Ojima I. (ed.) Fluorine in Medicinal Chemistry and Chemical Biology
Подождите немного. Документ загружается.

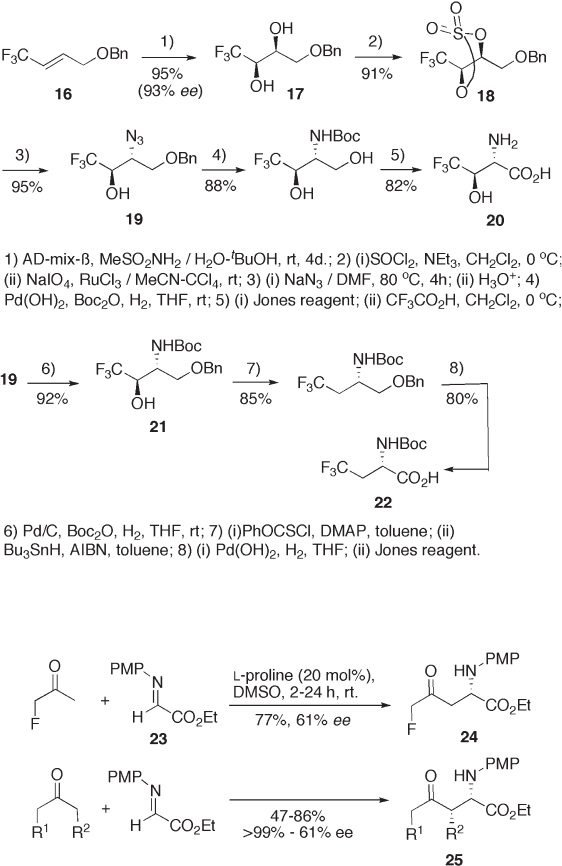
Recent Advances in the Syntheses of Fluorinated Amino Acids 217
9.8 shows two examples: one is synthesis of fl uorinated phenylalanines in which ben-
zylation to 26 proceeds in an excellent yield with almost perfect enantioselection under
dimeric Cinchona alkaloid phase - transfer catalysis ( α , α ′ - bis[ O (9) - allylcinchonidinum] -
o,m , or p - xylene) [20] ; the other is S
N
2 ′ reaction of 26 with 29 , which provides 30 with
moderate to good enantioselectivity [21] . Diastereoselective synthesis of fl uorinated amino
acids using 2 - hydroxypinanone glycine Schiff base is described in section 9.2 .
Scheme 9.6
Scheme 9.7
218 Fluorine in Medicinal Chemistry and Chemical Biology
Scheme 9.8
Scheme 9.9
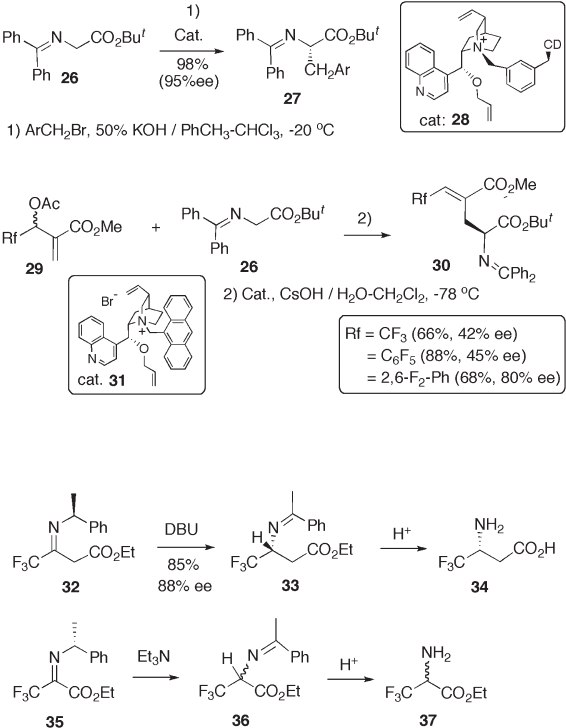
9.2.2 Chiral Transposition
Both enantiomers of α - phenethyl amines have most frequently been used as one of the
easily available and economically feasible chiral auxiliaries for diastereoselective synthe-
ses. Soloshonok developed a useful 1,3 - proton shift methodology in which chirality is
transposed concertedly from α - phenethyl amines to newly formed trifl uoromethyl amines.
