Ortiz de Montellano Paul R.(Ed.) Cytochrome P450. Structure, Mechanism, and Biochemistry
Подождите немного. Документ загружается.


144
Mark J.I. Paine et ai.
127.
Li, H.Y., K. Darwish, and XL. Poulos (1991).
Characterization of recombinant Bacillus mega-
terium cytochrome P-450 BM-3 and its two func-
tional domains. J. Biol. Chem. 266, 11909-11914.
128.
Govindaraj, S. andT.L. Poulos (1997). The domain
architecture of cytochrome P450BM-3. J. Biol.
Chem.
212,1915-7921.
129.
Noble, M.A., C.S. Miles, S.K. Chapman, D.A.
Lysek, A.C. MacKay, G.A. Reid et al (1999).
Roles of key active-site residues in flavocy-
tochrome P450 BM3. Biochem. J. 339, 371-379.
130.
Shirane, N., Z. Sui, J.A. Peterson, and PR. Ortiz
de Montellano (1993). Cytochrome P450BM-3
(CYP102): Regiospecificity of oxidation of omega-
unsaturated fatty acids and mechanism-based
inactivation. Biochemistry 32, 13732-13741.
131.
Ost, T.W., C.S. Miles, A.W. Munro, J. Murdoch,
G.A. Reid, and S.K. Chapman (2001). Phenyl-
alanine 393 exerts thermodynamic control over the
heme of flavocytochrome P450
BM3.
Biochemistry
40,13421-13429.
132.
Murataliev, M.B. and R. Feyereisen (2000).
Functional interactions in cytochrome P450BM3.
Evidence that NADP(H) binding controls redox
potentials of
the
flavin cofactors. Biochemistry 39,
12699-12707.
133.
Murataliev, M.B. and R. Feyereisen (1999).
Mechanism of cytochrome P450 reductase from
the house fly: Evidence for an FMN semiquinone
as electron donor. FEBS
Lett.
453, 201-204.
134.
Murataliev, M.B. and R. Feyereisen (2000).
Interaction of NADP(H) with oxidized and reduced
P450 reductase during catalysis. Studies with
nucleotide analogues. Biochemistry 39, 5066-5074.
135.
Munro, A.W., K. Malarkey, J. McKnight, A.J.
Thomson, S.M. Kelly, N.C. Price et al. (1994). The
role of tryptophan 97 of cytochrome P450 BM3
from Bacillus megaterium in catalytic function.
Evidence against the 'covalent switching' hypothesis
of P-450 electron transfer.
Biochem.
J.
303,423-428.
136.
Murakami, H.,
Y.
Yabusaki, T. Sakaki, M. Shibata,
and H. Ohkawa (1987). A genetically engineered
P450 monooxygenase: Construction of the func-
tional fused enzyme between rat cytochrome
P450c and NADPH-cytochrome P450 reductase.
DMA
6, 189-197.
137.
Fisher, C.W., M.S. Shet, D.L. Caudle, C.A. Martin-
Wixtrom, and R.W. Estabrook (1992). High-level
expression in Escherichia coli of enzymatically
active fusion proteins containing the domains of
mammalian cytochromes P450 and NADPH-P450
reductase flavoprotein. Proc. Natl.
Acad.
Sci. USA
89,
10817-10821.
138.
Shet, M.S., C.W. Fisher, PL. Holmans, and
R.W. Estabrook (1993). Human cytochrome
P450 3A4: Enzymatic properties of a purified
recombinant fiision protein containing NADPH-
P450 reductase. Proc. Natl.
Acad.
Sci. USA 90,
11748-11752.
139.
Chun, Y.J., T. Shimada, and FP Guengerich
(1996).
Construction of a human cytochrome
P450 lAl: Rat NADPH-cytochrome P450 reduc-
tase fusion protein cDNA and expression in
Escherichia coli, purification, and catalytic prop-
erties of the enzyme in bacterial cells and after
purification. Arch. Biochem. Biophys. 330, 48-58.
140.
Shet, M., C.W. Fisher, PL. Holmans, and R.W.
Estabrook (1996). The omega-hydroxlyation of lau-
ric acid: Oxidation of 12-hydroxylauric acid to
dodecanedioic acid by a purified recombinant fusion
protein containing P450 4A1 and NADPH-P450
reductase.
Arch.
Biochem. Biophys. 330, 199-208.
141.
Helvig, C. and J.H. Capdevila (2000). Biochemical
characterization of rat P450 2C11 fused to rat or
bacterial NADPH-P450 reductase domains.
Biochemistry 39, 5196-5205.
142.
Sakaki, T, S. Kominami, S. Takemori, H. Ohkawa,
M. Akiyoshi-Shibata, and Y. Yabusaki (1994).
Kinetic studies on a genetically engineered fused
enzyme between rat cytochrome P4501A1 and
yeast NADPH-P450 reductase. Biochemistry 33,
4933-4939.
143.
Hayashi, K., T. Sakaki, S. Kominami, K. Inouye,
and Y. Yabusaki (2000). Coexpression of geneti-
cally engineered fused enzyme between yeast
NADPH-P450 reductase and human cytochrome
P450 3A4 and human cytochrome b5 in yeast.
Arch.
Biochem. Biophys. 381, 164-170.
144.
Deeni, YY, M.J. Paine, A.D. Ayrton, S.E. Clarke,
R. Chenery, and C.R. Wolf (2001). Expression,
purification, and biochemical characterization
of a human cytochrome P450 CYP2D6-NADPH
cytochrome P450 reductase fusion protein. Arch.
Biochem. Biophys. 396, 16-24.
145.
Sanborn, R.C. and CM. William (1950). The
cytochrome system in the Cecropia silkworm, with
special reference to the properties of a new com-
ponent. J. Gen. Physiol. 33, 579-588.
146.
Porter, T.D. (2002). The roles of cytochrome b5 in
cytochrome P450 reactions. J. Biochem. Mol.
Toxicol. 16,311-316.
147.
Vergeres, G. and L. Waskell (1995). Cytochrome
b5,
its functions, structure and membrane topol-
ogy. Biochimie 11, 604-620.
148.
Hildebrandt, A. and R.W. Estabrook (1971).
Evidence for the participation of cytochrome b5
in hepatic microsomal mixed-function oxidation
reactions. Arch. Biochem. Biophys. 143, 66-79.
