Ojima I. (ed.) Fluorine in Medicinal Chemistry and Chemical Biology
Подождите немного. Документ загружается.


10
Fluorinated Moieties for Replacement
of Amide and Peptide Bonds
Takeo Taguchi and Hikaru Yanai
10.1 Introduction
Chemical modifi cations of peptides to improve their biological activity, in vivo stability
or bioavailability have been studied extensively [1, 2] . As one of the promising approaches,
replacement of a specifi c amide bond in the target peptide molecule by an appropriate
amide bond surrogate can not only bring about enhanced recognition by the target enzyme,
which induces the parent peptide to exhibit biological responses, but also facilitate pepti-
dase resistance, conformational control or increase of the lipophilicity of the target peptide
(see Figure 10.1 ) [1, 2] .
Isosteric amide replacements are commonly shown using the symbol Ψ [ ], where
the Ψ indicates the absence of an amide bond and the structure that is replacing the amide
is shown in the brackets. The alkene dipeptide isostere Ψ [CH = CH], which is conforma-
tionally fi xed and enzymatically nonhydrolyzable (peptidase resistance), has been well
studied because of its close similarity to the amide bond with regard to the steric demand,
bond lengths, and bond angles, as described in Chapter 1 [3] . However, the low polarity
of the alkene moiety compared with the amide bond, as well as the small dipole moment
and the lack of ability to form hydrogen bonds are quite different characteristics of
Ψ [CH = CH] as a replacement of the amide bond. Introduction of fl uorine into the double
bond remarkably changes the electronic nature of the double bond to one much closer
to that of the amide bond, while still keeping steric similarity [4 – 7] . In addition to the
fl uoro - olefi n dipeptide isostere Ψ [CF = CH], several types of fl uorinated moieties such
Fluorine in Medicinal Chemistry and Chemical Biology Edited by Iwao Ojima
© 2009 Blackwell Publishing, Ltd. ISBN: 978-1-405-16720-8
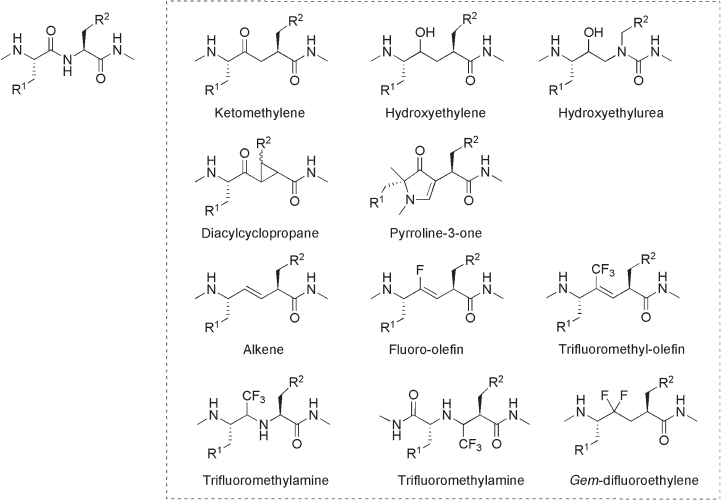
258 Fluorine in Medicinal Chemistry and Chemical Biology
as trifl uoromethyl - olefi n Ψ [C(CF
3
) = CH], trifl uoromethylamine Ψ [CH(CF
3
) - NH] and
difl uoroethylene Ψ [CF
2
- CH
2
] have been developed as dipeptide isosteres. In this chapter,
recent achievements in fl uoro - olefi n Ψ [CF = CH] and trifl uoromethyl - olefi n Ψ [C(CF
3
) = CH]
dipeptide isosteres are reviewed. Developing bioorganic chemistry of fl uoroarenes as
replacement for nucleoside bases is also briefl y described.
