Ojima I. (ed.) Fluorine in Medicinal Chemistry and Chemical Biology
Подождите немного. Документ загружается.

Recent Advances in the Syntheses of Fluorinated Amino Acids 237
synthon for the hydroxymethyl group. The arylsulfi nylmethylene moiety is introduced to,
for example, acyl halides, imines, and imidoyl halides by diastereoselective nucleophilic
addition of arylsulfi nylmethyl lithium. Then, nonoxidative desulfi nylation followed by
oxidation or conventional desulfi nylation leads to the synthesis of enantio - enriched amino
acids (see Scheme 9.43 ).
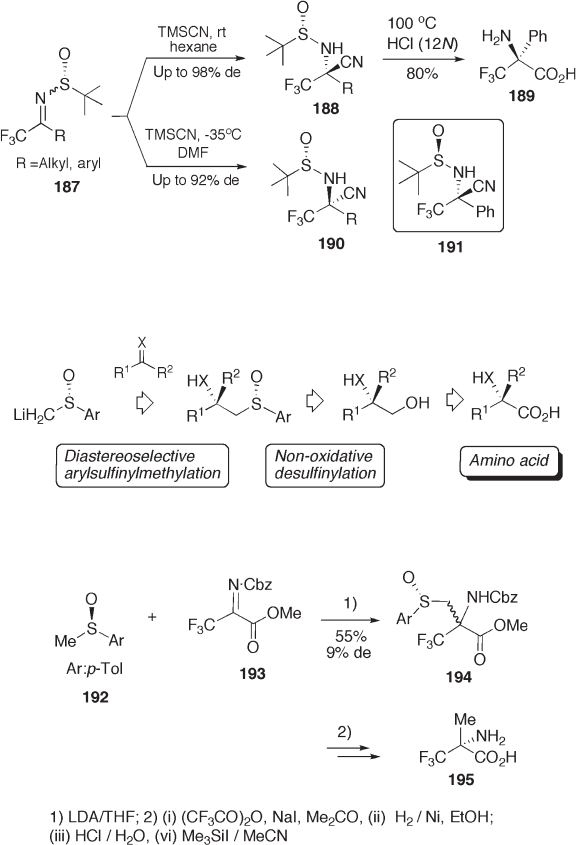
A typical example is shown in Scheme 9.44 . Arylsulfi nylmethyl lithium is
introduced diastereoselectively to imine 193 to give 194 . Then, enantio - enriched
Scheme 9.42
Scheme 9.43
Scheme 9.44
238 Fluorine in Medicinal Chemistry and Chemical Biology
Scheme 9.45
Scheme 9.46
2 - trifl uoromethylalanine 195 is synthesized, where arylsulfi nyl group is transformed to a
methyl group [71] .
Another example is shown in Scheme 9.45 , in which arylsulfi nylmethyl lithium
couples with polyfl uoroalkylimidoyl chlorides 197 . Diastereoselective reduction of imine
198 to amine 199 followed by nonoxidative desulfi nylative hydroxylation and oxidation
of hydroxymethyl group provides trifl uoro - and difl uoroalanines 202 (see Scheme 9.45 )
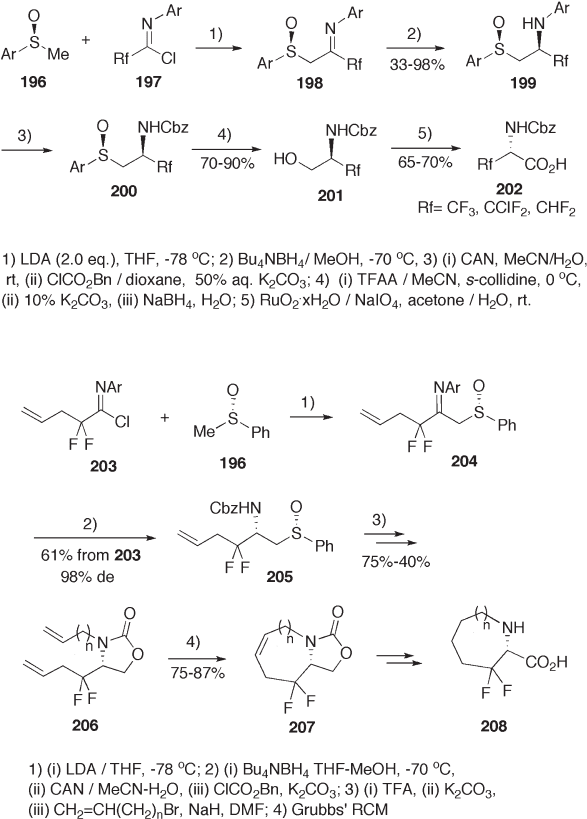
[72] . The same protocol is applicable for the synthesis of 3,3 - difl uorocyclic amino acids
208 (see Scheme 9.46 ) [72, 73] . The borohydride reduction of sulfi nylimine 204 proceeds
with almost complete diastereoselectivity ( > 98% de). The conventional chemical modifi -
