Ojima I. (ed.) Fluorine in Medicinal Chemistry and Chemical Biology
Подождите немного. Документ загружается.

Recent Advances in the Syntheses of Fluorinated Amino Acids 227
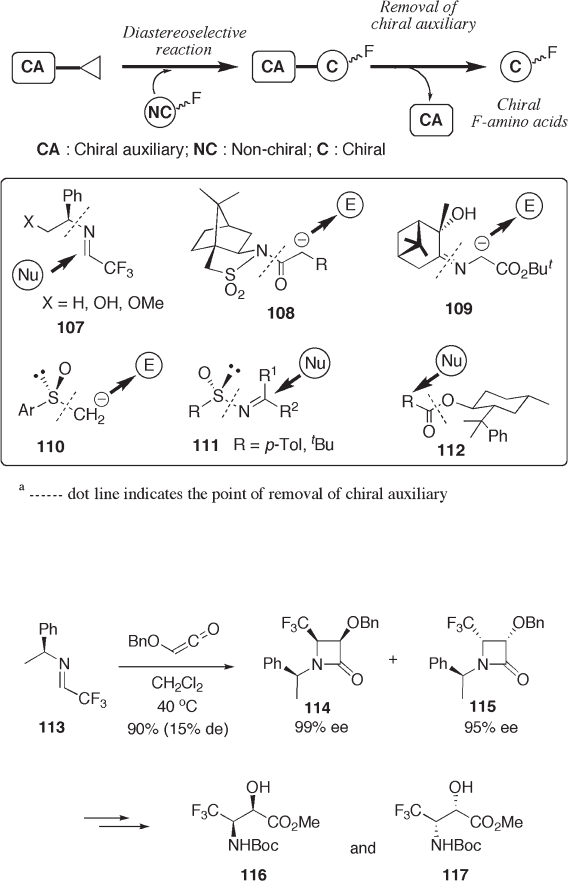
aldimines and ketoimines bearing a fl uorine - functionality. The corresponding aldimine 113
of trifl uoroacetaldehyde undergoes cycloaddition or electrophilic addition with nucleo-
philes. Cycloaddition of 113 with ketene provides highly enantiomerically enriched tri-
fl uoromethylated lactams 114 and 115 , although diastereoselectivity is poor [49] . The
lactams are transformed into syn - 2 - hydroxy - 3 - aminobutanoates 116 and 117 , respectively
(see Scheme 9.24 ).
Figure 9.1 Chiral auxiliary approach. The dotted lines indicate the point of removal of the
chiral auxiliary.
Scheme 9.24
228 Fluorine in Medicinal Chemistry and Chemical Biology
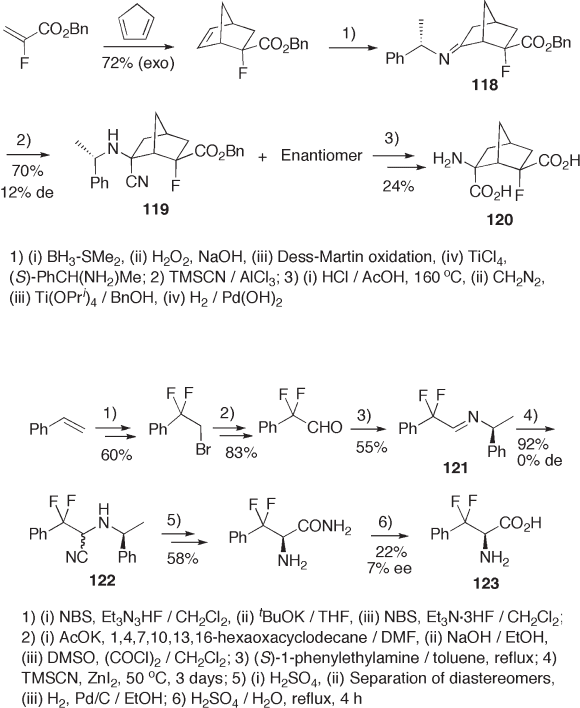
Likewise, imine 118 was transformd into 120 , a mimic of a conformationally rigid
glutamic acid, via cyanide 119 (see Scheme 9.25 ) [50] . 1 - Phenyl - 1,1 - difl uoroacetaldehyde
imine 121 was also used as a precursor of 3,3 - difl uorophenylalanine 123 via cyanide 122 ,
but no diastereoselectivity was observed in the cyanation step (see Scheme 9.26 ) [51] .