10.2 Fluoro - olefi ns Ψ [CF = CH] as Dipeptide Isosteres
10.2.1 Synthetic Methods
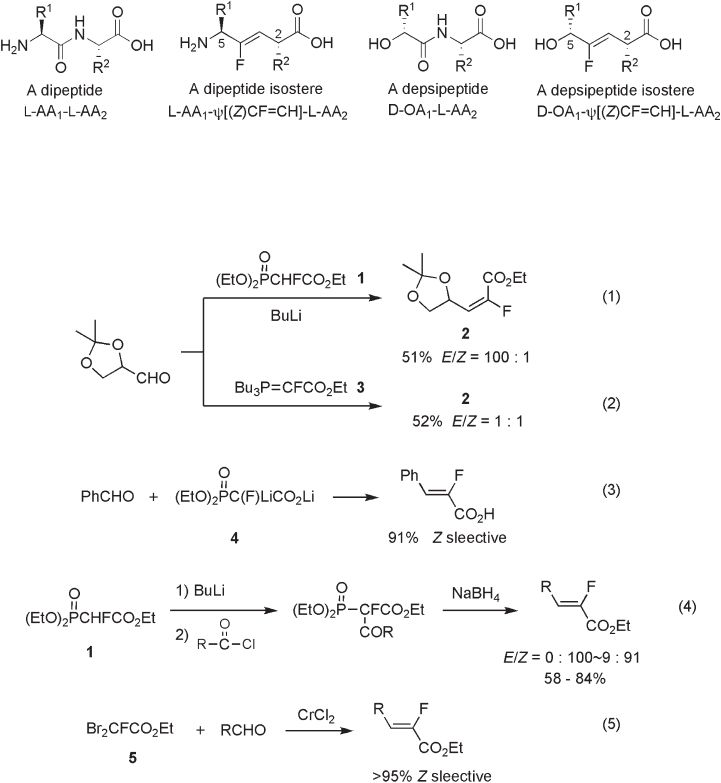
As described above, the fl uoro - olefi ns are the most studied fl uorinated peptide mimetics.
For the synthesis of the fl uoro - olefi n Ψ [CF = CH] dipeptide isostere or the depsipeptide
isostere (5 - hydroxy derivative, see Figure 10.2 ), stereochemical control of the olefi n con-
fi guration (either E or Z ) and the relative stereochemistry of the two chiral centers at C - 2
and C - 5 (either syn or anti ) is a major issue to be solved. Furthermore, the use of readily
available starting material is also important. Since the pioneering work by Allmenginger
et al . in the early 1990s [8] , many research groups, as described in this section, have con-
tributed to this development of facile synthetic methods for fl uorinated dipeptide isosteres,
in particular for optically pure forms, but this subject is still challenging. In this section
are summarized (i) synthetic methods mainly focused on the olefi nation reactions of car-
Figure 10.1 Various peptide bond mimics.
Fluorinated Moieties for Replacement of Amide and Peptide Bonds 259
bonyl compounds and related reactions, (ii) utilization of fl uoroalkenyl building blocks,
and (iii) application to the modifi cations of the target peptides of biological interest.
10.2.1.1 Utilization of Organophosphorus and Organosilyl Reagents
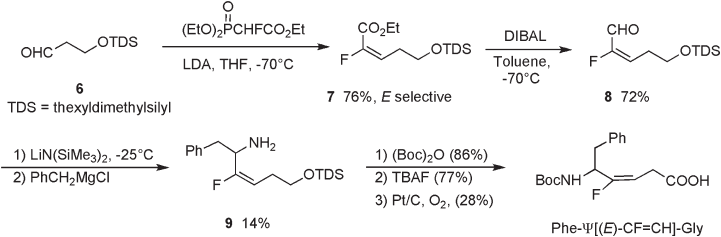
The Honor – Wadsworth – Emmons (HWE) reaction of α - fl uoro - α - phosphonoacetate 1 with
aldehydes or ketones, providing α - fl uoro - α , β - unsaturated carbonyl compounds, is one of
commonest methods for fl uoro - olefi ns having suitable functionality for further elaboration
to the target molecules (see equation 1, Scheme 10.1 ) [9] . The stereochemical outcome of
this reaction with both aliphatic and aromatic aldehydes is generally excellent, giving rise
Figure 10.2 Replacement of amide bonds by ( Z ) - fl uoro - olefi ns.