Recent Advances in the Syntheses of Fluorinated Amino Acids 239
cation and Grubbs ’ RCM afford bicyclooxazolidone 207 , which is fi nally transformd to
208 . Starting from sulfi nylketone 209 , enantiomerically pure difl uoroalanine 213 is syn-
thesized by the same sequence of reactions as shown in Scheme 9.47 [74] . Stereospecifi c
nucleophilic substitution of the hydroxyl group in sulfi nyl alcohol 214 [75] with azide ion
and subsequent reduction of the azide group with thiol gave sulfi nyl amine 216 . The non-
oxidative desulfi nylation followed by oxidation of the hydroxymethyl group afforded
monofl uoroalanine 218 (see Scheme 9.48 ) [76] .
9.3.1.8 Menthyl Group as a Chiral Auxiliary
The menthyl group has often been employed as an easily available chiral auxiliary.
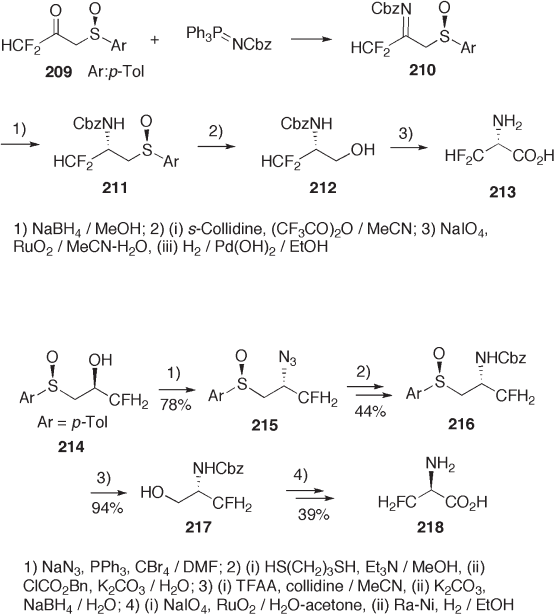
8 - Phenylmenthyl 2 - amino - 3,3 - difl uorocyclopentenecarboxylate 222 prepared from 219
via 221 was hydrogenated diastereoselectively to form cis - 2 - aminocarboxylate 223
(see Scheme 9.49 ) [77] . The higher diastereoselectivity induced by the 8 - phenylmenthyl
group was also observed in ZnI
2
- catalyzed NaBH
4
reduction of open - chain β - amino - α , β -
unsaturated esters 224 . In contrast, the unsubstituted menthyl ester was reduced with no
practical diastereoselectivity (see table in Scheme 9.49 ).
Scheme 9.47
Scheme 9.48

240 Fluorine in Medicinal Chemistry and Chemical Biology
A similar approach was employed for the synthesis of racemic m = 1 and higher
members of cyclic β - amino esters 229 starting from 227 (see Scheme 9.50 ) [78] .