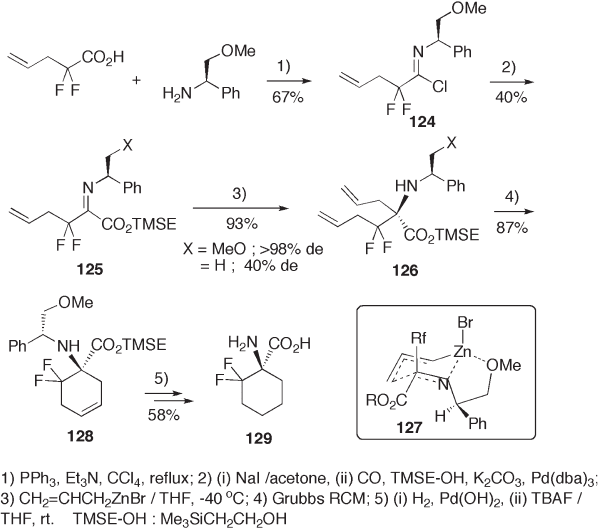
The 2 - methoxy group of 2 - methoxy - 1 - phenylethylamine sometimes plays an impor-
tant role in diastereoselection. Thus, imine 125 (X = OMe), which is prepared convention-
ally from imidoyl chloride 124 , undergoes allylation with allylzinc reagent in a
diastereoselective manner ( > 98% de), affording 126 in a high yield. On the other hand,
nonmethoxylated imine 125 (X = H) gives the product with a poor diastereoselectivity
(40% de) (see Scheme 9.27 ). The methoxy group will chelate with the zinc atom tightly,
making the transition state 127 favorable for the stereo - controlled allylation [52, 53] .
However, the effect of the stereoselectivity enhancement by the methoxy group is not
necessarily applicable for the stereochemical outcome of the Strecker - type cyanation of
Scheme 9.25
Scheme 9.26
Recent Advances in the Syntheses of Fluorinated Amino Acids 229
the Schiff base of N - (1 - phenyl - 2 - methoxyethyl)amine with trifl uoromethyl ketones since
the reaction of the Schiff base with TMS - cyanide is strongly affected not only by substitu-
ent (X) but also by Lewis acid [54] . Grubbs ’ ring - closing metathesis (RCM) of 126 leads
to cyclohexene skeleton 128 , which is fi nally converted to 1 - amino - 6,6 - difl uorocyclohex-
ane - 1 - carboxylic acid 129 . Fluoride ion - promoted desilylation is useful for the deprotec-
tion of the trimethylsilylethyl group (TMSE) from 2 - (trimethylsilyl)ethyl carboxylate 128
under mild conditions.
9.3.1.2 Chiral Oxazolidines as Auxiliaries
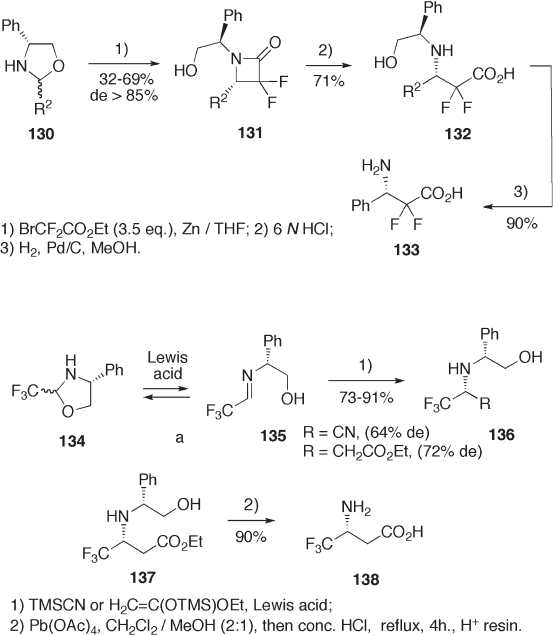
Oxazolidine 130 is a masked aldimine bearing a chiral N - (2 - hydroxy - 1 - phenyl)ethyl
moiety and is readily available from ( R ) - phenylglycinol. Mannich reaction of 130 with
Reformatsky reagent of ethyl bromodifl uoroacetate produces difl uorolactam 131 in high
diastereoselectivity, which is then transformed to enantio - enriched ( S ) - 3 - amino - 2,2 -
difl uoro - 3 - phenylpropanoic acid 133 (see Scheme 9.28 ) [55] .