149.
Correia, M.A. and G.J. Mannering (1973).
Reduced diphosphopyridine nucleotide synergism
of the reduced triphosphopyridine nucleotide-
dependent mixed-function oxidase system of

Electron Transfer Partners of Cytochrome P450
145
hepatic microsomes. II. Role of the type I
drug-binding site of cytochrome P-450. Mol.
Pharmacol. 9, 470-485.
150.
Yamazaki, H., M. Nakamura, T. Komatsu,
K. Ohyama, N. Hatanaka, S. Asahi et al. (2002).
Roles of NADPH-P450 reductase and apo- and
holo-cytochrome b5 on xenobiotic oxidations cat-
alyzed by 12 recombinant human cytochrome
P450s expressed in membranes of Escherichia
coli. Protein Expr
Purif.
24, 329-337.
151.
Voice,
M.W.,
Y.
Zhang, C.R.
Wolf,
B. Burchell, and
T. Friedberg (1999). Effects of human cytochrome
b5 on CYP3A4 activity and stability in vivo. Arch.
Biochem. Biophys. 366, 116-124.
152.
Hlavica, P and D.F. Lewis (2001). Allosteric phe-
nomena in cytochrome P450-catalyzed monooxy-
genations. Eur J. BioChem. 268, 4817-^832.
153.
Mitoma, J. and A. Ito (1992). The carboxy-termi-
nal 10 amino acid residues of cytochrome b5 are
necessary for its targeting to the endoplasmic retic-
ulum.
EMBOJ.
11, 4197-4203.
154.
Keyes, S.R. and D.L. Cinti (1980). Biochemical
properties of cytochrome b5-dependent microso-
mal fatty acid elongation and identification of
products. ^ 5/o/. Chem. 255, 11357-11364.
155.
Oshino,
N.,
Y.
Imai, and R. Sato (1971). A function
of cytochrome b5 in fatty acid desaturation
by rat liver microsomes. J. Biochem. (Tokyo) 69,
155-167.
156.
Reddy, VV, D. Kupfer, and E. Caspi (1977).
Mechanism of
C-5
double bond introduction in the
biosynthesis of cholesterol by rat liver micro-
somes. J. Biol. Chem. 252, 2797-2801.
157.
Hegesh, E., J. Hegesh, and A. Kaftory (1986).
Congenital methemoglobinemia with a deficiency
of cytochrome b5. N. Engl. J. Med. 314,
757-761.
158.
Manabe, J., R. Arya, H. Sumimoto,
T.
Yubisui, A.J.
Bellingham, D.M. Layton et al. (1996). Two novel
mutations in the reduced nicotinamide adenine
dinucleotide (NADH)-cytochrome b5 reductase
gene of a patient with generalized type, hereditary
methemoglobinemia. Blood SS, 3208-3215.
159.
Tamburini, P.P., S. MacFarquhar, and J.B.
Schenkman (1986). Evidence of binary complex
formations between cytochrome P-450, cyto-
chrome b5, and NADPH-cytochrome P-450 reduc-
tase of hepatic microsomes. Biochem. Biophys.
Res.
Commun. 134, 519-526.
160.
Stayton, PS. and S.G. Sligar (1990). The
cytochrome P-450cam binding surface as defined
by site-directed mutagenesis and electrostatic
modeling. Biochemistry 29, 7381-7386.
161.
Omata, Y, H. Sakamoto, R.C. Robinson, M.R.
Pincus, and F.K. Friedman (1994). Interaction
between cytochrome P450 2B1 and cytochrome
b5:
Inhibition by synthetic peptides indicates a role
for P450 residues Lys-122 and Arg-125. Biochem.
Biophys. Res. Commun. 201, 1090-1095.
162.
Omata, Y, R.C. Robinson, H.V Gelboin, M.R.
Pincus, and F.K. Friedman (1994). Specificity of
the cytochrome P-450 interaction with cytochrome
b5.
FEBSLett. 346, 241-245.
163.
Guengerich, F.P. (1983). Oxidation-reduction
properties of rat liver cytochromes P-450 and
NADPH-cytochrome p-450 reductase related to
catalysis in reconstituted systems. Biochemistry
22,2811-2820.
164.
Yamazaki, H., W.W. Johnson, Y.-F. Ueng, T
Shimada, and F.P. Guengerich (1996). Lack of
electron transfer from cytochrome b5 in stimula-
tion of catalytic activities of cytochrome P450
3A4.
Characterization of a reconstituted cyto-
chrome P450 3A4/NADPH-cytochrome P450
reductase system and studies with apo-cytochrome
b5.
J. Biol. Chem. 271, 27438-27444.
165.
Yamazaki, H., T. Shimada, M.V Martin, and F.P
Guengerich (2001). Stimulation of cytochrome
P450 reactions by apocytochrome b5. Evidence
against transfer of heme from cytochrome P450
3A4 to apo-cytochrome b5 or heme oxygenase.
J. Biol. Chem. 276, 30885-30891.
166.
Yamazaki, H., M. Nakano, Y Imai, Y.F. Ueng,
F.P.
Guengerich, and T. Shimada (1996). Roles of
cytochrome b5 in the oxidation of testosterone and
nifedipine by recombinant cytochrome P450 3A4
and by human liver microsomes. Arch. Biochem.
Biophys. 325, 174-182.
167.
Bell, L.C. and EP Guengerich (1997). Oxidation
kinetics of ethanol by human cytochrome P450
2E1.
Rate-limiting product release accounts for
effects of isotopic hydrogen substitution and
cytochrome b5 on steady-state kinetics. J. Biol.
Chem.
272,29643-29651.
168.
Schenkman, J.B., A.I. Voznesensky, and I. Jansson
(1994).
Influence of ionic strength on the P450
monooxygenase reaction and role of cytochrome
b5 in the process. Arch. Biochem. Biophys. 314,
234-241.
169.
Perret, A. and D. Pompon (1998). Electron shuttle
between membrane-bound cytochrome P450 3A4
and
b5
rules uncoupling mechanisms. Biochemistry
37,
11412-11424.
170.
