Ojima I. (ed.) Fluorine in Medicinal Chemistry and Chemical Biology
Подождите немного. Документ загружается.

186 Fluorine in Medicinal Chemistry and Chemical Biology
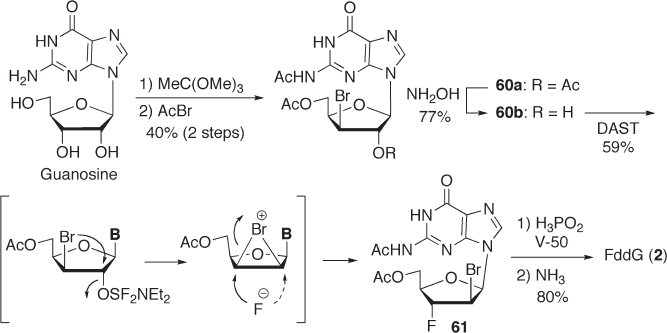
7.3.3.2.2 Synthesis of F dd G with Bromine Rearrangement during Fluorination W e
reported that the bromohydrin derivative 60b , which was easily prepared from guanosine
via 60a , gave the C - 3 ′ - α - fl uorinated compound 61 in 59% yield accompanied by the for-
mation of a regioisomer (21%) under fl uorination conditions (see Scheme 7.21 ) [84] . The
reaction can be explained by considering the rearrangement of the bromine atom from the
C - 3 ′ - β - to the C - 2 ′ - β - position via a bromonium ion intermediate on which the fl uoride
anion attacks from the α - side of the C - 3 ′ - or C - 2 ′ - position. The C - 3 ′ - α - fl uorinated product
61 was converted to FddG ( 2 ) by radical debromination and deprotection.
We further investigated the fl uorination conditions to improve the yield of C - 3 ′ - α -
product 61 . We then found that the fl uorination of compound 60b with SF
4
furnished the
C - 3 ′ - α - product 61 in 79% yield in an 3 ′ - F:2 ′ - F ratio of 7.8 : 1 [85] . This suggests that
the diethylamino group of DAST may have a negative infl uence on the regioselectivity
of the reaction. To our surprise, we also found that the reaction of compound 60b with
NfF in the presence of NEt
3
did not afford fl uorination product 61 , but produced a 2 ′ ,3 ′ -
didehydro - 2 ′ ,3 ′ - dideoxyguanosine derivative in 62% yield. This method has advantages
with respect to the number of reaction steps and high overall yield. However, one serious
drawback is that this method uses SF
4
- type reagents which require special equipment for
handling.
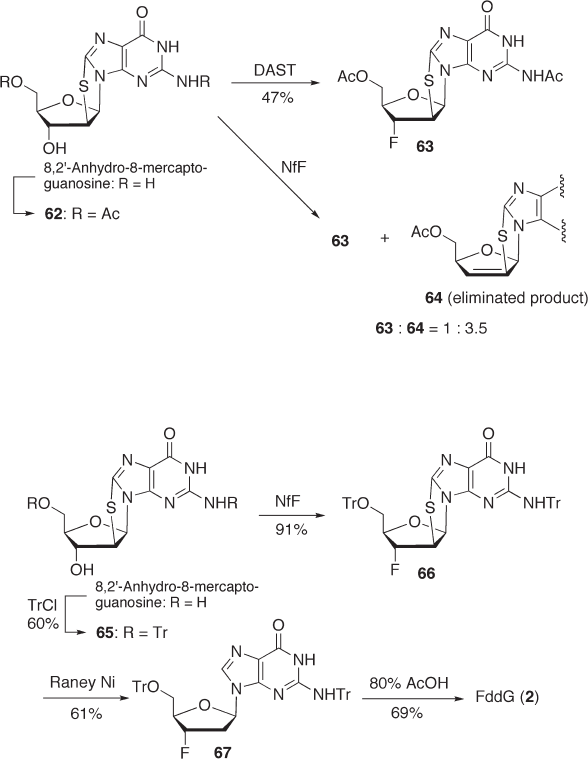
7.3.3.2.3 Synthesis of F dd G via Retentive Fluorination at the C - 3 ′ - α Position After
concluding that the fl uorination with bromine rearrangement described above might
proceed via a bromonium ion intermediate, we considered that a similar reaction might
take place via a sulfonium ion intermediate. We chose readily available 8,2 ′ - anhydro - 8 -
mercaptoguanosine as the starting material, anticipating that fl uorination might proceed
with the participation of a sulfur atom, which would facilitate the attack of fl uoride ion to
the C - 3 ′ - α - rather than the C - 2 ′ - α - position for steric reasons [86] . 8,2 ′ - Anhydro - 8 -
mercaptoguanosine was fi rst converted to the N
2
, O
5 ′
- diacetyl derivative 62 which was
subjected to fl uorination with DAST (see Scheme 7.22 ). As anticipated, we obtained the
Scheme 7.21
Synthesis and Biological Activity of Fluorinated Nucleosides 187
C - 3 ′ - α - fl uorinated product 63 in 47% yield without any formation of the C - 2 ′ - α - fl uori-
nated product. In the case of fl uorination with NfF, however, we found that elimination
predominated ( 63 : 64 = 1 : 3.5) (see Scheme 7.22 ).