Oxazolidine 134 is a masked trifl uoroacetaldehyde imine that generates in situ the
corresponding imine 135 under Lewis acid catalysis conditions. Lewis acid - catalyzed
reactions of 134 with TMS - cyanide and ketene silylacetal provide adducts 136 in high
yields with good diastereoselectivities (see Scheme 9.29 ) [56] . Conventional chemical
transformation of 137 produces 3 - amino - 4,4,4 - trifl uorobutanoic acid 138 . Similarly, tri-
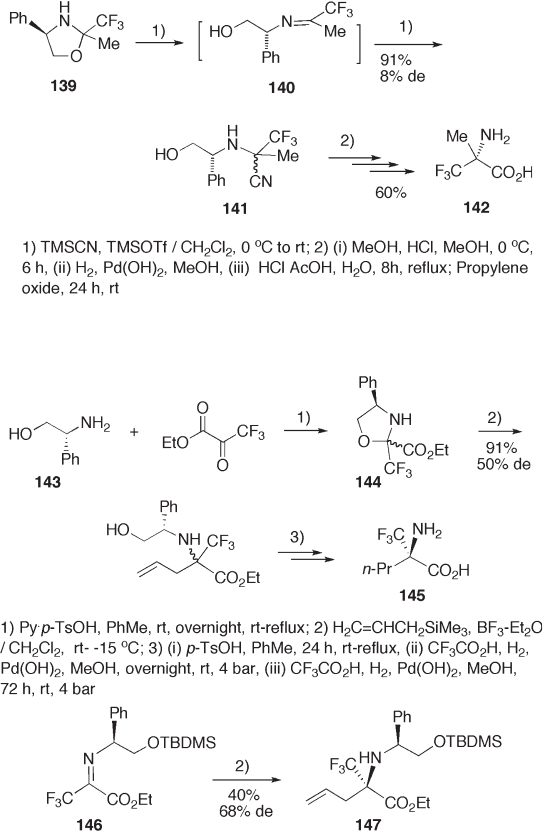
fl uoroacetone oxazolidine 139 is used for the synthesis of 2 - trifl uoromethylalanine 142
Scheme 9.27
230 Fluorine in Medicinal Chemistry and Chemical Biology
via 141 . The stereochemistry of C - 2 in 139 does not affect diastereoselectivity in cyanation
to ketimine 140 (see Scheme 9.30 ) [54] .
Oxazolidine 144 obtained from amino alcohol 143 and ethyl trifl uoropyruvate is also
a synthetic intermediate for 2 - amino - 2 - trifl uoromethylpentanoic acid 145 . Lewis acid -
catalyzed allylation of 144 with allyl silane occurs in excellent yield with a moderate ste-
reoselectivity. Meanwhile, O - tert - butyldimethylsilyl - protected imine 146 gives better
diastereoselectivity although yield is poor (see Scheme 9.31 ) [57] .
A 1 - phenylethylamino moiety is used for diastereomeric control not only in addition
of nucleophiles to N - (1 - phenylethyl)imines but also in diasteroselective Michael addition
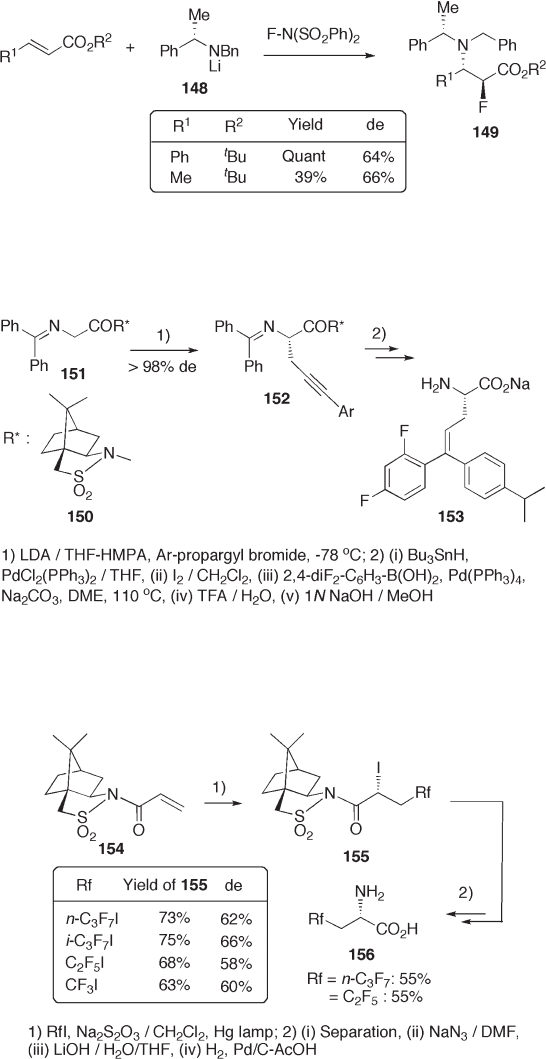
to α , β - unsaturated esters. Thus, lithium N - (1 - phenylethyl) - N - benzylamide 148 is employed
for a one - pot tandem Michael addition - fl uorination reaction (see Scheme 9.32 ) [58] . The
reaction provides anti - 3 - amino - 2 - fl uoroesters 149 exclusively, whose diastereoselectivi-
ties (64 – 66% de) to the chiral carbon of the 1 - phenylethyl - group are good enough.