Scheme 10.1
260 Fluorine in Medicinal Chemistry and Chemical Biology
to the ( E ) - isomer. In contrast, the Wittig reaction of phosphorane 3 (Bu
3
P = CFCO
2
Et) with
aldehyde proceeds in nonstereoselective manner (see equation 2, Scheme 10.1 ). The reac-
tions of the dianion of α - fl uoro - α - phosphonoacetic acid 4 , with aromatic aldehydes give
Z - isomers exclusively, while those with aliphatic aldehydes are nonstereoselective (see
equation 3, Scheme 10.1 ) [10] . Sano and Nagao reported the Z - selective synthesis through
acylation of 1 with acyl chloride, followed by NaBH
4
reduction of the ketone group (see
equation 4, Scheme 10.1 ) [11] . Falck and Mioskowski reported a high yield and Z - selec-
tive synthesis of α - haloacrylates by the Cr(II) - mediated Reformatsky type reaction of tri-
haloacetate, including dibromofl uoroacetate 5 for fl uoro derivatives, with aldehydes (see
equation 5, Scheme 10.1 ) [12] .
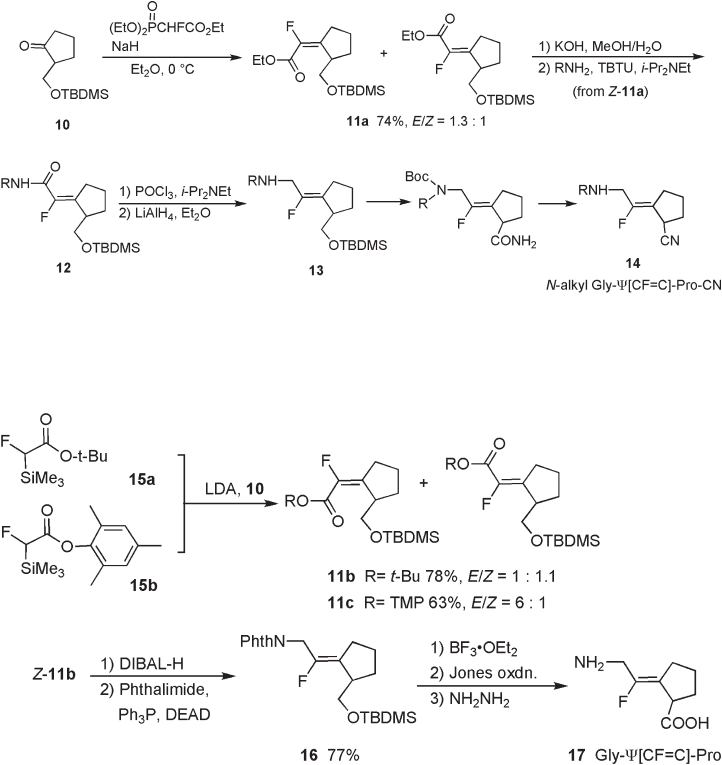
Allmenginger reported the preparation of Phe - ψ [( E ) - CF = CH] - Gly in racemic form
via E - selective HWE reaction, yielding α - fl uoroenoate 7 . Conversion of the ( E ) - aldehyde
8 to N - silylimine, followed by the addition of the Grignard reagent afforded amine 9
without isomerization of the double bond (see Scheme 10.2 ) [13] .
The HWE reaction of (EtO)
2
P(O)CHFCO
2
Et ( 1 ) with ketones proceeds in nonselec-
tive manner. For example, Augustyns reported the HWE reaction of 1 with cyclopentanone
derivative 10 to give a mixture of α - fl uoroenoate 11a ( E / Z = 1.3: 1), which, after separa-
tion of isomers, was converted to N - alkyl Gly - ψ [CF = C] - Pro - CN ( 14 ) for their SAR studies
on dipeptidyl peptidase (DPP) inhibitors (see Scheme 10.3 ) [14] . In this synthesis, conver-
sion of amide 12 to amine 13 was achieved by LiAlH
4
reduction of the imidoyl derivative
formed by treating 12 with POCl
3
. Without this pre - treatment, LiAlH
4
reduction of 12
gave 13 in very low yield because of several side - reactions (see Scheme 10.3 ).
For the preparation of Gly - ψ - [CF = CH] - Pro in relation to the study of cyclophilin
A inhibitors, Welch and co - workers employed the Peterson reaction of α - fl uoro - α -
trimethylsilyl acetate ( 15a,b ) with ketone 10 . E / Z selectivity was found to be infl uenced
by the ester part of the acetate (see Scheme 10.4 ) [15] . The reaction of tert - butyl ester 15a
gave almost an equal amount of the isomers ( 11b , E : Z = 1 : 1.1), while moderate E
selectivity was observed when trimethylphenyl ester 15b was used ( 11c , E : Z = 6 : 1).