Gruenke, L.D., K. Konopka, M. Cadieu, and
L. Waskell (1995). The stoichiometry of the
cytochrome P-450-catalyzed metabolism of
methoxyflurane and benzphetamine in the presence
and absence of cytochrome b5. J. Biol. Chem. 270,
24707-24718.
171.
Wang, M.H., C.J. Patten, G.Y Yang, S.R.
Paranawithana, Y Tan, and C.S. Yang (1996).
Expression and coupling of human cytochrome
P450 2E1 and NADPH-cytochrome P450

146
Mark J.I. Paine et al.
oxidoreductase in dual expression and co-infection
systems with baculovims in insect cells. Arch.
Biochem. Biophys. 334, 380-388.
172.
Cooper, M.T. andT.D. Porter (2001). Cytochrome
b(5) coexpression increases the CYP2E1-depend-
ent mutagenicity of dialkylnitrosamines in methyl-
transferase-deficient strains of Salmonella
typhimurium. Mutat. Res. 484, 61-68.
173.
Jansson, I. and J.B. Schenkman (1987). Influence
of cytochrome b5 on the stoichiometry of the
dif-
ferent oxidative reactions catalyzed by liver micro-
somal cytochrome P-450. Drug
Metab.
Dispos. 15,
344-348.
174.
Grinberg, A.V, F. Hannemann, B. Schiffler, J.
Muller, U. Heinemann, and R. Bernhardt (2000).
Adrenodoxin: Structure, stability, and electron
transfer properties. Proteins 40, 590-612.
175.
Poulos, T.L., B.C. Finzel, and A.J. Howard (1987).
High-resolution crystal structure of cytochrome
P450cam. J. Mol Biol. 195, 687-700.
176.
Mathews, FS., A.G. Mauk, and G.R. Moore
(2000).
In C. Kleanthous (ed.), Protein-Protein
Recognition. Oxford University Press, Oxford,
pp.
60-101.
177.
Shimada, H., S. Nagano, H. Hori, and
Y.
Ishimura
(2001).
Putidaredoxin-cytochrome P450cam inter-
action. J. Inorg. Biochem. 83, 255-260.
178.
Trower, M.K., R. Lenstra, C. Omer, S.E. Buchholz,
and FS. Sariaslani (1992). Cloning, nucleotide
sequence determination and expression of the
genes encoding cytochrome P-450soy (soyC) and
ferredoxinsoy (soyB) from Streptomyces griseus.
Mol. Microbiol. 6, 2125-2134.
179.
Green, A.J., A.W. Munro, M.R. Cheesman,
G.A. Reid, C. von Wachenfeldt, and S.K. Chapman
(2003).
Expression, purification and characterisa-
tion of a Bacillus subtilis ferredoxin: A potential
electron transfer donor to cytochrome P450 Biol.
J. Inorg. Biochem. 93, 92-99.
180.
Matocha, M.F and M.R. Waterman (1984).
Discriminatory processing of the precursor forms
of cytochrome P-450scc and adrenodoxin by
adrenocortical and heart mitochondria. J. Biol.
Chem.
259, 8672-8678.
181.
Matsubara, H. and K. Saeki (1992). Structural and
functional diversity of ferredoxins and related pro-
teins.
Adv. Inorg Chem. 38, 223-280.
182.
Sevrioukova, I.F and T.L. Poulos (2002).
Putidaredoxin reductase, a new function for an old
protein. J. Biol. Chem. Ill, 25831-25839.
183.
Omura, T., E. Sanders, R.W. Estabrook, D.Y.
Cooper, and O. Rosenthal (1966). Isolation from
adrenal cortex of a nonheme iron protein and a
flavoprotein functional as a reduced triphosphopy-
ridine nucleotide-cytochrome P-450 reductase.
Arch.
Biochem. Biophys. Ill, 660-673.
184.
Hanukoglu, I., C.T. Privalle, and C.R. Jefcoate
(1981).
Mechanisms of ionic activation of adrenal
mitochondrial cytochromes P-450scc and P-45011
beta. J. Biol. Chem. 256, 4329^335.
185.
Ziegler, G.A., C. Vonrhein, I. Hanukoglu, and G.E.
Schulz (1999). The structure of adrenodoxin
reductase of mitochondrial P450 systems: Electron
transfer for steroid biosynthesis. J. Mol. Biol. 289,
981-990.
186.
Bossi, R.T., A. Aliverti, D. Raimondi, F Fischer,
G. Zanetti, D. Ferrari et al. (2002). A covalent
modification of NADP^ revealed by the atomic
resolution structure of FprA, a Mycobacterium
tuberculosis oxidoreductase, Biochemistry 41,
8807-8818.
187.
Hanukoglu, I. (1992). Steroidogenic enzymes:
Structure, function, and role in regulation of
steroid hormone biosynthesis.
J.
Steroid.
Biochem.
Mol. Biol. 43, 779-804.
188.
Pochapsky, T.C., N.U. Jain, M. Kuti, T.A. Lyons,
and J. Heymont (1999). A refined model for the
solution structure of oxidized putidaredoxin.
Biochemistry 38, 4681-4690.
189.
Fukuyama, K., T. Okada, Y. Kakuta, and Y.
Takahashi (2002). Atomic resolution structures of
oxidized [4Fe-4S] ferredoxin from Bacillus
thermoproteolyticus in two crystal forms:
Systematic distortion of [4Fe-4S] cluster in the
protein.
J.
Mol. Biol. 315, 1155-1166.
190.
Holden, M., M. Mayhew, D. Bunk, A. Roitberg,
and V Vilker (1997). Probing the interactions of
putidaredoxin with redox partners in camphor
P450 5-monooxygenase by mutagenesis of surface
residues. J 5/o/. Chem. 272, 21720-21725.
191.
Unno, M., J.F Christian, T. Sjodin, D.E. Benson,
I.D. Macdonald, S.G.
SMgdiV
etal.
(2002). Complex
formation of cytochrome P450cam with
Putidaredoxin. Evidence for protein-specific
interactions involving the proximal thiolate ligand.
J. Biol. Chem. Ill, 2547-2553.
192.
Roitberg, A., H.M. Holden, M.P Mayhew, I.V
Kurnikov, D.N. Beraten, and V.L. Vilker (1998).
Binding and electron transfer between putidare-
doxin and cytochrome P450cam. J. Am. Chem.
Soc. 120, 8927-8932.
193.
