drug diclofenac
to CYP2C5 that catalyzes the 4'-hydroxylation of
the dichlorophenyl ring of diclofenac. Diclofenac
is positioned in CYP2C5 so that the flat surface of
the dichlorophenyl ring faces the heme Fe^^. The
4'-hydroxylation of diclofenac is likely to proceed
through the formation of an epoxide intermediate
formed by the addition of the iron oxo intermedi-
ate to the pi-electron system. The epoxide inter-
mediate would rearrange to form the 4'-hydroxy
product. The 3' and 4' carbons are 4.4 and 4.7 A
from the heme Fe, respectively^^.
Multiple sites of oxidation are frequently seen
for substrates of drug metabolizing enzymes. This
is likely to reflect either the capacity of
the
active
site to bind the substrate in more than one orienta-
tion/location or motion of substrate within the
active site cavity. The former case is evident for
the structure of CYP2C5 complex with DMZ,
structures of Cytochrome P450 Enzymes
P450cam
101
P450BM3
Figure 3.16. Active site structures of various P450-substrate complexes. The arrow indicates the carbon atom that
is hydroxylated.
4-methyl-7V-methyl-A^-(2-phenyl-2i/-pyrazol-3-
yl)benzenesulfonamide (see ref.
[63],
Wester et al.
2003b). DMZ is a substrate for CYP2C5 as well
as for each of
the
four human CYP2C enzymes^"^.
Electron density maps and in silico docking stud-
ies indicate that the substrate binds in two alter-
nate conformations, Figure 3.22. For DMZ, the
primary site of hydroxylation (>98%) is the ben-
zyllic methyl group. One conformation of DMZ
places the benzylic methyl group 4.4 A from the
heme iron, and the reaction is likely to occur effi-
ciently by a hydrogen abstraction mechanism. The
second conformation places the other end of the
molecule closest to the heme. However, DMZ is
102
Thomas L. Poulos and Eric F. Johnson
Figure 3.17. The substrate complex in P450BSp^^. This P450 utiUzes peroxide to oxidize the atom indicated by
the arrow. The I helix utilizes an arginine to help stabilize the carboxyl group of
the
substrate.
not located optimally for hydroxylation in this
conformation as it is >5.9 A from the heme iron,
and the phenolic product accounts for <
1%
of the
total products. The two conformations of DMZ
overlap but occupy distinct portions of the active
site cavity, which is larger than the substrate.
CYP2C5 exhibits adaptive changes when sub-
strates bind. The largest, adaptive changes involve
alterations in the conformations of the helix B-C
region, in the locations of helices F and G, and the
conformation of the region between the F and G
helices. A comparison of
the
diclofenac and DMZ
complexes illustrates differences that occur when
bound substrates that are different in size, shape,
and polarity (Figure 3.23). The change in the posi-
tion of
the
F helix reflects differences in the space
occupied by the two substrates near the heme Fe.
Changes in the conformation of the B' helix reflect
adaptations that maximize interactions with each
substrate and, in the case of diclofenac, that
accommodate residual hydration in the distal por-
tion of the active site that is not occupied by the
substrate (Figure 3.23). These active site water
molecules are ordered in a pattern that permits
extensive intermolecular hydrogen bonding
between water molecules, the protein and the sub-
strate. This hydrogen bonding network is anchored
by the polar carboxyl group of diclofenac and
polar side chains in the distal portion of
the
active
site.
These interactions contribute to the affinity of
the enzyme for diclofenac and the regioselective
hydroxylation of the dichlorophenyl group.
Human CYP2C9 exhibits similar regioselectivity
for the oxidation of diclofenac and displays a high
catalytic efficiency for the reaction. In contrast, the
other human CYP2C enzymes generally exhibit a
much lower catalytic efficiency and a more relaxed
regioselectivity in that they hydroxylate both rings
of
the
substrate.
8. Conformational Dynamics
for Substrate Access
Substrate binding may involve rather large con-
formational changes. Once the P450cam structure
became available, an immediate puzzle was how
camphor gains access to the active site since the
substrate is buried and there is no obvious opening.
The substrate-free and -bound structures showed
no differences although substrate-free P450cam
exhibited much higher thermal motion in the B', F,
and G helices suggesting that the these regions
must move to allow substrate to enter the active
site^^.
That this region is quite flexible was demon-
strated by binding of
a
ferrocene label to Cys85^^.
The positioning of the ferrocene into the active site
results in unfolding of the B' helix (Figure 3.24)
and exposure of the active site. Although these
changes may not mimic what happens when
structures of Cytochrome P450 Enzymes
103
P450cam
OH
Camphor
Fatty acid
P450BM3
P450eryF
6-deoxyerythronolide B
OH
O OH O
Epothilone B
Figure 3.18. Reactions catalyzed by various P450s.
substrate binds and product leaves, this result does
support the view that the B' helix region can open
to allow substrate to enter. Additional support for
this view is provided by the structures of P450cam
complexed with tether compounds. The tether
compounds are designed to position a fluorescent
reporter group at the enzyme surface by attaching
it to a substrate analog that binds in the active site
using a linker of the approximate length and size of
the substrate access channel^^' ^^. As shown in
Figure 3.25, the tether molecule occupies a contin-
uous channel from the active site to the surface of
