Ojima I. (ed.) Fluorine in Medicinal Chemistry and Chemical Biology
Подождите немного. Документ загружается.

166 Fluorine in Medicinal Chemistry and Chemical Biology
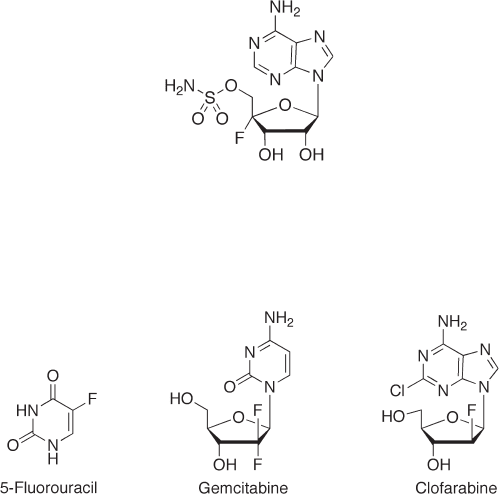
Natural products bearing fl uorine atoms are so rare that only fi ve compounds have
been discovered. Interestingly, a nucleoside analogue, nucleocidin, is one of these fl uori-
nated naturally occurring compounds [1] (see Figure 7.1 ). Its discovery motivated organic
chemists to synthesize fl uorinated nucleosides. Additionally, the practical uses of 5 - fl uo-
rouracil [2] , gemcitabine [3] , and clofarabine [4] as anticancer drugs have also attracted
interest (see Figure 7.2 ). A fl uorinated nucleoside, clevudine (L - FMAU, Levovir) [5] , is
expected to get FDA approval as an anti - hepatitis B virus agent in the near future. Since
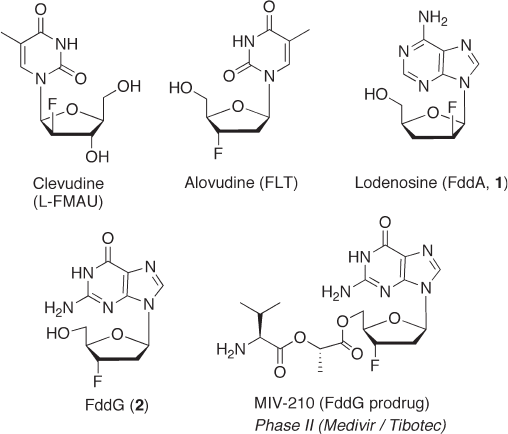
fl uoronucleosides have been shown to be useful in clinical studies, novel antiviral fl uoro-
nucleosides such as FddA ( 1 , lodenosine) [6] , MIV - 210 (a prodrug of FddG ( 2 )) [7] , and
FLT (alovudine, MIV - 310) [8] , are currently under development (see Figure 7.3 ).
There are a number of excellent reviews on the synthesis of nucleoside analogues
bearing fl uorine atom(s) in their sugar moieties [9 – 14] . Therefore, in this chapter, we
describe the syntheses of several fl uorinated nucleoside analogues from common ribonu-
cleosides such as adenosine, guanosine, and uridine, focusing on synthetic issues. As
practical applications, possible process - scale syntheses of FddA (9 - (2,3 - dideoxy - 2 - fl uoro -
β - d - threo - pentofuranosyl)adenine) and FddG (2 ′ ,3 ′ - dideoxy - 3 ′ - α - fl uoroguanosine) are
also described. There are good reviews on the conformational analysis of nucleosides [15] ,
but in this chapter the relationship between the conformations and biological activities of
fl uorinated nucleosides is discussed.
Figure 7.1 Structure of nucleocidin.
Figure 7.2 Structures of antitumor fl uorinated nucleoside analogues.
Synthesis and Biological Activity of Fluorinated Nucleosides 167
7.2 Synthesis and Biological Activity of
2 ′ - Deoxy - 2 ′ - α - fl uororibonucleosides
This section describes the synthesis of 2 ′ - deoxy - 2 ′ - α - fl uoronucleosides using α - selective
fl uorination at C - 2 ′ by several types of nucleophilic substitutions.