This protocol is applicable for enantio - enriched amino acid synthesis, as shown in Scheme
9.9 [22] . The driving force for the proton shift is the thermodynamically lower stability
of trifl uoromethyl imine 32 than that of phenyl imine 33 [23] . Ketones and imines with a
strongly electron - withdrawing α - substituent such as the trifl uoromethyl group are, in
general, unstable and are transformed to the corresponding hydrate as a stable form on
exposure to water. The Schiff base of trifl uoropyruvate 35 readily undergoes 1,3 - proton
Recent Advances in the Syntheses of Fluorinated Amino Acids 219
shift with Et
3
N, but trifl uoroalanine derivative 36 was racemic. This undesired stereo-
chemical outcome would arise from facile racemization of 36 under the reaction conditions
due to the higher acidity of the methine proton of 36 than that of 33 [24] .
9.2.3 Introduction of Fluorine Functionality into Nonfl uorinated
Chiral Building Blocks
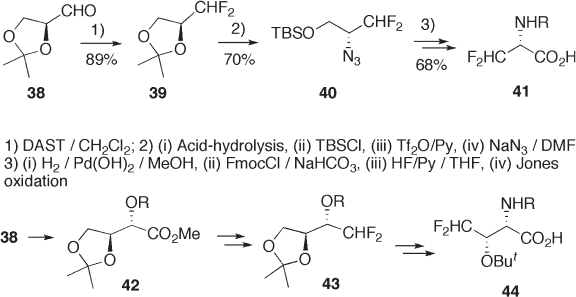
9.2.3.1 Introduction of Chirality by Stereospecifi c Nucleophilic Substitution
Stereospecifi c nucleophilic substitution of a chiral sec - hydroxyl group is sometimes reli-
able when the hydroxyl group is preactivated by a strongly electron - withdrawing group.
Some examples are shown in Schemes 9.10 , 9.11 , and 9.12 . sec - Trifl uoromethanesulfonate
derived from 38 undergoes stereospecifi c S
N
2 reaction with sodium azide, affording 40 ,
which is subsequently transformed to difl uoroalanine derivative 41 (see Scheme 9.10 ) [25] .
The carbomethoxy group of 42 is modifi ed to difl uoromethyl group of 43 via the formyl
group so that 43 can be transformed into 44 in a similar manner [25] .
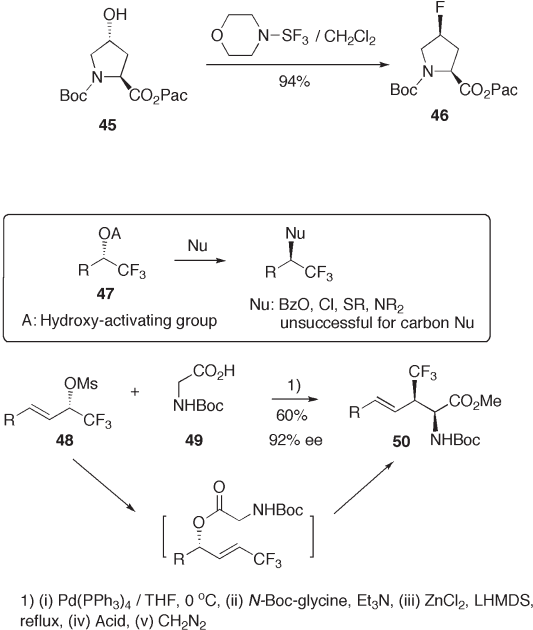
The hydroxyl group of sec - alcohols is, in general, replaced with a fl uorine atom ste-
reospecifi cally (inversion) by reaction with DAST [26] . Thus, the hydroxyl group of 45
can be replaced stereospecifi cally with fl uorine by morpho - DAST to give (4 S ) - fl uoropro-
line ester 46 (see Scheme 9.11 ) [27] . Its enantiomer (4 R ) - fl uoroproline, a mimic of (4 R ) -
hydroxyproline, which controls the thermal stability of the collagen - like triple - helical
structure [28] , is also prepared in a similar manner (79%) [27] .