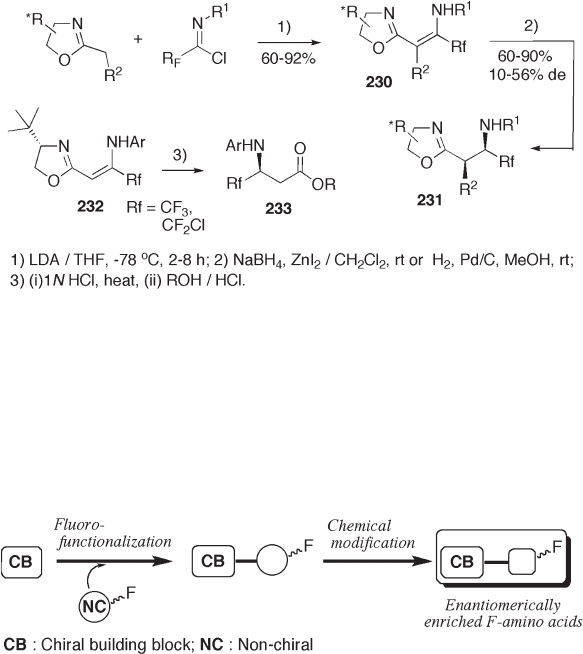
The oxazoline moiety is a masked carboxyl group. A chiral oxazoline moiety attached
to enamine 230 confers chirality to the double bond on reduction. Separation of the dia-
stereomers followed by hydrolysis of the oxazoline moiety provide 3 - amino - 4,4 - difl uoro -
or 3 - amino - 4,4,4 - trifl uorobutanoates 233 in enantiomerically pure forms (see Scheme
9.51 ) [79] .
Scheme 9.49
Scheme 9.50
Recent Advances in the Syntheses of Fluorinated Amino Acids 241
9.3.2 Building Blocks
Chiral fl uorinated building blocks are in general less readily available, so that fl uoro -
functionalization of available chiral building blocks is one feasible approach for the
asymmetric synthesis of fl uorinated amino acids (see Fig. 9.2 ).
Scheme 9.51
Figure 9.2 Chiral building block approach.
9.3.2.1 Garner ’ s Aldehyde
Garner ’ s aldehyde is readily available from serine. Synthetic processes so far reported for
fl uoroamino acids mostly consist of (1) fl uoro - functionalization of the Garner ’ s aldehyde
at the formyl group, (2) acid - catalyzed hydrolytic opening of the oxazoline ring, and (3)
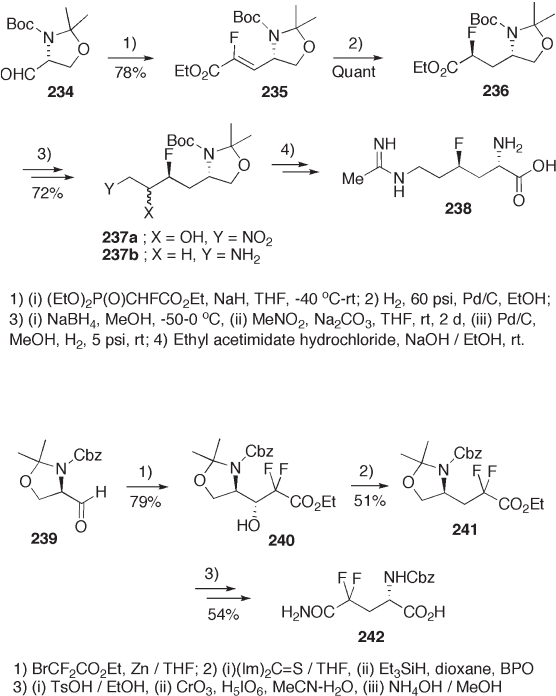
oxidation of the hydroxymethyl group to a carboxyl group. Reaction of 234 with ethyl 2 -
fl uoro - 2 - phosphonoacetate followed by diastereoselective hydrogenation of 235 provides
monofl uoroester 236 . A sequence of reactions – condensation of 236 with nitromethane,
catalytic reduction of 237a , reaction of 237b with acetimidate, and transformation of the
oxazoline ring to an α - aminocarboxyl moiety – synthesize 4 - fl uoro - L - lysine 238 (see
Scheme 9.52 ) [80] . 4,4 - Difl uoroglutamine 242 is synthesized similarly (see Scheme 9.53 )
[81] .