7.2.1 Synthesis of 2 ′ - Deoxy - 2 ′ - α - fl uoro Pyrimidine Nucleosides via
Cleavage of Cyclonucleosides
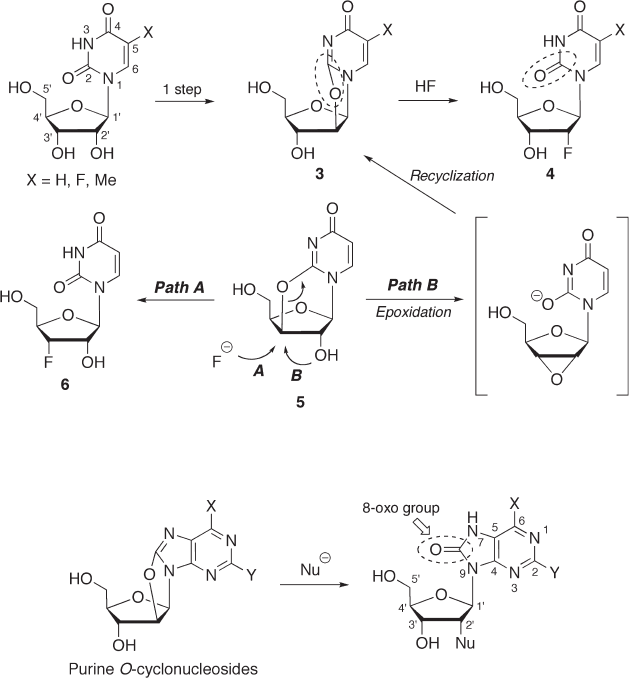
In 1964, Fox and co - workers synthesized 2 ′ - deoxy - 2 ′ - α - fl uorouridine ( 4 , X = H) via cleav-
age of 2,2 ′ - O - cyclouridine ( 3 ) with hydrogen fl uoride in 41 – 46% yield (see Scheme 7.1 )
[16a] . Compound 3 was readily prepared from uridine in one step. In general, a condensa-
tion method, in which sugars are subjected to fl uorination prior to the introduction of
nucleobases, has been used to prepare fl uorinated nucleosides because the direct fl uorina-
tion of nucleosides is diffi cult. There are thus several reasons why Fox ’ s direct fl uorination
is successful. The conversion of the cleaved ether oxygen into the carbonyl group at the
2 - position of uracil is one of the most important. The stability of pyrimidine nucleosides
under severe reaction conditions (anhydrous HF, 100 ° C) also makes this reaction possible.
Thus, it is advisable to use 2,2 ′ - O - cyclouridine to prepare 2 ′ - α - substituted analogues of
uridine; in fact, various substitutions (e.g., by other halogen and azide groups) have been
reported [16a] . Interestingly, the cleavage of 2,3 ′ - O - cyclouridine ( 5 ) with hydrogen fl uo-
ride in the presence of aluminum fl uoride afforded the desired 3 ′ - deoxy - 3 ′ - α - fl uorouridine
( 6 ) in only 31% yield (path A); the major product was 2 ′ - fl uoro analogue 4 (X = H) (47%
Figure 7.3 Structures of antiviral fl uorinated nucleoside analogues.
168 Fluorine in Medicinal Chemistry and Chemical Biology
yield). In contrast, in the reaction of the 2 ′ ,5 ′ - di - O - trityl derivative of 5 , the desired 3 ′ -
deoxy - 3 ′ - α - fl uorouridine analogue 6 was obtained in good yield as a sole product [17] .
Based on these results, the formation of 4 from 5 can be explained by the isomerization
of 5 to 3 through an epoxidation – recyclization pathway (path B) [16b] .