Encouraged by these results, we further investigated the fl uorination of ditrityl pro-
tected compound 65 using several fl uorinating reagents (see Scheme 7.23 ). Although the
reaction with DAST gave a complex mixture, the desired C - 3 ′ - α - product 66 was obtained
in 63% yield with NfF/NEt
3
, along with the elimination product. Next, we optimized the
reaction conditions. When we used diisopropylethylamine (DIPEA) as the base, elimina-
tion was completely suppressed, albeit the yield was not improved. The best yield (91%)
was achieved when we used an excess amount of NfF and DIPEA.
Scheme 7.22
Scheme 7.23
188 Fluorine in Medicinal Chemistry and Chemical Biology
The fl uorination product 66 was then treated with acetic acid followed by reductive
desulfurization with Raney nickel in aqueous NaOH to give defl uorination product ddG,
but not FddG ( 2 ). Accordingly, we tried the desulfurization of compound 66 in various
solvent systems prior to deprotection with the acid. After several unsuccessful attempts,
we obtained the desired Tr
2
- FddG 67 in 61% isolated yield when we carried out Raney -
nickel reduction in toluene. This method is advantageous because fl uorination can be
achieved using the scalable reagent NfF.
In this section, synthetic approaches to FddG ( 2 ) with particular focus on industrially
applicable fl uorination methods are described. At the beginning of this study, these
approaches which used a nucleoside as a starting material required SF
4
reagents such as
DAST and morpholinosulfur trifl uoride (MOST) for fl uorination because other agents gave
mainly elimination products. However, SF
4
- type reagents are not desirable for industrial -
scale synthesis because of their poor availability and inherent toxicity. To overcome these
problems, we developed a new nucleoside fl uorination method that involved the participa-
tion of neighboring groups. With this methodology, we achieved the fl uorination of a
guanosine derivative at the C - 3 ′ position in good yield using readily available NfF. This
would provide a useful stereoselective method for introducing a fl uorine atom into the
sugar moiety of nucleosides.
7.4 Biological Activity of Fluorinated Nucleosides and Correlations with
Their Conformations
7.4.1 Sugar Ring Conformation of 2 ′ - Deoxy - 2 ′ - a - fl uoronucleosides
7.4.1.1 Properties of Modifi ed RNA Containing 2 ′ - Deoxy - 2 ′ - α - fl uoronucleosides
In 1968, four years after the fi rst synthesis of 2 ′ - deoxy - 2 ′ - α - fl uorouridine ( 4 ), Fox and
co - workers reported that 4 adopts the 3 ′ - endo conformation (N - form) more readily than
the 2 ′ - endo conformation (S - form) [87] . In the 1970s, some studies examined the relation-
ships between the conformations of 2 ′ - deoxy - 2 ′ - α - substituted nucleosides and the electro-
negativity of their 2 ′ - substituents, and revealed that an increase in electronegativity led to
a high proportion of the 3 ′ - endo conformation. According to these studies, the proportion
of the 3 ′ - endo conformer of 4 is approximately 85% [88] . With regard to 2 ′ - substituted
purine nucleosides [89] , Uesugi and co - workers also reported that 2 ′ - deoxy - 2 ′ - α - fl uoro-
adenosine ( 10b ) favors the 3 ′ - endo conformation, and this trend remained unchanged even
when 10b was contained in dinucleoside monophosphate [90] . Although ribonucleoside
generally favors the 3 ′ - endo conformation, 2 ′ - deoxy - 2 ′ - α - fl uoro analogues increase this
tendency more than ribonucleosides. However, despite the difference in sugar puckering,
the torsion angles of the C - 4 ′ – C - 5 ′ bond and the C - 5 ′ – O - 5 ′ bond in the 2 ′ - deoxy - 2 ′ - α -
fl uoro analogues are analogous to those of ribonucleosides. Thus, a nucleic acid that con-
tains the 2 ′ - deoxy - 2 ′ - α - fl uoronucleoside forms a structure similar to RNA, in which the
proportion of the 3 ′ - endo conformation is higher than that in RNA.
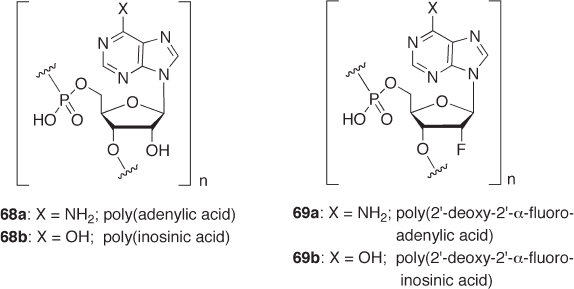
This property naturally infl uences their biological activities. For example, it has been
reported that poly(2 ′ - deoxy - 2 ′ - α - fl uoroadenylic acid) ( 69a ) is as good a template as
poly(adenylic acid) ( 68a ) for some reverse transcriptases and DNA polymerase [91]
Synthesis and Biological Activity of Fluorinated Nucleosides 189
(Figure 7.4 ). Likewise, poly(2 ′ - deoxy - 2 ′ - α - fl uoroinosinic acid) ( 69b ) and poly(2 ′ - deoxy -
2 ′ - α - fl uorocytidylic acid) are effective templates for reverse transcriptases [92] . Notably,
69a also functions as mRNA in protein - synthesizing systems in vitro , in which [
14
C]lysine
is incorporated into polypeptides [93] . Moreover, modifi ed mRNA that contains 2 ′ - deoxy -
2 ′ - α - fl uoroadenosine ( 10b ) can give rise to the corresponding protein (luciferase). Interest-
ingly, modifi ed RNA that contains 2 ′ - deoxy - 2 ′ - α - fl uorouridine ( 4 ) does not act as mRNA
[94] . A similar trend has been observed in a study on the induction of interferon by double -
helical RNA (poly I · poly C). The complex of poly(2 ′ - deoxy - 2 ′ - α - fl uoroinosinic acid)
( 69b ) · poly C shows higher interferon - inducing activity than that of poly I · poly C, while
the complex of poly I · poly(2 ′ - deoxy - 2 ′ - α - fl uorocytidylic acid) is not an effective inter-
feron inducer [95] .