Stereospecifi c nucleophilic substitution of the hydroxyl group of sec - trifl uorometh-
ylalcohol 47 with carbon nucleophiles has been a subject of active investigation; however,
until now no successful result has been reported, although the S
N
2 reaction of 47 with
some heteroatom nucleophiles is known [29] .
Konno et al. demonstrated that palladium catalysis accelerates the formal stereospe-
cifi c replacement of the sec - MsO group in 48 with a carbon nucleophile generated from N -
Boc - glycine 49 to give trifl uoromethylated amino acid derivatives 50 (see Scheme 9.12 )
[30] .
Scheme 9.10
220 Fluorine in Medicinal Chemistry and Chemical Biology
Scheme 9.11
Scheme 9.12
9.2.3.2 Introduction of Fluorine Functionality into Nonfl uorinated Chiral
Building Blocks
( R ) - Isopropylideneglyceraldehyde, Garner ’ s aldehyde, ( S ) - serine, and L - proline have been
most frequently employed as starting chiral building blocks. A fl uorine functionality is
introduced into the building blocks and the subsequent chemical modifi cation of fl uori-
nated chiral synthetic intermediates leads to syntheses of enantiomerically pure or enriched
fl uorinated amino acids as a fi nal product. Examples of the syntheses from ( R ) - isopropyli-
deneglyceraldehyde ( 51 ), Garner ’ s aldehyde ( 52 ), ( S ) - serine ( 53 ), and proline are shown
in Schemes 9.13 – 9.18 .
A fi ve - step chemical modifi cation of 51 gives N , O - protected α - amino - α ′ - hydroxy-
ketone 54 . Difl uorination of the ketone 54 at the carbonyl carbon with morpho - DAST
followed by conventional chemical modifi cation results in the synthesis of β - amino - α , α -
difl uorocarboxylic acid 55 (see Scheme 9.13 ) [31] . Enantiomerically pure 5,5,5,5 ′ ,5 ′ ,5 ′ -
hexafl uoroleucine 57 is effi ciently synthesized from Garner ’ s aldehyde 52 as shown in
Scheme 9.14 [32] . Triphenylphosphine - induced reductive coupling of 52 with hexafl uo-
rothioacetone produces 56 in an excellent yield, which is conventionally transformed to
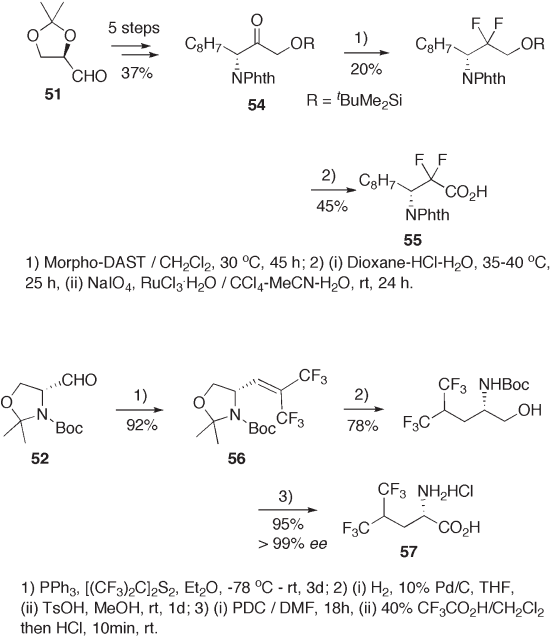
Recent Advances in the Syntheses of Fluorinated Amino Acids 221
57 . Reformatzky reaction of 52 followed by radical - initiated reductive hydrogenation of
the hydroxyl group in 58 via a thiocarbamate intermediate provides 59 . The usual chemical
modifi cation of 59 produces enantiomerically pure 4,4 - difl uoroglutamine 60 (see Scheme
15 ) [33] .