7.2.2 Synthesis of 2 ′ - Deoxy - 2 ′ - α - fl uoro Purine Nucleosides via Nucleophilic
Substitutions at the Carbon - 2 ′ Position
The synthesis of 2 ′ - deoxy - 2 ′ - α - fl uoro purine nucleosides via cleavage of purine O - cyclo-
nucleosides (see Scheme 7.2 ) has not been reported, presumably for the following reasons
[18] : (i) the synthesis of purine O - cyclonucleosides is not so easy as that of pyrimidine
O - cyclonucleosides; (ii) the glycosyl bond of purine nucleosides is rather unstable under
severe reaction conditions such as the use of HF; and (iii) it is not easy to remove the
Scheme 7.1
Scheme 7.2
Synthesis and Biological Activity of Fluorinated Nucleosides 169
resultant 8 - oxo group even if the C - 2 ′ – O bond is successfully cleaved by nucleophilic
substitution. Thus, another synthetic strategy for introducing fl uorine at C - 2 ′ - α has been
developed to achieve the synthesis of 2 ′ - deoxy - 2 ′ - α - fl uoro purine nucleosides. In this
strategy, 3 ′ ,5 ′ - di - O - protected arabanoside is initially prepared from 8,2 ′ - O - cyclo purine
nucleosides, and its 2 ′ - hydroxyl group is then inverted by a nucleophilic (F
−
) substitution
to give the desired 2 ′ - deoxy - 2 ′ - α - fl uoronucleoside.
7.2.2.1 Synthesis of 2 ′ - Deoxy - 2 ′ - α - fl uoroadenosine via an
8,2 ′ - O - Cycloadenosine Derivative
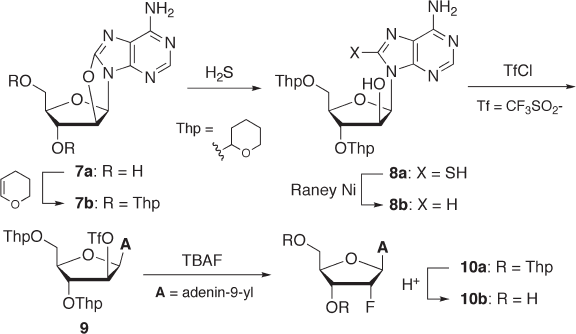
Ikehara and co - workers synthesized 2 ′ - deoxy - 2 ′ - α - fl uoroadenosine ( 10b ) via 8,2 ′ - anhydro -
8 - oxo - 9 - ( β - d - arabinofuranosyl)adenine (8,2 ′ - O - cycloadenosine, 7a ), as shown in Scheme
7.3. First, 7a was effi ciently prepared from 8 - bromoadenosine in three steps using a regi-
oselective 2 ′ - O - tosylation as a key reaction [19, 20] . After both hydroxyl groups of 7a
were protected with a tetrahydro - 2 - pyranyl (Thp) group, the resultant 7b was successively
treated with H
2
S and Raney nickel to afford arabinoside 8b as a key intermediate [21] .
Compound 8b was converted to trifl ate 9 , which, upon subsequent substitution with tetra-
butylammonium fl uoride (TBAF), afforded the S
N
2 product 10a in 60% yield. Finally,
removal of the Thp groups in 10a gave 2 ′ - deoxy - 2 ′ - α - fl uoroadenosine ( 10b ) in good yield
[22] . Notably, its guanosine analogue, 2 ′ - deoxy - 2 ′ - α - fl uoroguanosine, was also synthe-
sized by almost the same procedure [23] , although the yield of the fl uorination was
reduced. As an alternative method for the preparation of 8b , Ranganathan reported a syn-
thetic route that started from a sugar derivative [24] .
7.2.2.2 Synthesis of 2 ′ - Deoxy - 2 ′ - α - fl uoroadenosine Using a Protection Strategy with
3 ′ - and 5 ′ - Hydroxyl Groups
Various methods for protecting the hydroxyl groups of the ribofuranosyl moiety have
been reported to date, and 2 ′ ,3 ′ - O - isopropylidene and 5 ′ - O - trityl groups have been used
Scheme 7.3
170 Fluorine in Medicinal Chemistry and Chemical Biology
extensively in nucleoside chemistry [25a] . The synthesis of 3 ′ ,5 ′ - di - O - acylnucleosides via
selective deprotection of 2 ′ ,3 ′ ,5 ′ - tri - O - acylnucleosides has also been reported by Ishido et
al. [25b] .