7.4.1.2 Properties of 2 ′ - Deoxy - 2 ′ - α - fl uoronucleosides
Although the relationship between conformation and biological activity is not well docu-
mented at the nucleoside level, several 2 ′ - deoxy - 2 ′ - α - fl uoronucleosides are known to have
biological activity, such as antiviral activity. Tuttle and co - workers reported that several
2 ′ - deoxy - 2 ′ - α - fl uoropurine nucleosides exhibited antiviral potency against infl uenza A and
B viruses. The 2 - amino group of the purine moiety is critical [96a] for this activity, and
the antiviral activity is much better than those of amantadine and ribavirin [96b] . Notably,
2 ′ - deoxy - 2 ′ - α - fl uoro - 2 ′ - C - methylcytidine shows increased inhibitory activity in the hepa-
titis C virus (HCV) replicon assay compared to 2 ′ - C - methylcytidine, and low cellular
toxicity [97] . Infl uenza virus and HCV are classifi ed as RNA viruses, which are a major
target of current chemotherapy. However, the development of effective drugs against these
virus infections is still slow; further investigation of 2 ′ - deoxy - 2 ′ - α - fl uoronucleosides is
expected to accelerate the development of such drugs.
Another interesting biological activity of 2 ′ - deoxy - 2 ′ - α - fl uoropuromycin has been
observed. Unlike other nucleoside analogues, puromycin inhibits protein biosynthesis
without phosphorylation of the 5 ′ - hydroxyl group. As mentioned above (Section 7.2.2.3.1 ),
2 ′ - deoxy - α - 2 ′ - fl uoropuromycin ( 20b ) showed antitumor and antibacterial activity
Figure 7.4 Structures of polynucleotides and their analogues.
190 Fluorine in Medicinal Chemistry and Chemical Biology
comparable to that of puromycin. Since 2 ′ - deoxypuromycin, which favors the 2 ′ - endo
conformation, showed no biological activity, the 3 ′ - endo conformation is thought to be
essential for its activity.
In general, the 3 ′ - endo conformation of fl uoronucleoside is essential for antiviral
activity against RNA viruses although the anti - HIV activity is an exception (the details of
anti - HIV activity are addressed in the next section).
7.4.2 Sugar Ring Conformations of F dd A and F dd G and
Their Anti - HIV Activities
Mu and co - workers reported that the introduction of a fl uoro group to the C - 2 ′ - α position
of 2 ′ ,3 ′ - dideoxyadenosine 5 ′ - triphosphate (ddATP) led to the 3 ′ - endo conformation like a
nucleoside congener, while introduction to its β position led to the 2 ′ - endo conformation
[98] . In contrast, the 2 ′ - endo conformation was predominant when the fl uoro group was
introduced to the C - 3 ′ - α position of 2 ′ ,3 ′ - dideoxynucleosides [99] . Further conformational
analysis of alovudine (3 ′ - deoxy - 3 ′ - fl uorothymidine, FLT) (see Figure 7.3 ), a potent anti -
HIV agent, has revealed that approximately 90% of FLT is fi xed to the 2 ′ - endo conforma-
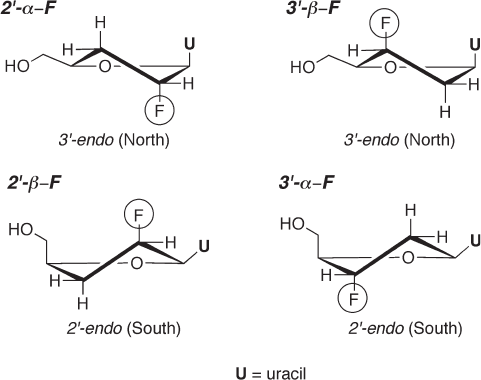
tion in aqueous solution [100] . Another conformational analysis of four monofl uorinated
analogues of 2 ′ ,3 ′ - dideoxyuridine (i.e., 2 ′ - α - F, 2 ′ - β - F, 3 ′ - α - F, and 3 ′ - β - F analogues, see
Figure 7.5 ) also revealed that the 2 ′ - up and 3 ′ - down analogues favor the 2 ′ - endo confor-
mation [101] . This preference can be explained by a gauche effect, in which a more elec-
tronegative substituent favors an axial orientation [15] .
We now discuss the relationship between these conformations and anti - HIV activity.
In 1989, Roey and co - workers reported the correlation between the sugar ring conforma-
Figure 7.5 Sugar - puckering of four monofl uorinated analogues of 2 ′ ,3 ′ - dideoxyuridine.