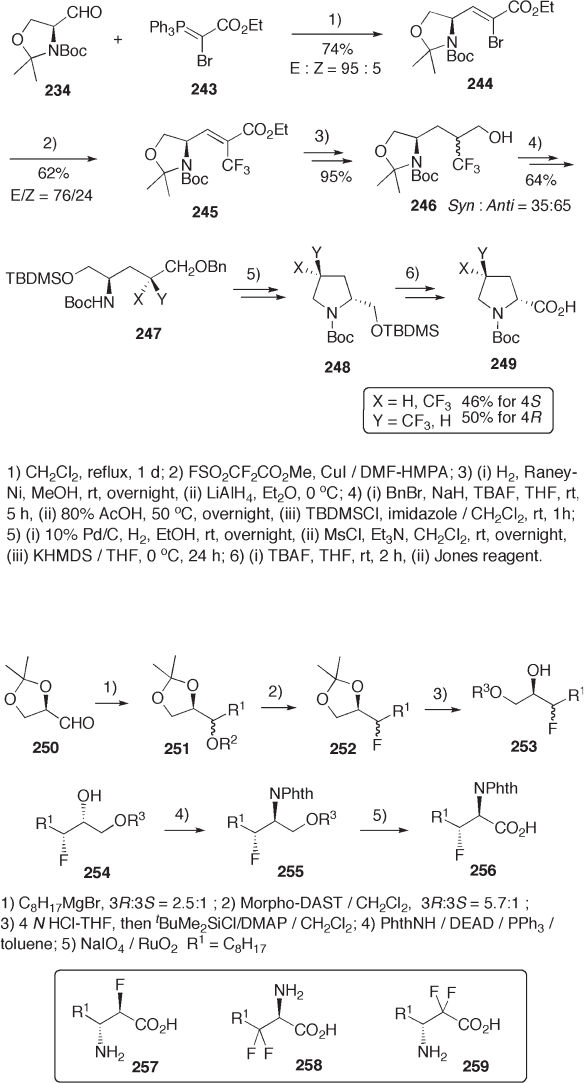
The aldehyde 234 is also applicable for construction of the proline skeleton. The
oxazoline moiety of 234 was transferred to the N - CHCO
2
H moiety of the prolines 249 ,
the trifl uoromethyl group of which was introduced by cross - coupling of bromide 244 with
trifl uoromethyl copper reagent (see Scheme 9.54 ) [82] .
242 Fluorine in Medicinal Chemistry and Chemical Biology
Scheme 9.52
Scheme 9.53
9.3.2.2 ( R ) - 2,3 - O - Isopropylideneglyceraldehyde
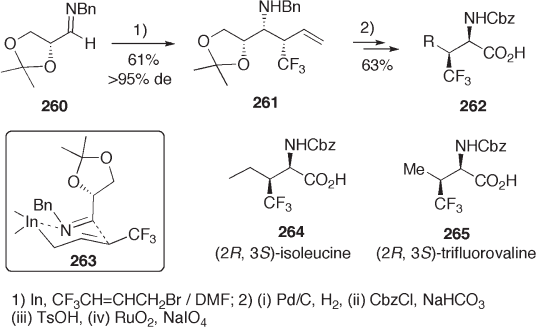
( R ) - 2,3 - O - Isopropylideneglyceraldehyde 250 (see Scheme 9.55 ) is also one of the most
readily available chiral building blocks for amino acid synthesis. Here again, a similar
sequence to that with Garner ’ s aldehyde is used: fl uoro - functionalization at the formyl
group and oxidation to carboxyl group of either the secondary or the primary hydroxyl
group involved in the masked glyceraldehydes 250 . The Mitsunobu protocol for transfor-
mation of a hydroxyl group to an amino group is used as a key reaction for synthesis of
fl uorinated amino acids [83] .