Davies, M.D. and S.G. Sligar (1992). Genetic vari-
ants in the putidaredoxin-cytochrome P-450cam
electron-transfer complex: Identification of the
residue responsible for redox-state-dependent
conformers. Biochemistry 31, 11383-11389.
194.
Stayton, PS. and S.G. Sligar (1990). The
cytochrome P-450cam binding surface as defined
by site-directed mutagenesis and electrostatic
modeling. Biochemistry 29, 7381-7386.
195.
Aoki, M., K. Ishimori, H. Fukada, K. Takahashi,
and I. Morishima (1998). Isothermal titration

Electron Transfer Partners of Cytochrome P450
147
calorimetric studies on the associations of puti-
daredoxin to NADH-putidaredoxin reductase and
P450cam. Biochim. Biophys. Acta. 1384, 180-188.
196.
Mueller, E.J., P.J. Loida, and S.G. Sligar (1995). In
P.R. Ortiz de Montellano (ed.), Cytochrome P450;
Structure, Mechanism and Biochemistry (2nd edn.).
Plenum Press, New York, pp. 83-124.
197.
Hintz, M.J. and J.A. Peterson (1981). The kinetics
of reduction of cytochrome P-450cam by reduced
putidaredoxin. J. Biol. Chem. 256, 6721-6728.
198.
Roome, PW., J.C. Philley, and J.A. Peterson
(1983).
Purification and properties of putidare-
doxin reductase. J. Biol. Chem. 49, 10592-10600.
199.
Sevrioukova, I.F., J.T. Hazzard, G. Tollin, and T.L.
Poulos (2001). Laser flash induced electron trans-
fer in P450cam monooxygenase putidaredoxin
reductase-putidredoxin interaction. Biochemistry
49,
10592-10600.
200.
Pochapsky, S.S., T.C. Pochapsky, and J.W. Wei
(2003).
A model for effector activity in a highly
specific biological electron transfer complex: The
cytochrome P450(cam)-putidaredoxin couple.
Biochemistry 42, 5649-5656.
201.
Tosha, T, S. Yoshioka, S. Takahashi, K. Ishimori,
H. Shimada, and I. Morishima (2003). NMR study
on the structural changes of cytochrome P450cam
upon the complex formation with putidaredoxin:
Functional significance of the putidaredoxin-
induced structural changes. J. Biol. Chem. 2, 2.
202.
Brewer, C.B. and J.A. Peterson (1988) Single
turnover kinetics of the reaction between oxycy-
tochrome P450cam and reduced putidaredoxin. J.
Biol. Chem. 263, 791-798.
203.
Muller, A., J.J. Muller, Y.A. Muller, H. Uhlmann,
R. Bernhardt, and U. Heinemann (1998). New
aspects of electron transfer revealed by the crystal
structure of a truncated bovine adrenodoxin,
Adx(4-108). Structure 6, 269-280.
204.
Pikuleva, I.A., K. Tesh, M.R. Waterman, and Y.
Kim (2000). The tertiary structure of fiill-length
bovine adrenodoxin suggests functional dimers.
Arch.
Biochem. Biophys. 373, 44-55.
205.
Beilke, D., R. Weiss, F. Lohr, P Pristovsek, E
Hannemann, R. Bernhardt et al. (2002). A new
electron transport mechanism in mitochondrial
steroid hydroxylase systems based on structural
changes upon the reduction of adrenodoxin.
Biochemistry 41, 7969-7978.
206.
Vickery, L.E. (1997). Molecular recognition and
electron transfer in mitochondrial steroid hydroxy-
lase systems. Steroids 62, 124-127.
207.
ZoUner, A., E Hannemann, M. Lisurek, and
R. Bernhardt (2002). Deletions in the loop sur-
rounding the iron-sulfiir cluster of adrenodoxin
severely affect the interactions with its native
redox partners adrenodoxin reductase and
cytochrome P450(scc) (CYPllAl). J. Inorg.
Biochem. 91, 644-654.
208.
Uhlmann, H., V Beckert, D. Schwarz, and
R. Bernhardt (1992). Expression of bovine adren-
odoxin in E. coli and site-directed mutagenesis of
/2 Fe-2S/ cluster ligands. Biochem. Biophys. Res.
Commun. 188, 1131-1138.
209.
Hara, T, C. Koba, M. Takeshima, and Y. Sagara
(2000).
Evidence for the cluster model of mito-
chondrial steroid hydroxylase system derived from
dissociation constants of the complex between
adrenodoxin reductase and adrenodoxin. Biochem.
Biophys. Res. Commun. 276, 210-215.
210.
Lambeth, J.D., D.W Seybert, J.R. Lancaster, Jr.,
J.C. Salerno, and H. Kamin (1982). Steroidogenic
electron transport in adrenal cortex mitochondria.
Mol. Cell Biochem. 45,
13-31.
211.
Muller, J.J., A. Lapko, K. Ruckpaul, and
U. Heinemann (2003). Modeling of electrostatic
recognition processes in the mammalian mito-
chondrial steroid hydroxylase system. Biophys.
Chem.
100, 281-292.
212.
Muller, J.J., A. Lapko, G. Bourenkov, K. Ruckpaul,
and U. Heinemann (2001). Adrenodoxin reduc-
tase-adrenodoxin complex structure suggests elec-
tron transfer path in steroid biosynthesis. J. Biol.
Chem.
276, 2786-2789.
213.
Aoki, M., K. Ishimori, and I. Morishima (1998).
Roles of negatively charged surface residues of
putidaredoxin in interactions with redox partners
in p450cam monooxygenase system. Biochim.
Biophys. Acta. 1386, 157-167.
214.
Sibbesen, O., J.J. De Voss, and PR. Ortiz de
Montellano (1996). Putidaredoxin reductase-
putidaredoxin-cytochrome P450cam triple fusion
protein. Construction of a self-suff'icient Escheri-
chia coli catalytic system. J. Biol. Chem. 211,
22462-22469.
215.
Song, WC, CD. Funk, and A.R Brash (1993).
Molecular cloning of an allene oxide synthase: A
cytochrome P450 specialized for the metabolism
of fatty acid hydroperoxides. Proc. Natl.
Acad.
Sci.
t/&4 90, 8519-8523.
216.