In 1978, Markiewicz and co - workers developed a novel diol - protecting group, that
is, a tetraisopropyldisiloxan - 1,3 - diyl (TIDS) group, for regioselective protection of the
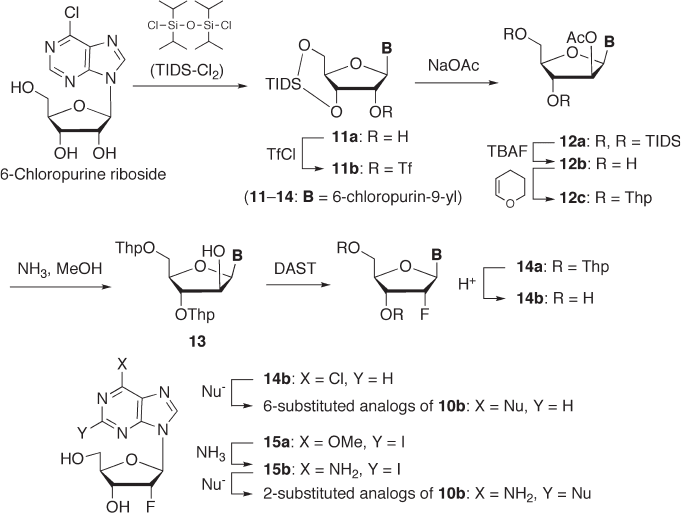
3 ′ ,5 ′ - diol moiety [26] . We applied Markiewicz ’ s strategy to the synthesis of base - modifi ed
analogues of 2 ′ - deoxy - 2 ′ - α - fl uoronucleosides, as shown in Scheme 7.4 . 3 ′ ,5 ′ - O - TIDS -
protected 6 - chloropurine riboside derivative 11a was converted to trifl ate 11b , which was
subsequently transformed to 2 ′ - β - acetate 12a by S
N
2 substitution with acetate ion. Since
a TIDS group is unstable against fl uoride ion, the TIDS of 12a should be changed prior
to fl uorination at C - 2 ′ . Thus, 12a was treated with TBAF to afford 12b , whose hydroxyl
groups were, in turn, protected again with Thp groups to provide 12c . Subsequent hydro-
lysis of the 2 ′ - acetate moiety gave 3 ′ ,5 ′ - di - O - Thp arabinoside 13 , which is a substrate for
fl uorination. Compound 13 was treated with diethylaminosulfur trifl uoride (DAST) to give
14a , and further deprotection provided 2 ′ - α - fl uoride 14b , which was subsequently treated
with various nucleophiles to give the desired base - modifi ed analogues (i.e., 6 - substituted
2 ′ - deoxy - 2 ′ - α - fl uoroadenosine analogues) [27] . This synthetic method can also be applied
to the synthesis of 2 - substituted analogues. Thus, compound 15a , which was prepared
from 2 - iodo - 6 - methoxypurine riboside by almost the same procedure, was converted to
2 - iodoadenine analogue 15b ; its C - 2 position was subjected to various nucleophilic sub-
Scheme 7.4
Synthesis and Biological Activity of Fluorinated Nucleosides 171
stitutions to give a variety of 2 - substituted 2 ′ - deoxy - 2 ′ - α - fl uoroadenosine analogues [28] .
Unfortunately, these 2 - or 6 - substituted compounds did not show any signifi cant anti - HIV
activity [27, 28] .