Synthesis and Biological Activity of Fluorinated Nucleosides 191
tion and the anti - HIV activity of some nucleoside analogues. They discovered that the 2 ′ -
endo conformation is preferable for anti - HIV activity [102] . In the 2 ′ - endo conformation,
the C - 5 ′ moiety takes an axial orientation, which directs its 5 ′ - hydroxyl group to an advan-
tageous position for the kinase - mediated phosphorylation process, or to a favorable posi-
tion for its affi nity with reverse transcriptase (RT). Mu and co - workers reported a molecular
dynamics simulation of ternary complexes of an HIV - 1 - RT, a double - strand DNA, and
2 ′ ,3 ′ - dideoxy - 2 ′ - fl uoroadenosine 5 ′ - triphosphate (FddATP), and discussed the activity of
FddATPs (2 ′ - α - or 2 ′ - β - forms) as inhibitors [98] . Based on the preference of the RT to
bind incoming dNTP (or ddNTP), a 3 ′ - endo conformer (i.e., α FddATP) is believed to fi t
the polymerase site better than β FddATP. Interestingly, however, their study has revealed
that Tyr115 of the RT, which appears to function as a steric gate against the incoming
dNTP, prevents the effective binding of α FddATP, while the 2 ′ - endo conformer, β FddATP,
can pass the gate without hindrance to the RT. Consequently, the 2 ′ - endo conformer,
β FddATP, shows stronger anti - HIV activity, although this conformation shows a worse fi t
to the polymerase site of HIV - RT.
As mentioned above, nucleoside analogues have to clear many hurdles to exhibit
biological activity. For example, those hurdles include phosphorylation by kinase, further
conversion to triphosphate, penetration to the active site of the target enzyme through a
steric gate, and strong affi nity to the active site of the enzyme. Several dideoxynucleosides
like ddI and ddC, which have been approved as anti - HIV agents, are fl exible enough to
clear all of these hurdles and show good anti - HIV activity. However, the introduction of
a fl uoro group to the sugar moiety of nucleoside brings about rigid 2 ′ - endo or 3 ′ - endo
conformation, and such a fi xed conformation makes it diffi cult to clear all of these hurdles
in some cases. If these hurdles can be overcome, the many advantages of fl uoronucleoside,
such as chemical and pharmacological stability, could lead to stronger biological
activity.
7.5 Conclusion
Since the discovery of nucleocidin, a large number of fl uorinated nucleosides have been
synthesized and have contributed to the development of biologically active agents. The
practical use of gemcitabine as an anticancer agent and also the upcoming use of clevudine
as an anti - HBV agent refl ect this development. Early in this chapter, we described the
syntheses and biological activities of 2 ′ - deoxy - 2 ′ - α - fl uoronucleosides and their oligomers.
Such fundamental studies led to the use of fl uorinated nucleosides in other fi elds, such as
antisense and ribozymes, due to the chemical and biological stability of fl uorinated nucleo-
sides. Next, we described the synthesis of a potent antiviral agent, FddA, with special
focus on synthetic issues (e.g., the diffi culty of β - side - selective fl uorination) and its
process - scale synthesis. The possible process - scale synthesis of FddG was also described.
The relationships between the sugar ring conformations and the biological activities of
several fl uorinated nucleosides were also discussed. We hope that this chapter provides a
better understanding of the usefulness of fl uorinated nucleosides as well as valuable refer-
ences for their syntheses.
192 Fluorine in Medicinal Chemistry and Chemical Biology
References
Morton , G. O. , Lancaster , J. E. , Van Lear , G. E. , et al. ( 1969 ) Structure of nucleocidin. III.
Revised structure . J. Am. Chem. Soc. , 91 , 1535 – 1537 .
Heidelberger , C. , Chaudhuri , N. K. , Danneberg , P. , et al. ( 1957 ) Fluorinated pyrimidines, a
new class of tumour - inhibitory compounds . Nature , 179 ( 4561 ), 663 – 666 .
Hertel , L. W. , Kroin , J. S. , Misner , J. W. and Tustin , J. M. ( 1988 ) Synthesis of 2 - deoxy - 2,2 -
difl uoro - d - ribose and 2 - deoxy - 2,2 - difl uoro - d - ribofuranosyl nucleosides . J. Org. Chem. , 53 ,
2406 – 2409 .
Bonate , P. L. , Arthaud , L. , Cantrell , W. R. , Jr. , et al. ( 2006 ) Discovery and development of
clofarabine: a nucleoside analogue for treating cancer . Nat. Rev. Drug Discov. , 5 , 855 – 863 .
Korba , B. E. , Furman , P. A. and Otto , M. J. ( 2006 ) Clevudine: a potent inhibitor of hepatitis
B virus in vitro and in vivo . Expert Rev. Anti - Infect. Ther. , 4 , 549 – 561 .
Billich , A. ( 1999 ) Lodenosine . Curr. Opin. Anti - Infect. Invest. Drugs , 1 , 179 – 185 .
De Clercq , E. ( 2005 ) Emerging anti - HIV drugs . Expert Opin. Emerg. Drugs , 10 , 241 – 274 .
Rusconi , S. ( 2003 ) Alovudine (Medivir) . Curr. Opin. Investig. Drugs (Thomson Current Drugs)
4 , 219 – 223 .
Takamatsu , S. , Maruyama , T. and Izawa , K. ( 2005 ) Development of an industrial process for
synthesizing lodenosine (FddA) . Yuki Gosei Kagaku Kyokaishi , 63 , 864 – 878 .
Izawa , K. , Takamatsu , S. , Katayama , S. , et al. ( 2003 ) An industrial process for synthesizing
lodenosine (FddA) . Nucleosides, Nucleotides Nucleic Acids , 22 , 507 – 517 .