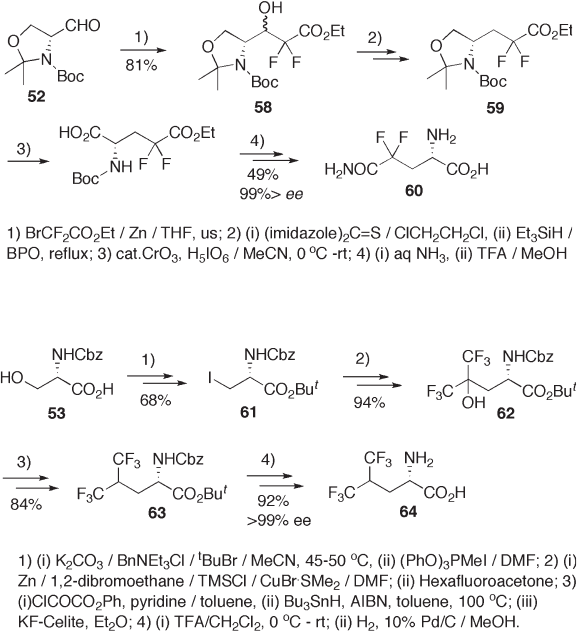
The hydroxyl group in serine 53 has a high potential for the various modifi cations in
the synthesis of β - substituted α - amino acids. One example is shown in Scheme 9.16 , in
which the C – O bond on C - 3 in serine 53 was at fi rst converted into a C – I bond and then
into a C – C bond. The overall transformation from serine 53 via 62 and 63 leads to the
synthesis of hexafl uoroleucine 64 [34] . L - Serine was also used for the synthesis of enan-
tiomerically pure 4,4 - difl uoroglutamic acid 66 (see Scheme 9.17 ), where the carboxyl
group in 53 was protected as an orthoester 65 and a difl uoromethylene moiety was sup-
plied from bromodifl uoroacetate via Reformatzky reaction [35] . Interestingly, aldehyde 67
[36] , readily prepared from L - serine, couples with 1,1 - bis( N , N - dimethylamino) - 2,2 - difl uo-
roethene 68 at room temperature to give adduct 69 as the sole stereoisomer. A bulky N -
protecting group (PhFI) effectively controls approach of nucleophile 68 to the hindered
carbonyl group of 67 .
Scheme 9.13
Scheme 9.14
222 Fluorine in Medicinal Chemistry and Chemical Biology
Scheme 9.15
Scheme 9.16
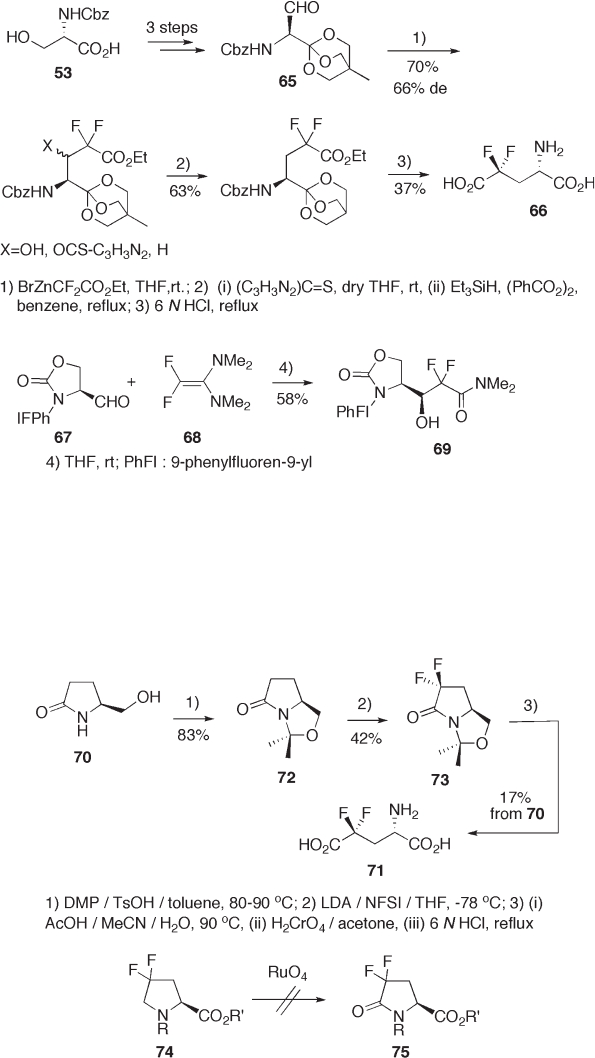
Pyroglutaminol 70 is easily available from L - glutamic acid and is useful for amino
acid synthesis as a functionalized chiral building block related to proline. Active methylene
hydrogens are readily replaced with fl uorine step - by - step via monofl uoride by electrophilic
fl uorinating reagents such as N - fl uorosulfone imines and N - fl uoroammonium salts like
Selectfl or [37] . Thus, 4, 4 - difl uoroglutamic acid 71 was successfully synthesized from the
readily available bicyclolactam 72 [38] . This synthesis involves electrophilic difl uorination
as a key step. Meanwhile, oxidation of 7 4 to 75 was found to be diffi cult due to the lower
reactivity of the methylene group inactivated by the electron - withdrawing effect of the
difl uoromethylene group (see Scheme 9.18 ).