7.2.2.3 Synthesis of Various 2 ′ - Deoxy - 2 ′ - α - fl uororibosides
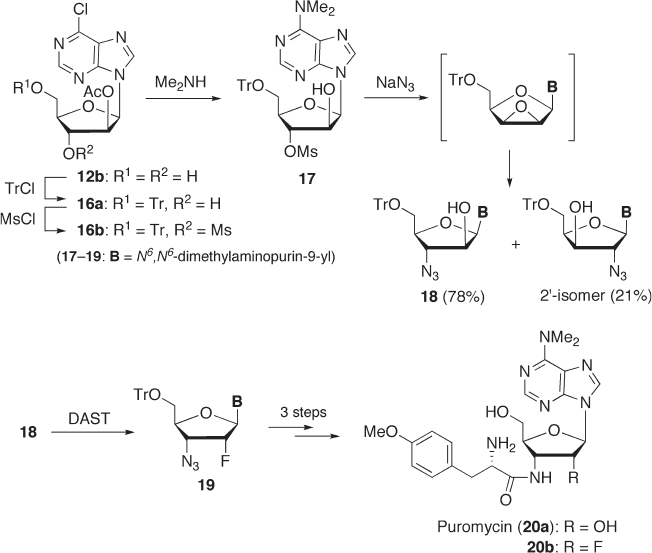
7.2.2.3.1 2 ′ - Deoxy - 2 ′ - α - fl uoropuromycin Puromycin ( 20a ), a broad - spectrum antibi-
otic, inhibits protein biosynthesis at the translation stage by prematurely terminating a
peptide chain (see Scheme 7.5 ) [29] . It is generally accepted that puromycin can bind to
the A site of a ribosome, where the peptidyl chain of a peptidyl - tRNA is transferred to the
amino group of the p - methoxyphenylalanine moiety of puromycin. However, the role of
the 2 ′ - hydroxyl group in this puromycin reaction is still unclear. Thus, we performed the
synthesis and biological evaluation of 2 ′ - deoxy - 2 ′ - α - fl uoropuromycin ( 20b ) to gain a
better understanding. The 5 ′ - and 3 ′ - hydroxyl groups of 2 ′ - O - acetate 12b were modifi ed
with trityl and mesyl groups, respectively, to give 16b , which was subsequently reacted
with dimethylamine to afford 3 ′ - O - mesyl arabinoside 17 (see Scheme 7.5 ). Treatment of
17 with sodium azide gave a mixture of 3 ′ - azido - arabinoside 18 and its 2 ′ - isomer (78%
and 21% yields, respectively) via an epoxide intermediate. The major product 18 was
Scheme 7.5
172 Fluorine in Medicinal Chemistry and Chemical Biology
treated with DAST to give 19 , which was converted to the desired 20b in a three - step
sequence (hydrogenation of the 3 ′ - N
3
group, aminoacylation of the resultant 3 ′ - NH
2
group,
and removal of the protective groups) [30] . The biological activity of 20b was evaluated.
Interestingly, 20b showed appreciable antitumor activity in vitro and also exhibited anti-
bacterial potency that was comparable to that of puromycin. These activities are likely
caused by the inhibition of protein biosynthesis.
It has been recognized that the 2 ′ - hydroxyl group of puromycin is indispensable for
its biological potency since 2 ′ - deoxypuromycin showed no signifi cant activity [31] .
However, on the basis of our present result, it seems that the hydroxyl group at the 2 ′ - posi-
tion is not essential for the biological potency of puromycin. The coupling constant of
1
H
NMR between 1 ′ - H and 2 ′ - H in 20b ( J
1
’
– 2
’
) is nearly zero, which means that 20b has the
3 ′ - endo conformation ( N - conformation), similar to that of ribonucleosides composing
RNA. Therefore, the biological potency of puromycin does not seem to require the 2 ′ -
hydroxyl group itself, but the 3 ′ - endo conformation of the sugar moiety, which can be
formed by the presence of the hydroxyl or fl uoro group at C - 2 ′ , is important.