Izawa , K. , Torii , T. , Onishi , T. and Maruyama , T. ( 2007 ) The synthesis of an antiviral fl uori-
nated purine nucleoside: 3 ′ - α - fl uoro - 2 ′ ,3 ′ - dideoxyguanosine . Current Fluoroorganic Chemis-
try, ACS Symposium Series 949 , pp. 363 – 378 .
Herdewijn , P. , Van Aerschot , A. and Kerremans , L. ( 1989 ) Synthesis of nucleosides fl uorinated
in the sugar moiety. The application of diethylaminosulfur trifl uoride to the synthesis of fl uo-
rinated nucleosides . Nucleosides Nucleotides , 8 , 65 – 96 .
Pankeiwicz , K. W. ( 2000 ) Fluorinated nucleosides . Carbohydr. Res. , 327 , 87 – 105 .
Pankeiwicz , K. W. and Watanabe , K. A. ( 1993 ) Synthesis of 2 ′ - β - fl uoro - substituted nucleosides
by a direct approach . J. Fluorine Chem. , 64 , 15 – 36 .
Saenger , W. ( 1984 ) Pronciples of Nucleic Acid Structure . Springer - Verlag , New York .
(a) Codington , J. F. , Doerr , I. L. and Fox , J. J. ( 1964 ) Nucleosides. XVIII. Synthesis of 2 ′ -
fl uorothymidine, 2 ′ - fl uorodeoxyuridine, and other 2 ′ - halogeno - 2 ′ - deoxy nucleosides . J. Org.
Chem. , 29 , 558 – 564 . (b) Kowollik , G. , Gaertner , K. and Langen , P. ( 1975 ) Nucleosides of
fl uorocarbohydrates. XIII. Synthesis of 3 ′ - deoxy - 3 ′ - fl uorouridine . J. Carbohydr., Nucleosides,
Nucleotides , 2 , 191 – 195 .
Misra , H. K. , Gati , W. P. , Knaus , E. E. and Wiebe , L. I. ( 1984 ) Reaction of 1 - (2 ′ ,3 ′ - epoxy - β -
d - lyxofuranosyl)uracil with hydrogen fl uoride. The unexpected formation of 1 - (3 ′ - fl uoro - 3 ′ -
deoxy - β - d - ribofuranosyl)uracil . J. Heterocyclic Chem. , 21 , 773 – 775 .
(a) Ikehara , M. and Maruyama , T. ( 1976 ) Studies of nucleosides and nucleotides. LXIX. Purine
cyclonucleosides. (30). Elimination of the 8 - oxy function of purine nucleosides . Chem. Pharm.
Bull. , 24 , 565 – 569 . (b) Ikehara , M. , Maruyama , T. , Miki , H. and Takatsuka , Y. ( 1977 ) Studies
of nucleosides and nucleotides. LXXXV. Purine cyclonucleosides. (35). Synthesis of purine
nucleosides having 2 ′ - azido and 2 ′ - amino functions by cleavage of purine cyclonucleosides .
Chem. Pharm. Bull. , 25 , 754 – 760 .
Wagner , J. , Verheyden , J. P. H. and Moffatt , J. G. ( 1974 ) Preparation and synthetic utility of
some organotin derivatives of nucleosides . J. Org. Chem. , 39 , 24 – 30 .
Ikehara , M. and Maruyama , T. ( 1975 ) Studies of nucleosides and nucleotides – LXV: Purine
cyclonucleosides - 26 a versatile method for the synthesis of purine O - cyclo - nucleosides. The
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
Synthesis and Biological Activity of Fluorinated Nucleosides 193
fi rst synthesis of 8,2 ′ - anhydro - 8 - oxy 9 - β - d - arabinofuranosylguanine . Tetrahedron , 31 ,
1369 – 1372 .
Ikehara , M. , Maruyama , T. and Miki , H. ( 1976 ) A new method for the synthesis of 2 ′ - substi-
tuted purine nucleosides. Total synthesis of an antibiotic 2 ′ - amino - 2 ′ - deoxyguanosine . Tetra-
hedron Lett. , 17 , 4485 – 4488 .
Ikehara , M. and Miki , H. ( 1978 ) Studies of Nucleosides and Nucleotides. LXXXII. Cyclonu-
cleosides. (39). Synthesis and Properties of 2 ′ - Halogeno - 2 ′ - deoxyadenosines . Chem. Pharm.
Bull. , 26 , 2449 – 2453 .
Ikehara , M. and Imura , J. ( 1981 ) Studies on nucleosides and nucleotides. LXXXVII. Purine
cyclonucleosides. XLII. Synthesis of 2 ′ - deoxy - 2 ′ - fl uoroguanosine . Chem. Pharm. Bull. , 29 ,
1034 – 1038 .
Ranganathan , R. ( 1977 ) Modifi cation of the 2 ′ - position of purine nucleosides: syntheses of
2 ′ - α - substituted - 2 ′ - deoxyadenosine analogs . Tetrahedron Lett. , 18 , 1291 – 1294 .
(a) McOmie , J. F. W. ( 1973 ) Protective Groups in Organic Chemistry . Plenum Press , New
York . (b) Nishino , S. , Takamura , H. and Ishido , Y. ( 1986 ) Regioselective protection of carbo-
hydrate derivatives. Part 20. Simple, effi cient 2 ′ - O - deacylation of fully acylated purine and
pyrimidine ribonucleosides through tert - butoxide . Tetrahedron , 42 , 1995 – 2004 .