In the synthesis of 3 - fl uoroamino acid 256 (Scheme 9.55 ), fl uorination of alcohol 251
(R
2
= H) with morpho - DAST afforded the desired alcohol only in low yield (10 – 25%).
The same reaction of trimethylsilyl ether 251 (R
2
= TMS) improved the yield (50%). The
Mitsunobu amination of the secondary hydroxyl group in 254 successfully gives 255 in
89% yield. Starting from 250 , several fl uorinated α - and β - amino acids 257 – 259 have
been prepared [31] .
Recent Advances in the Syntheses of Fluorinated Amino Acids 243
Scheme 9.54
Scheme 9.55
244 Fluorine in Medicinal Chemistry and Chemical Biology
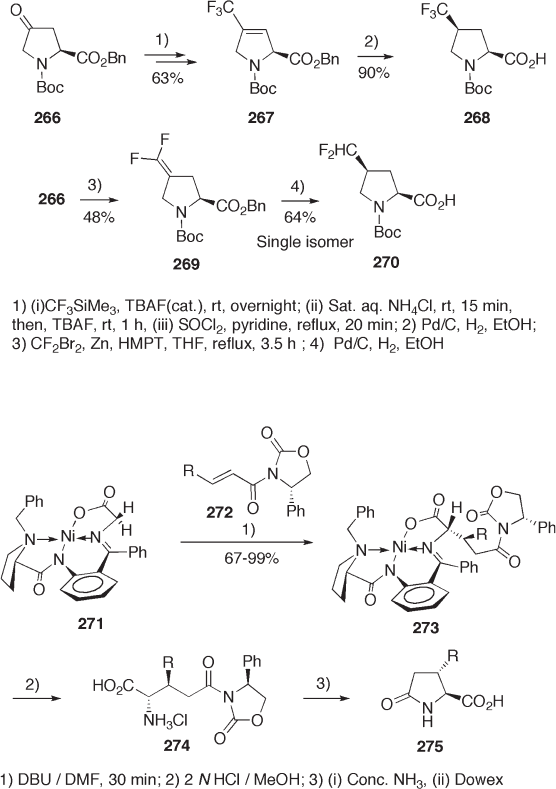
The benzyl imine 260 of aldehyde 250 is an excellent building block for the synthesis
of enantiomerically pure trifl uoromethylated isoleucine 264 and valine 265 (see Scheme
9.56 ). The In - mediated alkylation of imine 260 with 4,4,4 - trifl uorocrotyl bromide in DMF
proceeds with excellent diastereoselectivity ( > 95% de), affording 261 . In contrast, poor
diastereoselectivity (20% de) is obtained in the same In - mediated aldol reaction of alde-
hyde 250 . The transition state structure 263 is proposed to explain the exclusive stereo-
control [84] .
9.3.2.3 Proline Derivatives
Diastereoselective fl uoro - functionalization of proline derivatives produces fl uorinated pro-
lines. Both enantiomerically pure 4 - trifl uoromethyl and 4 - difl uoromethyl prolines 268 and
270 have been prepared (see Scheme 9.57 ) [85] . The key reaction for the stereo - controlled
synthesis is diastereoselective Pd - catalyzed hydrogenation of 267 and 269 .