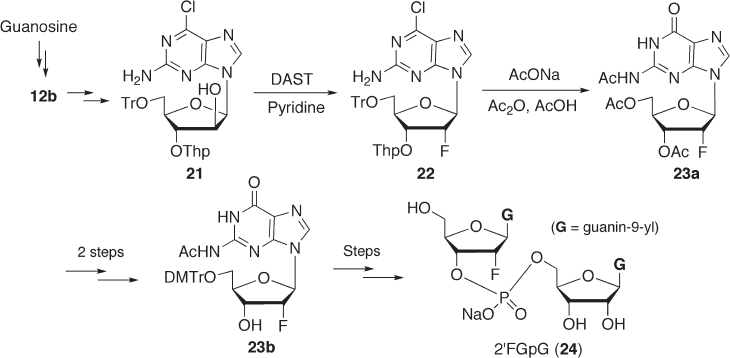
7.2.2.3.2 2 ′ FG p G Since some RNases have gained considerable attention because of
their specifi c cytotoxicity toward cancer cells and their use as cancer chemotherapy drugs
[32] , there have been reported many x - ray crystallographic analyses of complexes of
RNase with dinucleoside monophosphate seeking to better understand the structure – activ-
ity relationship (SAR) of such ribonucleases. Among them, dinucleoside monophosphates
containing a 2 ′ - deoxy - 2 ′ - α - fl uoronucleoside are expected to be a good substrate for study
because of their enzymatic resistance to RNases [33] . This resistance is likely due to the
lack of the 2 ′ - hydroxyl group, which is essential for the cleavage by RNase. Notably, it
seems that dimer analogues composed by 2 ′ - deoxy - 2 ′ - α - fl uoronucleosides retain the 3 ′ -
endo conformation (RNA - type conformation). We reported the synthesis of a GpG ana-
logue 24 (2 ′ FGpG) containing 2 ′ - deoxy - 2 ′ - α - fl uoroguanosine (see Scheme 7.6 ). In our
Scheme 7.6
Synthesis and Biological Activity of Fluorinated Nucleosides 173
synthesis, 2 - amino - 6 - chloropurine riboside was used as a precursor for guanine, since
previous reports revealed that attempted fl uorination of the 2 ′ - hydroxy group in arabino-
furanosylguanine (ara - G) derivatives resulted in low yield [22, 34, 35] . Thus, 2 - amino - 6 -
chloropurine riboside, which was readily prepared from guanosine, was transformed to
2 ′ - O - acetyl arabinoside ( 12b ’ , B = 2 - amino - 6 - chloropurine) and further converted to 21
in three steps. Compound 21 was reacted with DAST in the presence of pyridine to afford
2 ′ - α - fl uoro compound 22 in 60% yield, which was treated with sodium acetate in a mixture
of acetic acid and acetic anhydride to afford N
3
, O
3 ′
, O
5 ′
- triacetyl - 2 ′ - deoxy - 2 ′ - α - fl uoro-
guanosine ( 23a ). Under these reaction conditions, the following transformations were
performed in one pot: (a) hydrolysis of the 6 - chloro moiety to the corresponding 6 - oxo
group (via 6 - acetoxylation); (b) deprotection of the 3 ′ - and 5 ′ - hydroxyl groups; and (c)
acetylation of the resulting hydroxyl groups and the 2 - amino group. After the protecting
groups of 23a were changed to those of 23b in two steps, compound 23b was coupled
with a guanosine derivative (3 ′ - component) by a phosphoroamidate method to give the
desired dimer 24 (2 ′ FGpG) [36] . The 2 ′ - deoxy - 2 ′ - α - fl uoroguanosine moiety in 24 seems
to have the 3 ′ - endo conformation ( J
1
’
– 2
’
= 3.0 Hz). Thus, compound 24 is expected to have
a conformation similar to that of GpG.