Conversion of ester Z - 11b to amino derivative 16 was achieved via the Mitsunobu reaction
of phthalimide with the alcohol formed by the DIBAL - H reduction of Z - 11b .
Scheme 10.2
Fluorinated Moieties for Replacement of Amide and Peptide Bonds 261
Scheme 10.3
Scheme 10.4
10.2.1.2 Ring Opening Reaction of Oxygenated Chlorofl uorocyclopropanes to
( Z ) - α - Fluoroenal
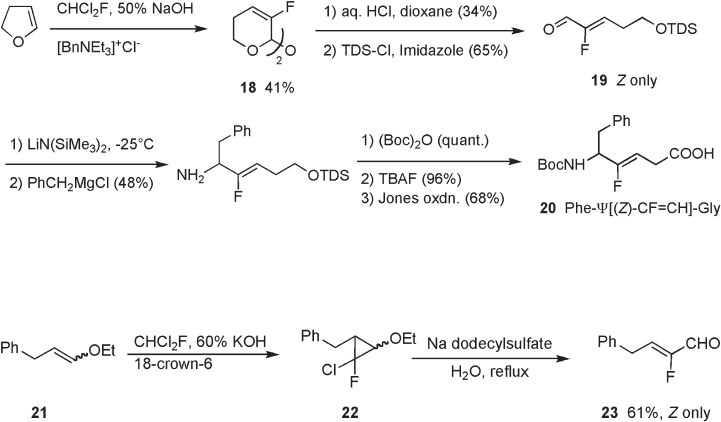
Under acidic conditions, α - fl uoro - α , β - unsaturated aldehydes form the thermodynamically
stable Z - isomer. For the preparation of ( Z ) - aldehyde 19 , acid hydrolysis of pyrane deriva-
tive 18 , whose olefi n - confi guration corresponds to the E confi guration, was affected due
to facile isomerization under the reaction conditions. Pyrane 18 was obtained by chloro-
fl uorocarbene addition to dihydrofuran under phase - transfer conditions (see Scheme 10.5 )
[13] . ( Z ) - Aldehyde 19 was converted to Ph - ψ [( Z ) - CF = CH] - Gly ( 20 ) using the same reac-
tions for the preparation of the ( E ) - isomer as described above (see Scheme 10.2 ).
262 Fluorine in Medicinal Chemistry and Chemical Biology
Carbene addition to an E / Z mixture of vinyl ether 21 , followed by solvolysis of the
resultant cyclopropane 22 afforded ( Z ) - α - fl uoroenal 23 (Scheme 10.6 ) [16] . ( Z ) - α - Fluo-
roenal 23 was used for the preparation of Substance P analogues containing a Phe - Ψ [( Z ) -
CF = CH] - Gly dipeptide unit as described next.
10.2.1.3 Imidate [3,3] - Sigmatropic Rearrangement
Transposition of an allylic hydroxyl group into an amino group through [3,3] - sigmatropic
rearrangement is well known as the Overmann rearrangement, which has been applied to
the total synthesis of a variety of nitrogen - containing natural products. In general, the
rearrangement proceeds via a six - membered chair - like transition state, resulting in a
perfect chirality transfer of the stereogenic center at the allylic hydroxyl moiety. For the
synthesis of a Phe - Ψ [( Z ) - CF = CH] - Gly dipeptide unit in optically pure form to be applied
to Substance P analogues, Allemendinger employed enantioselective aldol reactions of
chiral enolates 24 and 25 with ( Z ) - α - fl uoroenal 23 , giving rise to both enantiomers ( R ) - 26
and ( S ) - 26 , respectively, with high optical purities (see Scheme 10.7 ) [16] .
Enantiomerically pure imidate 27 was subjected to thermal rearrangement at 140 ° C
to give the desired amide derivative 28 in good yield, retaining the optical purity of 26
(see Scheme 10.8 ). Jones oxidation of the primary alcohol to carboxylic acid afforded
dipeptide isostere 29 in optically pure form, which was applied to the preparation of Sub-
stance P analogues 30 [16] . It should be noted that nonfl uorinated analogues easily undergo
isomerization of the double bond to form α , β - unsaturated amides, while the fl uorinated
double bond in these compounds and the corresponding intermediates is stable under basic
and acidic conditions employed, refl ecting the stabilizing effect of fl uorine on the double
bond [17] .