Nickel(II) complex 271 of the Schiff bases of glycine with o - [ N - α - pycolylamine]be
nzophenone plays two roles in stereocontrolled C – C bond formation; an excellent chiral
auxiliary and a building block. Its diastereoselective Michael addition to 272 occurs
cleanly at the α - position of the glycine moiety, affording 273 almost quantitatively. The
adduct 273 is hydrolyzed by acid - catalysis to give 274 , which is then cyclized to pyroglu-
tamic acid 275 [86] . During the two hydrolysis steps, both o - [ N - α - pycolylamine]benzoph
enone and ( S ) - 5 - phenyl - 2 - oxazolidone can be recovered for recycling (see Scheme
9.58 ).
9.3.3 Other Diastereoselective Syntheses
Other interesting diastereoselective syntheses of fl uorinated amino acids are briefl y sum-
marized in this section.
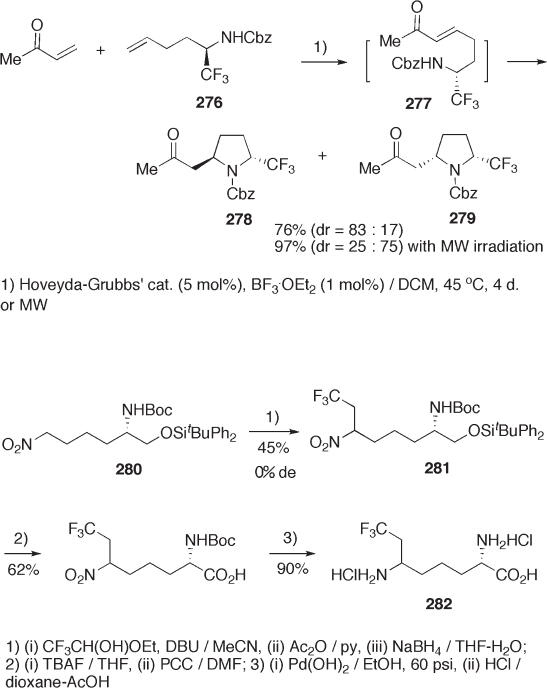
Tandem alkene metathesis – intramolecular Michael addition of ( R ) - amine 276 pro-
ceeds diastereoselectively, providing pyrrolidines 278 and 279 . These pyrrolidines can be
Scheme 9.56
Recent Advances in the Syntheses of Fluorinated Amino Acids 245
Scheme 9.57
Scheme 9.58
promising precursors of 5 - CF
3
- prolines and the related amino acids (see Scheme 9.59 )
[87] .
Introduction of a trifl uoroethyl group at the α - carbon of the nitro group of 280 by a
sequence of reactions – (1,5 - diazabicyclo[4.3.0]non - 5 - ene) (DBU) - catalyzed alkylation
with trifl uoroacetaldehyde hemiacetal in MeCN, dehydration with acetic anhydride – pyri-
dine, and reduction of the C – C double bond with NaBH
4
– produces 281 . 6 - Trifl uoroethyl -
l - lysine 282 can be prepared from 281 (see Scheme 9.60 ) [88] .
Diastereoselective fl uorination of enolate 283 , subsequent epoxidation of 284 , and
separation of diastereomers provide 285 . Activation of epoxy - oxygen with Et
2
AlCl
and deprotonation from the CHF group in 285 with LDA induces stereospecifi c
246 Fluorine in Medicinal Chemistry and Chemical Biology
cyclopropanation, affording bicyclo product 286 , which is further transformed to bicyclic
α - amino acid 287 (see Scheme 9.61 ) [89] .
9.4 Racemic Amino Acids
In this section, recent advances in the syntheses of racemic fl uorinated amino acids and
the related peptides that involve conceptually new synthetic designs are briefl y
summarized.
Base - catalyzed deprotonation – alkylation of trifl uoroalanine is not easy because α -
trifl uoromethylated carbanions readily undergo defl uorination in general [90] . Therefore,
α - alkylated trifl uoroalanines have been prepared either by alkylation of imines obtained
from trifl uoropyruvates [54, 57] or by Strecker cyanation [56] to trifl uoroketimines fol-
Scheme 9.59
Scheme 9.60