Lee, D.S., A. Yamada, H. Sugimoto, I. Matsunaga,
H. Ogura, K. Ichihara et al. (2003). Substrate
recognition and molecular mechanism of fatty
acid hydroxylation by cytochrome P450 from
Bacillus subtilis. Crystallographic, spectroscopic,
and mutational studies. J. Biol. Chem. 278,
9761-9767.
217.
Green, A.J., A.W Munro, M.R Cheesman, G.A.
Reid, C. von Wachenfeldt, and S.K. Chapman
(2003).
Expression, purification and characterisa-
tion of a Bacillus subtilis ferredoxin: A potential
electron transfer donor to cytochrome P450 Biol.
J. Inorg. Biochem. 93, 92-99.

148
Mark J.I. Paine et al.
218.
Hawkes, D.B., G.W. Adams, A.L. Burlingame,
P.R. Ortiz de Montellano, and J.J. De Voss (2002).
Cytochrome P450(cin) (CYP176A), isolation,
expression, and characterization. J. Biol. Chem.
277,
27725-27732.
219.
Mclver, L., C. Leadbeater, D.J. Campopiano, R.L.
Baxter, S.N.
Daff,
S.K. Chapman et al. (1998).
Characterisation of flavodoxin NADP^ oxido-
reductase and flavodoxin; key components of
electron transfer in Escherichia coli. Eur. J.
Biochem. 257, 577-585.
220.
Jackson, C.J., D.C. Lamb, T.H. Marczylo, A.G.
Warrilow, N.J. Manning, D.J. Lowe et al. (2002).
A novel sterol 14alpha-demethylase/ferredoxin
fusion protein (MCCYP51FX) from Methylo-
coccus capsulatus represents a new class of the
cytochrome P450 superfamily. J. Biol. Chem. 277,
46959^6965.
221.
Roberts, G.A., G. Grogan, A. Greter, S.L. Flitsch,
and N.J. Turner (2002). Identification of a new
class of cytochrome P450 from a Rhodococcus sp.
J. Bacteriol. 184, 3898-3908.
5
Activation of Molecular Oxygen by
Cytochrome P450
Thomas M. Makris, Ilia Denisov, lime Schlichting, and
Stephen G. Sligar
Life depends on the kinetic barriers to oxygen reactions
RJ.R Williams, in the preface to the Oxygen Chemistry by
Donald
T.
Sawyer^
1.
Introduction to Oxygen
Activation
The cytochromes P450 have occupied a central
focal point in biochemistry, pharmacology, toxicol-
ogy, and biophysics for more than three decades.
This rich history is faithfully conveyed in accom-
panying articles appearing in this volume as well as
those of the first and second editions of
Cytochrome
P450 edited by Ortiz de Montellano^'
^.
In addition,
there have been innumerable review articles and
book chapters devoted to the subject^'
^.
Interest in
the cytochromes P450 perhaps began due to the
importance of these catalysts in human physiology
and pharmacology. The interest of chemists and
biophysicists peaked due to the many unique spec-
tral features of these heme enzymes. One of the
most intriguing aspects of P450 systems is the
range of difficult chemistry efficiently catalyzed.
the importance of oxygen as a cosubstrate, and the
thoughts of overall common mechanisms with the
heme monooxygenases and other systems that
effect reductive metabolism of atmospheric dioxy-
gen^'
^.
Thus, from the very beginning, the phrase
"active oxygen" was used to label a holy grail of
cytochrome P450 research: the documentation
of the intermediate states of oxygen and heme
iron that give rise to the diversity of metabolic
chemistries observed.
The chemistry of "oxygen activation" cat-
alyzed by a variety of metal centers has a long and
rich history, and as many as eight variations of
structurally different metal-oxygen complexes
were discussed as early as
1965^.
This publication
of Cytochrome P450, Third Edition is particularly
timely. During the past 3-5 years, a great deal of
progress has been realized in the direct observa-
tion of
P450
intermediate states and the evolution
of a detailed understanding of the structural
Thomas M. Makris • Center for Biophysics, University of
Illinois,
Urbana, IL.
Ilia Denisov • Department of
Biochemistry,
University of
Illinois,
Urbana, IL.
lime Schlichting • Max Planck Institute for Medical Research, Department of Molecular
Mechanisms,
Germany.
Stephen G. Sligar • Departments of Biochemistry, Chemistry, the School of Medicine, and the Center for
Biophysics, University of
Illinois,
Urbana, IL.
Cytochrome
P450:
Structure,
Mechanism,
and
Biochemistry,
3e, edited by Paul R. Ortiz de Montellano
Kluwer Academic / Plenum Publishers,
New
York,
2005.
149

150
Thomas M. Makris ef al.
features of the protein, which control the popula-
tion and stepwise movement through the reduction
and protonation states of heme and oxygen. The
veil surrounding "oxygen activation" has been
lifted through the systematic application of
a
vari-
ety of biochemical and biophysical tools, such as
mutagenesis, high-resolution cryocrystallography,
multiparameter spectroscopic studies of interme-
diate states isolated using cryogenic or fast kinetic
techniques, and many rigorous quantum chemical
and molecular dynamics computational studies.
Equally important has been the explosion of
genomic and proteomic breakthroughs. No longer
are we dealing with a single microbial P450 and a
few membrane bound mammalian counterparts.
Rather, with over 3,000 P450 genes known, we
can find examples from unique environmental
niches where the enzymatic reaction cycle yields
newly observable features. Thus today, the current
view of oxygen activation mechanisms catalyzed
by metal centers in heme enzymes ensures a much
better opportunity to develop recurring catalytic
paradigms than was possible at the time of the
Cytochrome P450, Second Edition.