Scheme 10.5
Scheme 10.6
Fluorinated Moieties for Replacement of Amide and Peptide Bonds 263
Scheme 10.7
Scheme 10.8
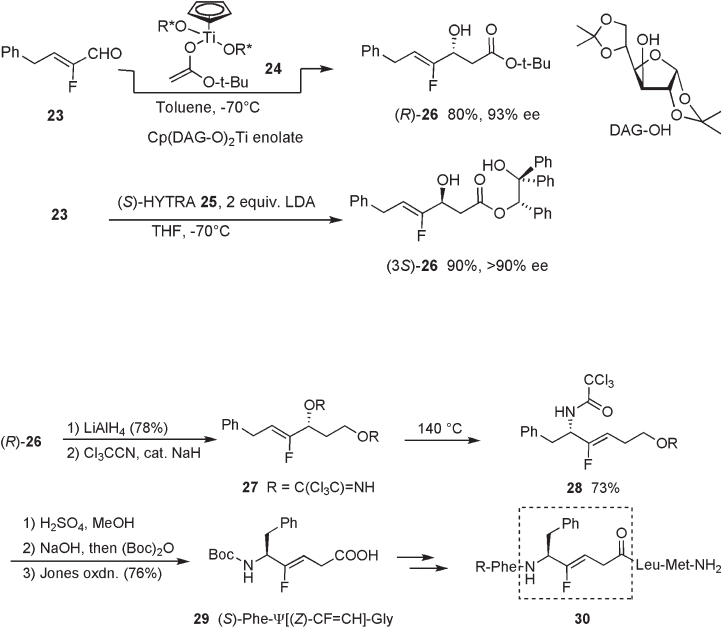
For the synthesis of fl uoro - olefi n dipeptide isosteres corresponding to dipeptides
consisting of two chiral amino acids in stereochemically pure forms with respect to both
the confi guration of the C – C double bond and the chiral centers at C - 2 and C - 5, we
reported the imidate rearrangement route starting from optically pure aldehydes derived
from, for example, commercially available ester 31 (see Scheme 10.9 ) [18] . Indium - medi-
ated difl uoroallylation of aldehyde 32 gave difl uorohomoallyl alcohol 33 as a mixture of
diastereomers, which were separated by column chromatography. In the presence of CuI
(20 mol%), Grignard reaction of 32 with difl uorohomoallyl alcohol 33 afforded allylic
substitution product 34 in a highly Z - selective manner, wherein both aliphatic and aromatic
Grignard reagents can be employed. Scheme 10.9 illustrates a typical example. The
imidate rearrangement of syn - 34c led to the formation of syn - 35c and the subsequent
conversion to the carboxylic acid proceeded smoothly to give Leu - Ψ [CF = CH] - Ala ( syn -
36c ) without any loss in optical purity. This method has some generality since a variety
of Grignard reagents can be used for the preparation of fl uoroallyl alcohols 34 from 33 .
Alternatively, the starting chiral aldehydes, such as 32 , could also be readily prepared by
hydroxymethylation of carboxylic acid derivatives using a chiral auxiliary protocol [19] .
264 Fluorine in Medicinal Chemistry and Chemical Biology
Preparation of N - Ac - Glu - Ψ [CF = CH] - Ala was also reported using the imidate rearrange-
ment protocol [20] .
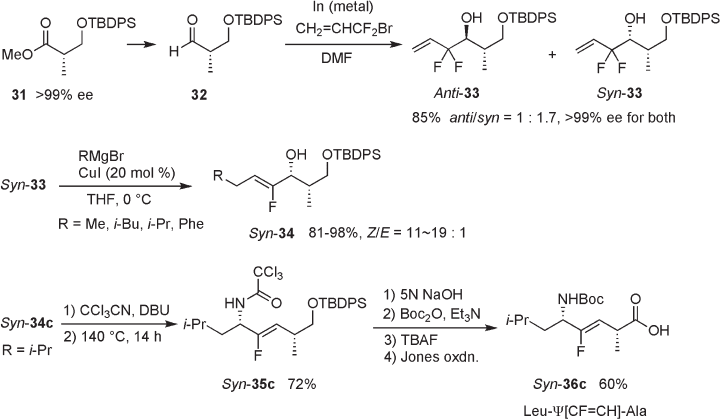
10.2.1.4 Aldolization of Fluorooxaloacetate
Bartlett and Otake employed aldol reaction of an in - situ - generated fl uorooxaloacetate with
a chiral aldehyde derived from 37 to prepare α - fl uoroenoate 38 . Stereoselectivity was
moderate ( Z : E = 2.3 : 1), with preferential formation of the Z - isomer (see Scheme 10.10 )
[19] . Further elaboration led to the preparation of tripeptide analogues, Cbz - Gly - Ψ [( Z ) -
CF = CH] - Leu - Xaa ( 39 ).