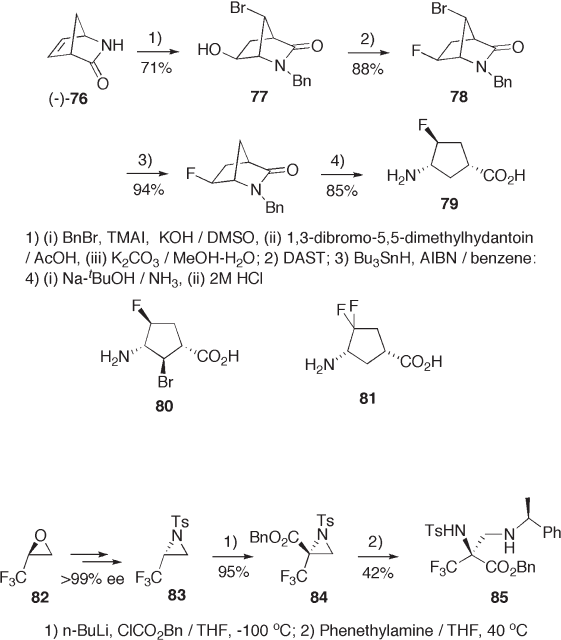
The commercially available lactam 76 is usable for amino acid synthesis as a chiral
cyclic building block. 4 - Fluoro - and 4,4 - difl uoro - 3 - aminocyclopentane carboxylic acids
79 and 81 , potential inhibitors of γ - aminobutanoic acid (GABA) aminotransferase, were
synthesized as shown in Scheme 9.19 [39] . In this process, replacement of hydroxyl or
carbonyl groups with fl uorine was achieved by the use of DAST. Interestingly, the stereo-
chemistry in substitution with DAST is retained [40] , although it is by inversion in most
cases [26] . Hydrolysis of 78 gave 80 .
Recent Advances in the Syntheses of Fluorinated Amino Acids 223
Scheme 9.17
Scheme 9.18
224 Fluorine in Medicinal Chemistry and Chemical Biology
9.2.4 Modifi cation of Chiral Fluorinated Building Block
( S ) - Trifl uoropropene Oxide
( S ) - Trifl uoropropene oxide (75% ee) [41] is commercially available. Both enantiomers are
readily prepared from racemic TFPO by Jacobsen ’ s co - catalyzed enantioselective ring
opening reaction [42] . The enantiomerically pure aziridine 83 is prepared from 82 by ring
opening with amine and recyclization with inversion of stereochemistry [43] . Carboben-
zyloxylation of 83 was achieved via lithiation followed by alkylation with benzyl chloro-
formate with complete retention of confi guration [44] . Ring opening of 84 with
( S ) - 1 - phenethylamine provided 85 (see Scheme 9.20 ).