A broader view of heme-oxygen catalysis with
the understanding of how proximal ligand, protein
structure, and subtle control of electron/proton
stoichiometry contribute to very different reactiv-
ities is now within reach^. Common mechanistic
hypotheses for oxygen activation in metalloen-
zymes are currently emerging. Recent progress in
mechanistic studies on other heme enzymes,
which use different forms of so-called "active
oxygen intermediates," such as peroxidases'^' •',
heme oxygenases (HOs)'^' '^, catalases'"^, nitric
oxide synthases (NOS)'^, and peroxygenases'^' '^,
have provided an impressive degree of unifica-
tion. A fundamental question that has always
plagued the interpretation of experimental data
has been the functional differentiation of these
enzymes, which efficiently catalyze a range of
diverse chemistries yet utilize similar highly reac-
tive heme-oxygen complexes. The comparison of
similar reactive intermediates in different enzymes
has helped to distinguish between the essential fea-
tures of each of the enzymes. This in turn has pro-
vided important clues into the role of protein
structure in the heme-oxygen catalytic events. The
recent progress in actually observing, through
kinetic isolation and cryogenic techniques, these
fundamental intermediate states in the cytochrome
P450s brings us to a much deeper understanding of
P450 monooxygenase catalysis that was available
at the time of the previous edition of this volume.
In discussing the reaction cycle of the
cytochrome P450s, it is useful to incorporate
similar states which appear to be more or less
stable in other heme-oxygen systems. The sequen-
tial steps of "oxygen activation" are those that
follow the formation of the ferrous reduced iron-
porphyrin state that is generated by electron
transfer from the physiological redox donor. These
steps are formally:
1.
Dioxygen binding to the ferrous heme
center to form an oxygenated complex which can
be written as either Fe^^-OO or Fe^^-00~.
2.
One-electron reduction of this complex
to form the Fe^^-00^~, ferric-peroxo complex.
3.
Protonation of the distal oxygen of
this peroxo anion to generate the Fe^^-OOH~,
ferric-hydroperoxo complex.
4.
A second protonation of the Fe^^-OOH~
complex at the distal oxygen atom to form a
transient oxo-water adduct which immediately
undergoes heterolytic scission of the 0-0 bond.
This process generates a single oxygen atom con-
taining iron-porphyrin species, which is formally
two redox equivalents above the ferric heme start-
ing state. This intermediate has been variously
termed the "iron-oxo," "ferryl-oxo porphyrin
TT-cation radical," or "Compound I," the latter after
the pioneering discoveries made by Theorell,
Keilin, George, and Chance while studying the
peroxidases and catalases'^.
The intermediate states so defined have coun-
terpoints in nonheme oxygen activation and have
been also studied and reviewed in numerous arti-
cles over past decades. We note that a search of the
term "oxygen activation" in SciFinder Chemical
Abstracts Database returns almost 29,000 refer-
ences published after 1995! Thus, we will not
attempt to link nonheme iron chemistry, nor
oxygen activation in multicenter metalloenzymes
which was recently reviewed by Siegbahn'^, with
that of the P450 systems in this chapter. Rather,
we focus on those features which directly address
the important aspects of the oxygen activation
cascade in P450. The structure and properties of
relevant porphyrin complexes, including models
of cytochrome P450 and other oxygen-activation
enzymes, are extensively reviewed in the recent
edition of
the
Porphyrin Handbook^^'^^.
Activation of IVIolecular Oxygen by Cytochrome P450
151
2.
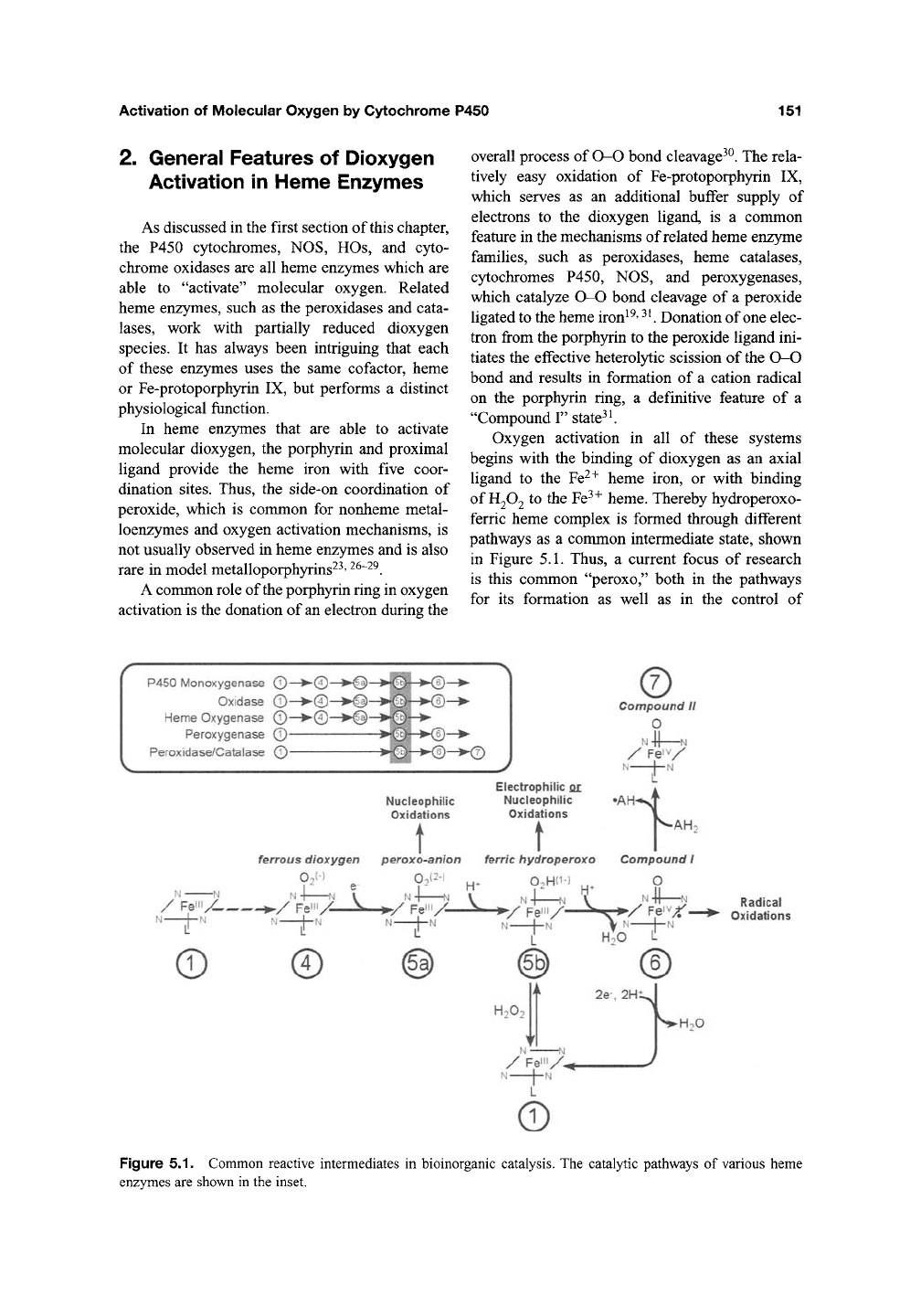
General Features of Dioxygen
Activation in Heme Enzymes
As discussed in the first section of this chapter,
the P450 cytochromes, NOS, HOs, and cyto-
chrome oxidases are all heme enzymes which are
able to "activate" molecular oxygen. Related
heme enzymes, such as the peroxidases and cata-
lases,
work with partially reduced dioxygen
species. It has always been intriguing that each
of these enzymes uses the same cofactor, heme
or Fe-protoporphyrin IX, but performs a distinct
physiological function.