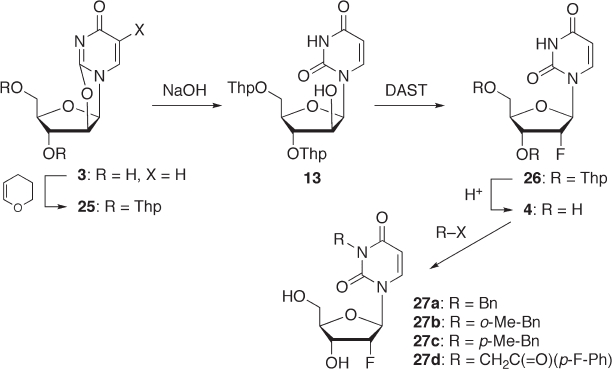
7.2.2.3.3 N
3
- Substituted 2 ′ - Deoxy - 2 ′ - α - fl uorouridine and Its Hypnotic Activity -
Uridine and N
3
- benzyluridine have been reported to show sleep - promoting activity [37]
and hypnotic activity [38] , respectively. We have been very interested in this topic and
have studied the hypnotic activity of N
3
- substituted 2 ′ - deoxy - 2 ′ - α - fl uorouridines to deter-
mine their SAR trends. For the synthesis of the key compound 4 (2 ′ - deoxy - 2 ′ - α - fl uorou-
ridine), an alternative synthetic route via arabinoside was developed since a previously
reported method [39] involving a ring - cleavage reaction of 2,2 ′ - O - cyclouridine ( 3 , X = H)
required severe reaction conditions, such as exposure to HF (see Scheme 7.1 ). The new
route is illustrated in Scheme 7.7. The hydroxyl groups of 2,2 ′ - O - cyclouridine ( 3 ) were
Scheme 7.7
174 Fluorine in Medicinal Chemistry and Chemical Biology
protected with Thp groups to afford 25 , which was subsequently treated with sodium
hydroxide to yield arabinoside 13 . Treatment of 13 with DAST in the presence of pyridine
gave 2 ′ - α - fl uoro compound 26 in 57% yield, which was deprotected to afford 2 ′ - deoxy -
2 ′ - α - fl uorouridine ( 4 ). Compound 4 was reacted with various alkyl halides such as benzyl
bromide and 2 - chloro - 4 ′ - fl uoroacetophenone to give the corresponding N
3
- alkyl deriva-
tives 27a – d [39] . Although these derivatives exhibited hypnotic activity, they were weaker
than the corresponding uridine derivatives. Since these derivatives are considered to have
the 3 ′ - endo conformation ( J
1
’
– 2
’
= 3.5 Hz), the 2 ′ - hydroxyl group seems to be important for
the hypnotic activity.
7.3 Syntheses and Antiviral Activities of F dd A and F dd G
7.3.1 Synthetic Issues Regarding 2 ′ - Deoxy - 2 ′ - b - fl uoroarabinosides
To introduce a β - fl uoro group at the C - 2 ′ position of a ribonucleoside, one may fi rst try
an S
N
2 reaction of a fl uoride ion with a 2 ′ - hydroxyl group activated as a leaving group,
such as trifl ate. However, in most cases, its nucleobase (both pyrimidine and purine) pre-
vents this reaction because (a) steric hindrance of the nucleobase interrupts the nucleo-
philic (F
−
) attack from the top face, and (b) the nucleobase reacts intramolecularly with
its own sugar moiety. Therefore, 2 ′ - β - fl uoro substitution is generally more diffi cult than
the corresponding α - fl uoro substitution.
7.3.1.1 Attempted synthesis of pyrimidine 2 ′ - deoxy - 2 ′ - β - fl uoroarabinosides
In the case of pyrimidine nucleosides, the S
N
2 reaction of 2 ′ - O - activated ribonucleoside
(e.g., 2 ′ - trifl ate) forms a 2,2 ′ - O - cyclo bond prior to substitution of a nucleophile such as
fl uoride ion. Therefore, with the hope of suppressing this intramolecular substitution, the
S
N
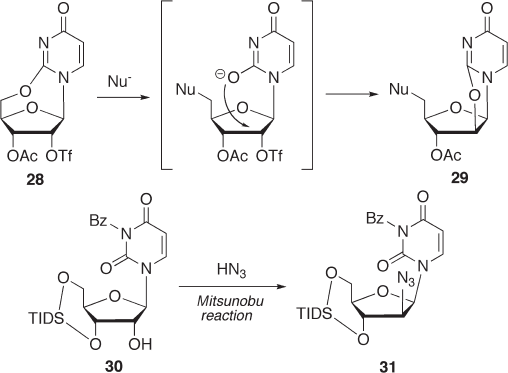
2 reaction of 2,5 ′ - O - cyclouridine derivative 28 , which places a trifl ate group at C - 2 ′ ,
was attempted (see Scheme 7.8 ). However, unexpectedly, a 5 ′ - substituted 2,2 ′ - O - cyclo
derivative 29 was obtained [40] . The reaction mechanism to form 29 can be explained as
follows. The nucleophilic attack at C - 5 ′ of 28 forms a 5 ′ - substituted intermediate, which
causes an intramolecular substitution of the 2 - oxo group at C - 2 ′ to give 29 . In some cases,
the introduction of an electron - withdrawing group at the N - 3 position is effective for
reducing the nucleophilicity of the 2 - oxo group. Only one successful example has been
reported by Matsuda and co - workers, in which an N
3
- benzoyl derivative 30 was condensed
with hydrogen azide using a Mitsunobu reaction to give 2 ′ - α - azido analogue 31 [41] .