9.3.1.3 (2 R ) - Bornane - 10,2 - sultam as an Auxiliary
Oppolzer ’ s (2 R ) - bornane - 10,2 - sultam 150 is a good auxiliary that transmits its chirality
to the α - carbon of an amide. The reaction of lithium enolate of 151 with the corresponding
Scheme 9.28
Scheme 9.29
Recent Advances in the Syntheses of Fluorinated Amino Acids 231
Scheme 9.30
Scheme 9.31
propargyl bromide affords 152 with excellent diastereoselectivity (see Scheme 9.33 ) [59] .
The subsequent reactions starting from 151 – hydrostannylation, iodine – tin exchange, and
Pd - catalyzed Suzuki – Miyaura coupling with aryl borane of 151 – furnish a total synthesis
of 153 , 5,5 - diaryl - 2 - amino - 4 - pentenoate, a novel class of biologically active molecules
targeted toward the recently cloned glycine reuptake transport system.
The radical perfl uoroalkyl - iodination reaction of α , β - unsaturated lactam 154 pro-
ceeds diastereoselectively. The stereospecifi c azide formation and subsequent chemical
transformation of the ( R ) - isomer result in the synthesis of a series of perfl uoroalkylated
α - amino acids 156 (see Scheme 9.34 ) [60] .
232 Fluorine in Medicinal Chemistry and Chemical Biology
Scheme 9.32
Scheme 9.33
Scheme 9.34
Recent Advances in the Syntheses of Fluorinated Amino Acids 233
9.3.1.4 2 - Hydroxy - 3 - Pinanone as an Auxiliary
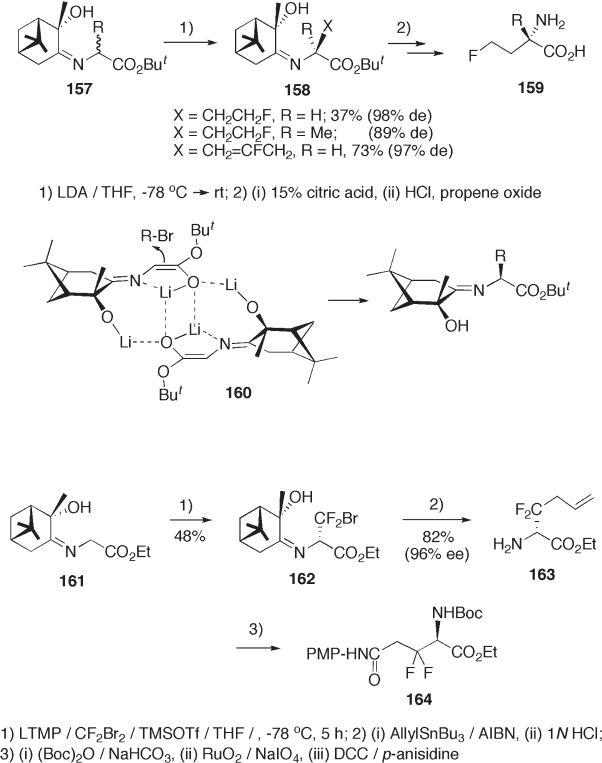
Alkylation of the Schiff base of glycine with 2 - hydroxy - 3 - pinanone proceeds in an
extremely diastereoselective manner. Thus, fl uoro - functionalization on the α - carbon of
the Schiff base followed by hydrolysis provides fl uorinated α - amino acids in a highly
enantiomerically enriched form. 2 - Fluoroethylation and 2 - fl uoroallylation of 157 (see
Scheme 9.35 ) and bromodifl uoromethylation of 161 (see Scheme 9.36 ) give the desired
adducts 158 and 162 , respectively, with excellent diastereoselectivities. Lithium enolate
dimer 160 has been proposed as a reactive intermediate for the stereocontrolled alkylation
[61] . The adducts 158 and 162 were transformed to 4 - fl uoro - 2 - amino acids ( > 96% ee) 159
[61] and 3,3 - difl uoroglutamine 164 [62] , respectively.