In heme enzymes that are able to activate
molecular dioxygen, the porphyrin and proximal
ligand provide the heme iron with five coor-
dination sites. Thus, the side-on coordination of
peroxide, which is common for nonheme metal-
loenzymes and oxygen activation mechanisms, is
not usually observed in heme enzymes and is also
rare in model metalloporphyrins^^'
^^~^^.
A common role of the porphyrin ring in oxygen
activation is the donation of an electron during the
overall process of O-O bond cleavage^^. The rela-
tively easy oxidation of Fe-protoporphyrin IX,
which serves as an additional buffer supply of
electrons to the dioxygen ligand, is a common
feature in the mechanisms of related heme enzyme
families, such as peroxidases, heme catalases,
cytochromes P450, NOS, and peroxygenases,
which catalyze 0-0 bond cleavage of a peroxide
ligated to the heme iron^^'
^K
Donation of one elec-
tron from the porphyrin to the peroxide ligand ini-
tiates the effective heterol3^ic scission of the O-O
bond and results in formation of a cation radical
on the porphyrin ring, a definitive feature of a
"Compound I" state^^
Oxygen activation in all of these systems
begins with the binding of dioxygen as an axial
ligand to the Fe^+ heme iron, or with binding
of
H2O2
to the Fe^^ heme. Thereby hydroperoxo-
ferric heme complex is formed through different
pathways as a common intermediate state, shown
in Figure 5.1. Thus, a current focus of research
is this common "peroxo," both in the pathways
for its formation as well as in the control of
P450 Monoxygenase ©
Oxidase ©
Heme Oxygenase ®-
Peroxygenase ©
Peroxfdase/Catalase ©
-MD
©
Compound
ft
O
ferrous dioxygen
02^J
Nucleophiiic
Oxidations
peroxo-mmon
N N
/ Fei"/L.
© ©
Etectrophiiic
QI
Nucleophlfic
Oxidations
/ Fe^v/
N f-~n
N-AH2
ferric hydroperoxo Compound i
\^
N f-N
^' »X
Fell'/—^
H9O,
/
Fe^
V^
N f-N
o
N4J
N
\
N
h-N
H,0 tl
®
2e .
2HU
rvH:>o
Radical
Oxidations
©
Figure 5.1. Common reactive intermediates in bioinorganic catalysis. The catalytic pathways of various heme
enzymes are shown in the inset.

152
Thomas M. Makris et al.
proton delivery events, which dictate the inherent
reactivity of the resulting "active oxygen" inter-
mediates. In the classic P450 reactivities of a
"Compound I like" intermediate, it is also this
critical acid-base chemistry which initiates dioxy-
gen bond scission to form the higher valent
iron-oxo state.
In addition, there are other redox active
pathways operant in the cytochromes P450
(Figure 5.1). First is an "oxidase" pathway using
atmospheric dioxygen and two additional redox
equivalents in addition to the two required for
heterolytic 0-0 bond cleavage. Additionally there
is a "peroxidase/peroxygenase" pathway wherein
hydrogen peroxide serves as an oxygen donor.
A detailed analysis of these pathways with respect
to oxygen activation in the cytochromes P450
was provided in earlier editions of
this
volume^' ^.
Obviously, these two mechanisms differ by the
overall redox state of oxygen vs peroxide and the
need in two additional reduction steps (one-
electron reductions by the physiological redox
partner) in the oxidase pathway. Perhaps most
important, however, is the difference in the proto-
nation states that necessitate specific delivery of
two protons to a bound peroxo intermediate when
beginning with dioxygen as opposed to a 1-2
proton shift to form the transient Fe-0-0H2 pre-
cursor when starting with hydrogen peroxide and
ferric heme.
2.1.
The Oxidase/Oxygenase
Pathway in Cytochrome
P450
The pivotal role of different protonation path-
ways to bound oxygen derivatives in bioinorganic
chemistry has been shown in various mechanistic
studies ^^' ^^' ^^. The same concept has also been
considered for the case of catalysis involving
H2O2,
in which the correlation between the ability
to efficiently deliver the proton to the metal-
bound hydroperoxide anion, and the efficiency of
catalytic decomposition of hydrogen peroxide has
been noted^'*. In heme-enzyme catalysis, however,
the ability to supply the necessary one or two
protons for the generation of the proper active
intermediate from the iron-bound peroxide/
hydroperoxide anion state is an essential and
crucial aspect of the detailed structural "fine-
tuning" provided by the enzyme active center.
Thus,
the relative spatial orientation of catalytic
residues and active-site water molecules may
facilitate or suppress various branches of the
microscopic pathways that contribute to the possi-
ble chemical reactions involving these activated
oxygen compounds. An encompassing scheme
that graphically illustrates the commonality of
these various processes is depicted in Figure 5.1.