However, no fl uorination studies using the S
N
2 - type reactions like this have been reported.
Therefore, the development of an effective method for suppressing the nucleophilicity of
the 2 - oxo group is still a challenging theme in the chemistry of pyrimidine nucleosides
(the details were reviewed by Pankiewicz et al. [14] .).
7.3.1.2 Synthetic Issues Regarding Purine 2 ′ - deoxy - 2 ′ - β - fl uoroarabinosides
The purine base also disturbs nucleophilic substitution at the sugar moiety, but the degree
of this disruption is controllable. The nucleophilicity of the N - 3 atom was reduced by the
Synthesis and Biological Activity of Fluorinated Nucleosides 175
introduction of electronegative group(s) to the purine base. In addition, the choice of the
protective group(s) of sugar - OHs is important to control side - reactions.
7.3.1.2.1 Participation of Purine N - 3 Atom In the case of purine ribonucleosides, the
participation of the purine N - 3 atom is a critical issue. For instance, the treatment of 32
with DAST furnished the desired 2 ′ - β - analogue 34a in only 30% yield because of an
undesirable side - reaction that formed by - product 33 in 51% yield [42] (see Scheme 7.9 ).
Compound 33 is probably formed as follows. The reaction of 32 with DAST gives the 2 ′ -
O - activated intermediate, which could receive not only the desired nucleophilic attack of
fl uoride ion, but also the intramolecular attack of the N - 3 atom. The latter forms a new
bond between N - 3 and C - 2 ′ of the sugar, which leads to an alternative attack of fl uoride
ion at the C - 1 ′ position to yield the 1 ′ - α - fl uoro product. Therefore, reducing the nucleo-
philicity of the N - 3 atom is effective for controlling this side - reaction. Pankiewicz and
co - workers successfully synthesized the desired purine 2 ′ - deoxy - 2 ′ - β - fl uoroarabinosides
34b in 63% yield by treating N
1
- benzylinosine derivative 35 with DAST [42] (79% yield
when tris(dimethylamino)sulfur(trimethylsilyl)difl uoride (TASF) was used as another fl uo-
rinating agent) [43] . We performed this conversion with 6 - chloropurine derivative 36 ,
whose N - 3 atom was expected to show weaker nucleophilicity than that of 32 , and then
the yield of 34c was indeed improved to 87% [44] . Consequently, effective β - fl uorination
at C - 2 ′ was achieved by the introduction of an electron - withdrawing group onto the base
moiety.
7.3.1.2.2 Elimination Another problem in the synthesis of 2 ′ - deoxy - 2 ′ - β - fl uoroarabi-
nosides is elimination. This issue seems to be infl uenced by the conformation of the sugar
moiety. For instance, the reaction of 37 with TASF did not give a 2 ′ - β - fl uoro derivative,
but rather two elimination products 38 and 39 [43] (see Scheme 7.10 ). The undesired
eliminations are probably caused by the 3 ′ - endo conformation of the sugar moiety in 37 ,
whose 3 ′ - hydrogen and 2 ′ - trifl ate are arranged in almost a trans - diaxial orientation that
Scheme 7.8