Scheme 9.35
Scheme 9.36
234 Fluorine in Medicinal Chemistry and Chemical Biology
Scheme 9.37
9.3.1.5 Oxazolidinone as an Auxiliary
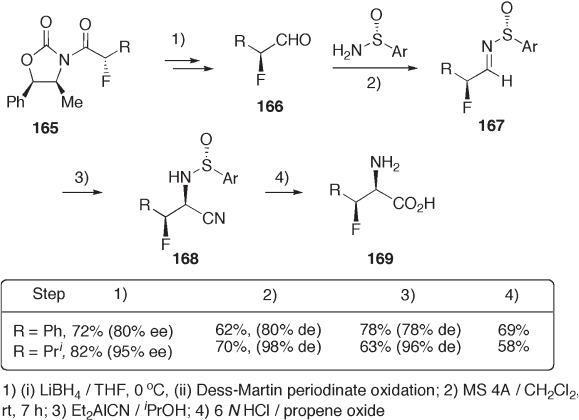
4 - Substituted 2 - oxazolidones 165 are useful chiral auxiliaries for diastereoselective func-
tionalization at the α - carbon of their amide carbonyl group. The α - fl uoroaldehydes 166 were
prepared by a series of reactions: electrophilic fl uorination of the corresponding oxazolidi-
none sodium enolates with N - fl uorobenzenesulfonimine; reductive removal of the auxiliary
with LiBH
4
; and Dess – Martin oxidation. The aldehydes are so unstable for isolation that they
are converted with ( R ) - p - toluenesulfi namide to p - toluenesulfi nimines 167 , which are isol-
able and satisfactorily enantio - enriched. Chiral sulfi nimine - mediated diastereoselective
Strecker cyanation with aluminum cyanide provided cyanides 168 in excellent diastereose-
lectivity, which were fi nally derived to 3 - fl uoroamino acids 169 (see Scheme 9.37 ) [63] .
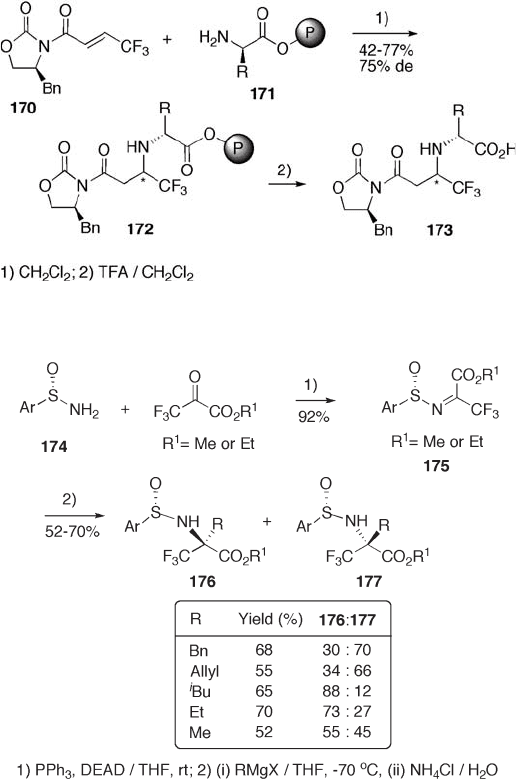
Diastereoslective Michael addition of the amino group in the polymer - supported
amino ester 171 to 4,4,4 - trifl uorocrotonamide 170 is another application of 4 - substituted
2 - oxazolidone for fl uoroamino acid synthesis (see Scheme 9.38 ) [64] .