Many laboratories have focused on the role of
protein structure in defining the pathways and
control points outlined in Figure 5.1. Recent
results, which are discussed in this chapter, have
greatly improved the understanding of these
events. For example, a hydrogen-bond network
has been implicated as the critical component of
the enzymatic mechanism which defines both cat-
alytic activity and the effective branching points
between productive and nonproductive pathways
for the camphor hydroxylation catalyzed by
cytochrome P450 CYPIOP^-^^ Modification of
this network, either through mutagenesis of the
CYPlOl enzyme, or by the use of substrate
analogs, points to the subtle active-site features of
the protein, which can in some cases have dire
consequences on the productive monooxygenase
pathway with resultant abortive processes that
short circuit the classic P450 mechanistic "wheel"
(Figure 5.2). These additional pathways, since
they bleed redox equivalents and dioxygen from
the pure monooxygenase stoichiometry, have been
termed "uncoupling" events. While the mecha-
nisms of uncoupling may have either a thermody-
namic and/or kinetic origin, it is most certainly
governed by the role of a protein-provided "proton
relay" system. These pathways of proton delivery
can involve not only backbone and side-chain
moieties from the protein structure, but also local-
ized water molecules, which are appropriately
stabilized in the active center of the enzyme.
The realization of how Nature uses specialized
water alludes to analogous discussion of proton
delivery in diverse enzymes such as cytochrome
oxidase"^^' ^^, and introduces these concepts into
the vernacular of P450 specialists. Again, geno-
mics and proteomics have helped identify critical
structural features, for instance the famous "acid-
alcohol pair," Asp251-Thr252 in the cytochrome
P450 CYPIOI. Only most recently, however, has
structural information shed critical light on how
these residues work in concert to deliver protons
at the right place and time.
Activation of Molecular Oxygen by Cytochrome P450
153
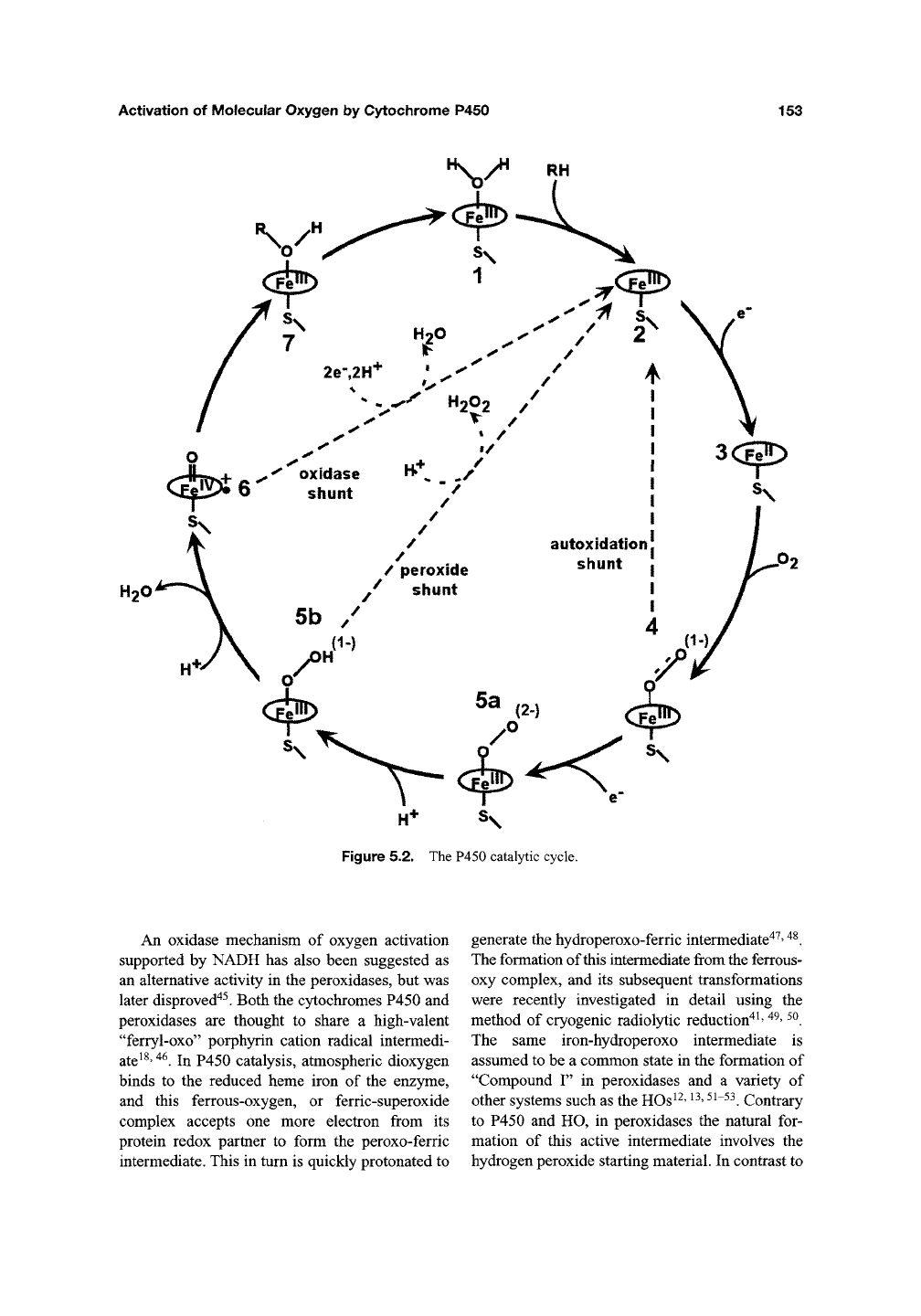
Figure 5.2. The P450 catalytic cycle.
An oxidase mechanism of oxygen activation
supported by NADH has also been suggested as
an alternative activity in the peroxidases, but was
later disproved"^^. Both the cytochromes P450 and
peroxidases are thought to share a high-valent
"ferryl-oxo" porphyrin cation radical intermedi-
ate^^'
^^.
In P450 catalysis, atmospheric dioxygen
binds to the reduced heme iron of the enzyme,
and this ferrous-oxygen, or ferric-superoxide
complex accepts one more electron from its
protein redox partner to form the peroxo-ferric
intermediate. This in turn is quickly protonated to
generate the hydroperoxo-ferric intermediate"*^'
^^.
The formation of this intermediate from the ferrous-
oxy complex, and its subsequent transformations
were recently investigated in detail using the
method of cryogenic radiol3^ic reduction"*^'
^^'
^^.
The same iron-hydroperoxo intermediate is
assumed to be a common state in the formation of
"Compound F' in peroxidases and a variety of
other systems such as the HOs^^'
^^' ^^~^^.
Contrary
to P450 and HO, in peroxidases the natural for-
mation of this active intermediate involves the
hydrogen peroxide starting material. In contrast to