(a) Markiewicz , W. T. and Wiewiorowski , M. ( 1978 ) A new type of silyl protecting groups in
nucleoside chemistry . Nucleic Acids Res., Spec. Publ. , 4 , 185 – 188 . (b) Markiewicz , W. T.
( 1975 ) Tetraisopropyldisiloxane - 1,3 - diyl, a group for simultaneous protection of 3 ′ - and 5 ′ -
hydroxy functions of nucleosides . J. Chem. Res., Synopses , 24 – 25 .
Maruyama , T. , Utzumi , K. , Sato , Y. and Richman , D. D. ( 1994 ) Synthesis and anti - HIV
activity of 6 - substituted purine 2 ′ - deoxy - 2 ′ - fl uororibosides . Nucleosides Nucleotides , 13 ,
527 – 537 .
Maruyama , T. , Utzumi , K. , Sato , Y. and Richman , D. D. ( 1994 ) Synthesis and anti - HIV activity
of 2 - substituted 2 ′ - deoxy - 2 ′ - fl uoroadenosines . Nucleosides Nucleotides , 13 , 1219 – 1230 .
Suhadolnik , R. J. ( 1979 ) Nucleosides as Biological Probes . John Wiley and Sons, Inc . , New
York , pp. 96 – 102 .
Maruyama , T. , Utsumi , K. , Tomioka , H. , et al. ( 1995 ) Synthesis, antiviral, antibacterial
and antitumor cell activities of 2 ′ - deoxy - 2 ′ - fl uoropuromycin . Chem. Pharm. Bull. , 43 ,
955 – 959 .
Koizumi , F. , Oritani , T. and Yamashita , K. ( 1990 ) Synthesis and antimicrobial activity of 2 ′ -
deoxypuromycin . Agric. Biol. Chem. , 54 , 3093 – 3097 .
(a) Ledoux , L. and Baltus , E. ( 1954 ) The effect of ribonuclease on the cells of the Ehrlich
carcinoma . Experientia , 10 , 500 – 501 . (b) Youle , R. J. and D ’ Alessio , G. ( 1997 ) Ribonucleases:
Structures and Functions . Academic Press , New York , pp. 491 – 514 .
Nonaka , T. , Nakamura , K. T. , Uesugi , S. , et al. ( 1993 ) Crystal structure of ribonuclease Ms
(as a ribonuclease T1 homolog) complexed with a guanylyl - 3 ′ ,5 ′ - cytidine analog . Biochemis-
try , 32 , 11825 – 11837 .
Ikehara , M. and Imura , J. ( 1981 ) Studies on nucleosides and nucleotides. LXXXIX. Purine
cyclonucleosides. (43). Synthesis and properties of 2 ′ - halogeno - 2 ′ - deoxyguanosines . Chem.
Pharm. Bull. , 29 , 3281 – 3285 .
Benseler , F. , Williams , D. M. and Eckstein , F. ( 1992 ) Synthesis of suitably - protected
phosphoramidites of 2 ′ - fl uoro - 2 ′ - deoxyguanosine and 2 ′ - amino - 2 ′ - deoxyguanosine for
incorporation into oligoribonucleotides . Nucleosides, Nucleotides, Nucleic Acids , 11 ,
1333 – 1351 .
Maruyama , T. , Kozai , S. , Nakamura , K. and Irie , M. ( 2002 ) Synthesis of 2 ′ - deoxy - 2 ′ -
fl uoroguanyl - (3 ′ ,5 ′ ) - guanosine . Nucleosides, Nucleotides, Nucleic Acids , 21 , 765 – 774 .
Honda , K. , Komoda , Y. , Nishida , S. , et al. ( 1984 ) Uridine as an active component of sleep -
promoting substance: its effects on nocturnal sleep in rats . Neurosci. Res. , 1 , 243 – 252 .
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
31.
32.
33.
34.
35.
36.
37.
194 Fluorine in Medicinal Chemistry and Chemical Biology
Yamamoto , I. , Kimura , T. , Takeoka , Y. , et al. ( 1987 ) N - Substituted oxopyrimidines and nucleo-
sides: structure – activity relationship for hypnotic activity as CNS depressant . J. Med. Chem. ,
30 , 2227 – 2231 .
Sato , Y. , Utsumi , K. , Maruyama , T. , et al. ( 1994 ) Synthesis and hypnotic and anti - human
immunodefi ciency virus - 1 activities of N
3
- Substituted 2 ′ - Deoxy - 2 ′ - fl uorouridines . Chem.
Pharm. Bull. , 42 , 595 – 598 .
Pankiewicz , K. W. and Watanabe , K. A. ( 1987 ) Nucleosides. CXLIII. Synthesis of 5 ′ - deoxy -
5 ′ - substituted - 2,2 ′ - anhydro - 1 - ( β - d - arabinofuranosyl)uracils. A new 2,5 ′ - to 2,2 ′ - anhydronucle-
oside transformation. Studies directed toward the synthesis of 2 ′ - deoxy - 2 ′ - substituted arabino
nucleosides. (4) . Chem. Pharm. Bull. , 35 , 4494 – 4497 .