9.3.1.6 Chiral Sulfi namides as Auxiliaries
Both ( S ) - p - toluenesulfi namide 174 [65] and ( R ) - tert - butylsulfi namide 182 (see Scheme
9.41 ) [66] are used for amino acid synthesis. Diastereoselective alkylation to their imines
is a key reaction for the creation of chiral amines.
Sulfi mines 175 are obtained in excellent yield by Staudinger condensation of sulfi n-
amide 174 with trifl uoropyruvates. In contrast, and quite surprisingly, the Staudinger
reaction of tert - butylsulfi namide 182 with the pyruvates under the same conditions does
not work. Sulfi mines 175 are alkylated with Grignard reagents in good yields but with
poor to moderate diastereoselectivities (see Scheme 9.39 ) [67] .
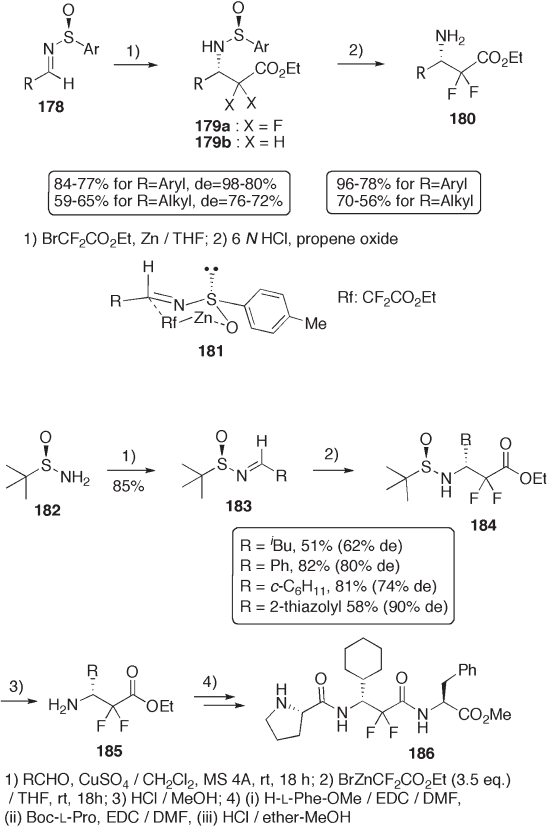
Meanwhile, Reformatsky reaction of sulfi nimines 178 with bromodifl uoroacetate
provides the adducts in excellent diastereoselectivities (see Scheme 9.40 ) [68] . The ste-
Recent Advances in the Syntheses of Fluorinated Amino Acids 235
Scheme 9.38
Scheme 9.39
reochemistry of ( S ) at C - 3 and the high diastereoselectivity arise from the six - membered
transition state shown in 181 . The same reaction with the corresponding nonfl uorinated
Reformatsky reagent (bromoacetate) provides 3 - aminoester with ( R ) - confi guration at C - 3
for 179b (R = aryl, 76%, 94% de), demonstrating that the fl uorine atoms of Reformatsky
reagent have no effect on the stereochemical outcome of the C – C bond formation. The
acid - catalyzed hydrolysis of the sulfi namide 179a produces enantiomerically enriched 3 -
amino - 2,2 - difl uorocarboxylates 180 [68] .
Likewise, tert - butylsulfi namide is also used for highly stereocontrolled syntheses of
3 - amino - 2,2 - difl uorocarboxylates 185 (see Scheme 9.41 ) [69] and 2 - phenyl - 3,3,3 - trifl uo-
roalanine 189 (see Scheme 9.42 ) [70] . Surprisingly, solvent is signifi cantly effective for
236 Fluorine in Medicinal Chemistry and Chemical Biology
Scheme 9.40
Scheme 9.41
the stereochemical outcome in the Strecker - type cyanation of 187 (see Scheme 9.42 ). The
use of ( R ) - sulfi namide provides the cyanide 191 with an opposite stereochemistry. Acid -
catalyzed hydrolysis of sulfi namides 184 and 188 generates amines 185 and 189 , respec-
tively. The difl uoroamino acid 185 is incorporated into oligopeptide 186 .
9.3.1.7 Optically Active Aryl Methyl Sulfoxide
The optically active arylsulfi nylmethylene moiety plays two important roles in asymmetric
synthesis of optically active amino acids: one is as chiral auxiliary, and the other is as