9.2.5 Enzymatic Resolution of Racemic Fluorinated Building Blocks
Both enzymatic esterifi cation and hydrolysis are useful tools for resolution of racemic
fl uorinated building blocks. Among them, lipase - catalyzed reaction is reliable and most
Scheme 9.19
Scheme 9.20
Recent Advances in the Syntheses of Fluorinated Amino Acids 225
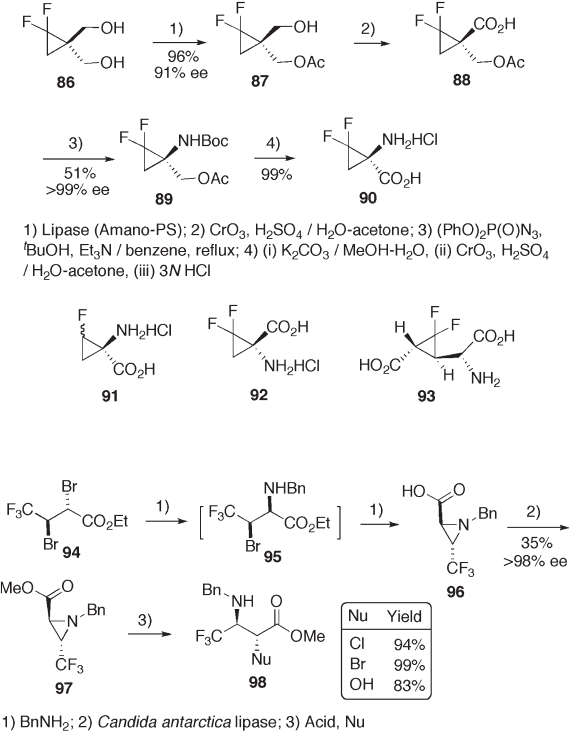
frequently used. Difl uorocyclopropane diol 86 is resolved by lipase (Amano - PS) - catalyzed
esterifi cation to give 87 in excellent yield and enantioselectivity. Further conventional
chemical transformation of 87 leads to enantiomerically pure difl uorocyclopropane amino
acid 90 (see Scheme 9.21 ) [45] . In relation to the biologically active 1 - aminocyclopropane -
1 - carboxylic acid, several related fl uorinated cyclopropane carboxylic acids 91 – 93 have
been synthesized.
Starting from ethyl 4, 4, 4 - trifl uorocrotonate, racemic aziridine carboxylic acid 96
was prepared as shown in Scheme 9.22 [46] and was then subjected to lipase - catalyzed
esterifi cation. Methyl ester 97 was obtained in 35% yield with excellent enantiomeric
purity. Acid - catalyzed ring opening of aziridine 97 proceeded regio - and stereoselectively,
affording 2 - substituted (2 R ,3 R ) - or (2 R ,3 S ) - 3 - amino - 4,4,4 - trifl uorobutanoates 98 in high
yields [47] .
Scheme 9.21
Scheme 9.22
226 Fluorine in Medicinal Chemistry and Chemical Biology
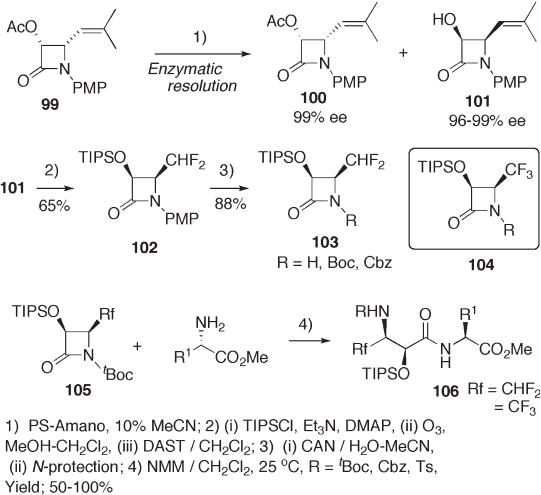
Likewise, lipase - catalyzed hydrolysis of racemic lactam 99 gave both 100 and 101
in almost enantiomerically pure form (see Scheme 9.23 ). Conventional chemical conver-
sion of the isobutenyl moiety of 101 to difl uoromethyl and trifl uoromethyl groups provided
lactams 103 and 104 , which were further transformed to dipeptide 106 by ring - opening
coupling with amino esters [48] .
9.3 Diastereoselective Synthesis of Fluorinated Amino Acids
9.3.1 Chiral Auxiliary Approach
Easy availability, high diastereoselection, and removal under mild conditions are essential
for auxiliaries that are feasible for synthetic organic chemistry. Very few chiral fl uorinated
building blocks are commercially available, so that highly diastereoselective fl uoro -
functionalization of nonfl uorinated building blocks bearing a chiral auxiliary is useful for
the synthesis of desired target fl uorinated amino acids. Chiral auxiliaries often used for
the synthesis of enantiomerically pure or enriched amino acids that appeared in references
published since 2000 are summarized in Figure 9.1 .
9.3.1.1 ( S ) - and ( R ) - 1 - Phenylethylamines and Their Related Amines as Auxiliaries
( S ) - and ( R ) - 1 - Phenylethylamines and the related 2 - hydroxy - and 2 - methoxyamines are
the most available and economically feasible auxiliaries and are frequently used as their
Scheme 9.23