Matsuda , A. , Yasuoka , J. and Ueda , T. ( 1989 ) A new method for synthesizing the antineoplastic
nucleosides 1 - (2 - azido - 2 - deoxy - β - d - arabinofuranosyl)cytosine (Cytarazid) and 1 - (2 - amino - 2 -
deoxy - β - d - arabinofuranosyl)cytosine (Cytaramin) from uridine . Chem. Pharm. Bull. , 37 ,
1659 – 1661 .
Pankiewicz , K. W. , Krzeminski , J. , Ciszewski , L. A. , et al. ( 1992 ) A synthesis of 9 - (2 - deoxy -
2 - fl uoro - β - d - arabinofuranosyl)adenine and - hypoxanthine. An effect of C3 ′ - endo to C2 ′ - endo
conformational shift on the reaction course of 2 ′ - hydroxyl group with DAST . J. Org. Chem. ,
57 , 553 – 559 .
Krzeminski , J. , Nawrot , B. , Pankiewicz , K. W. and Watanabe , K. A. ( 1991 ) Synthesis of 9 - (2 -
deoxy - 2 - fl uoro - β - d - arabinofuranosyl)hypoxanthine. The fi rst direct introduction of a 2 ′ - β -
fl uoro substituent in preformed purine nucleosides. Studies directed toward the synthesis of
2 ′ - deoxy - 2 ′ - substituted arabinonucleosides. 8 . Nucleosides Nucleotides , 10 , 781 – 798 .
Maruyama , T. , Sato , Y. , Oto , Y. , et al. ( 1996 ) Synthesis and antiviral activity of 6 - chloropurine
arabinoside and its 2 ′ - deoxy - 2 ′ - fl uoro derivative . Chem. Pharm. Bull. , 44 , 2331 – 2334 .
Uesugi , S. , Miki , H. , Ikehara , M. , et al. ( 1979 ) A linear relationship between electronegativity
of 2 ′ - substituents and conformation of adenine nucleosides . Tetrahedron Lett. , 20 ,
4073 – 4076 .
Maruyama , T. , Takamatsu , S. , Kozai , S. , et al. ( 1999 ) Synthesis of 9 - (2 - deoxy - 2 - fl uoro - β - d -
arabinofuranosyl)adenine bearing a selectively removable protecting group . Chem. Pharm.
Bull. , 47 , 966 – 970 .
Siddiqui , M. A. , Driscoll , J. S. and Marquez , V. E. ( 1998 ) A new synthetic approach to
the clinically useful, anti - HIV - active nucleoside, 9 - (2,3 - dideoxy - 2 - fl uoro - β - d - threo -
pentofuranosyl)adenine ( β - FddA). Introduction of a 2 ′ - β - fl uoro substituent via inversion of a
readily obtainable 2 ′ - α - fl uoro isomer . Tetrahedron Lett. , 39 , 1657 – 1660 .
Marquez , V. E. , Tseng , C. K. - H. , Kelley , J. A. , et al. ( 1987 ) 2 ′ ,3 ′ - Dideoxy - 2 ′ - fl uoro - ara - A. An
acid - stable purine nucleoside active against human immunodefi ciency virus (HIV) . Biochem.
Pharmacol. , 36 , 2719 – 2722 .
Marquez , V. E. , Tseng , C. K. - H. , Mitsuya , H. , et al. ( 1990 ) Acid - stable 2 ′ - fl uoro purine dide-
oxynucleosides as active agents against HIV . J. Med. Chem. , 33 , 978 – 985 .
Ruxrungtham , K. , Boone , E. B. , Ford , H. , Jr. , et al. ( 1996 ) Potent activity of 2 ′ - β - fl uoro - 2 ′ ,3 ′ -
dideoxyadenosine against human immunodefi ciency virus type 1 infection in hu - PBL - SCID
mice . Antimicrob. Agents Chemother. , 40 , 2369 – 2374 .
Graul , A. , Silvestre , J. and Castaner , J. ( 1998 ) Lodenosine: anti - HIV (reverse transcriptase
inhibitor) . Drugs Future , 23 , 1176 – 1189 .
Driscoll , J. S. , Mayers , D. L. , Bader , J. P. , et al. ( 1997 ) 2 ′ - Fluoro - 2 ′ ,3 ′ - dideoxyarabinosylade-
nine (F - ddA): activity against drug - resistant human immunodefi ciency virus strains and clades
A - E . Antivir. Chem. Chemother. , 8 , 107 – 111 .
Tanaka , M , Srinivas , R. V. , Ueno , T. , et al. ( 1997 ) In vitro induction of human immunodefi -
ciency virus type 1 variants resistant to 2 ′ - β - fl uoro - 2 ′ ,3 ′ - dideoxyadenosine . Antimicrob. Agents
Chemother. , 41 , 1313 – 1318 .
38.
39.
40.
41.
42.
43.
44.
45.
46.
47.
48.
49.
50.
51.
52.
53.
Synthesis and Biological Activity of Fluorinated Nucleosides 195
Singhal , D. , Morgan , M. E. and Anderson , B. D. ( 1997 ) Role of brain tissue localized
purine metabolizing enzymes in the central nervous system delivery of anti - HIV agents 2 ′ - β -
fl uoro - 2 ′ ,3 ′ - dideoxyinosine and 2 ′ - β - fl uoro - 2 ′ ,3 ′ - dideoxy - adenosine in rats . Pharmaceutical
Res. , 14 , 786 – 792 .
Driscoll , J. S. , Siddiqui , M. A. , Ford , H. , Jr. , et al. ( 1996 ) Lipophilic, acid - stable, adenosine
deaminase - activated anti - hiv prodrugs for central nervous system delivery. 3. 6 - Amino pro-
drugs of 2 ′ - β - fl uoro - 2 ′ ,3 ′ - dideoxyinosine . J. Med. Chem. , 39 , 1619 – 1625 .
Johnson , M. D. and Anderson , B. D. ( 1996 ) Localization of purine metabolizing enzymes in
bovine brain microvessel endothelial cells: an enzymic blood – brain barrier for dideoxynucleo-
sides? Pharm. Res. , 13 , 1881 – 1886 .
Ford , H. , Jr. , Siddiqui , M. , Driscoll , J. S. , et al. ( 1995 ) Lipophilic, acid - stable, adenosine
deaminase - activated anti - HIV prodrugs for central nervous system delivery. 2. 6 - Halo - and 6 -
alkoxy prodrugs of 2 ′ - β - fl uoro - 2 ′ ,3 ′ - dideoxyinosine . J. Med. Chem. , 38 , 1189 – 1195 .
Johnson , M. D. , Chen , J. and Anderson , B. D. ( 2002 ) Investigation of the mechanism of
enhancement of central nervous system delivery of 2 ′ - β - fl uoro - 2 ′ ,3 ′ - dideoxyinosine via a
blood – brain barrier adenosine deaminase - activated prodrug . Drug Metab. Dispos. , 30 ,
191 – 198 .
Herdewijn , P. , Pauwels , R. , Baba , M. , et al. ( 1987 ) Synthesis and anti - HIV activity of various
2 ′ - and 3 ′ - substituted 2 ′ ,3 ′ - dideoxyadenosines: a structure – activity analysis . J. Med. Chem. , 20 ,
2131 – 2137 .
Marquez , V. E. ( 1989 ) Design, synthesis, and antiviral activity of nucleoside and nucleotide
analogs . Nucleotide Analogues Antiviral Agents, ACS Symposium Series 401 , pp. 140 – 155 .
Wysocki , R. J. , Jr. , Siddiqui , M. A. , Barchi , J. J. , Jr. , et al. ( 1991 ) A more expedient approach
to the synthesis of anti - HIV - active 2,3 - dideoxy - 2 - fl uoro - β - d - threo - pentofuranosyl nucleosides .
Synthesis , 1005 – 1008 .
Siddiqui , M. A. , Marquez , V. E. , Driscoll , J. S. and Barchi , J. J. , Jr. ( 1994 ) A diastereo - selective
synthesis of ( S , S ) - α - fl uoro - 2,2 - dimethyl - 1,3 - dioxolane - 4 - propanoic acid methyl ester, a key
intermediate for the preparation of anti - HIV effective fl uorodideoxy nucleosides . Tetrahedron
Lett. , 35 , 3263 – 3266 .
Siddiqui , M. A. , Driscoll , J. S. , Abushanab , E. , et al. ( 2000 ) The “ β - fl uorine effect ” in the
non - metal hydride radical deoxygenation of fl uorine - containing nucleoside xanthates . Nucleo-
sides, Nucleotides, Nucleic Acids , 19 , 1 – 12 .
Caille , J. - C. , Miel , H. , Armstrong , P. and McKervey , M. A. ( 2004 ) A new synthetic approach
to 2,3 - dideoxy - 2 - fl uoro - β - d - threo - pentofuranose, the fl uorofuranose unit of the anti - HIV -
active nucleoside, β - FddA . Tetrahedron Lett. , 45 , 863 – 865 .
Shanmuganathan , K. , Koudriakova , T. , Nampalli , S. , et al. ( 1994 ) Enhanced brain delivery of
an anti - HIV nucleoside 2 ′ - F - ara - ddI by xanthine oxidase mediated biotransformation . J. Med.
Chem. , 37 , 821 – 827 .
Jin , F. , Wang , D. , Confalone , P. N. , et al. ( 2001 ) ( 2 R ,3 S ,5 S ) - 2 - Acetoxy - 3 - fl uoro - 5 - ( p - toluoy-
loxymethyl) tetrahydrofuran: a key intermediate for the practical synthesis of 9 - (2,3 - dideoxy -
2 - fl uoro - β - d - threo - pentofuranosyl)adenine (FddA) . Tetrahedron Lett. , 42 , 4787 – 4789 .
Choudhury , A. , Jin , F. , Wang , D. , et al. ( 2003 ) A concise synthesis of anti - viral agent F - ddA,
starting from ( S ) - dihydro - 5 - (hydroxymethyl) - 2(3 H ) - furanone . Tetrahedron Lett. , 44 ,
247 – 250 .
Takamatsu , S. , Maruyama , T. , Katayama , S. , et al. ( 2001 ) Improved synthesis of 9 - (2,3 -
dideoxy - 2 - fl uoro - β - d - threo - pentofuranosyl)adenine (FddA) using triethylamine trihydrofl uo-
ride . Tetrahedron Lett. , 42 , 2321 – 2324 .
Barton , D. H. R. , Jang , D. O. and Jaszberenyi , J. C. ( 1993 ) The invention of radical reactions.
Part 32. Radical deoxygenations, dehalogenations, and deaminations with dialkyl phosphites
and hypophosphorous acid as hydrogen sources . J. Org. Chem. , 58 , 6838 – 6842 .
54.
55.
56.
57.
58.
59.
60.
61.
62.
63.
64.
65.
66.
67.
68.
69.