10.2.1.5 Sulfoxide - elimination Reaction
Alkylation of the anion of α - fl uoro - α - phenylsulfi nylacetate 40 with an alkyl halide, fol-
lowed by thermal syn - elimination of sulfi nic acid, gave α - fl uoroenoate 41 in Z - selective
manner (see Scheme 10.11 ) [21] .
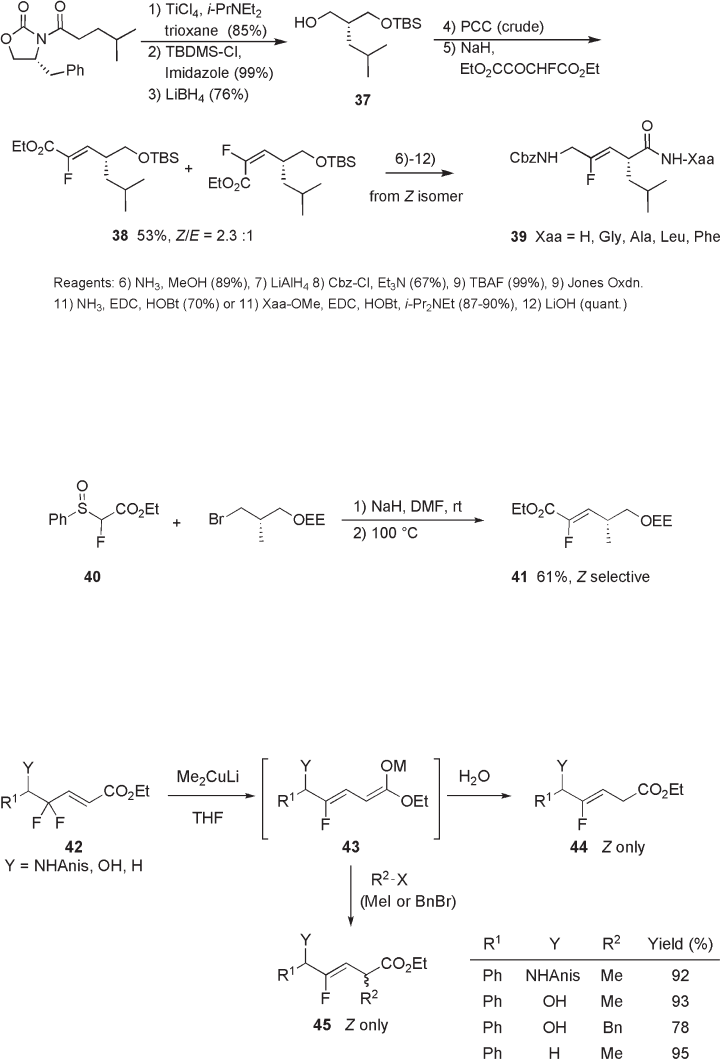
10.2.1.6 Defl uorinative Allylic Substitution and Related Reactions
Reaction of γ , γ - difl uoro - α , β - enoate derivatives 42 with Me
2
CuLi (5 equivalents), followed
by aqueous work - up afford the reductive defl uorination product 44 without forming the
α - methylated product. It is postulated that enolate intermediate 43 reacts with alkyl halide
smoothly and regioselectively to give the desired α - alkylated product 45 (see Scheme
10.12 ) [22, 23] . The reaction proceeded in an excellently Z - selective manner.
Otaka and Fujii demonstrated that alkyl transfer from Me
2
CuLi can be promoted by
air oxidation of intermediate 43 (see Scheme 10.13 ) [24] .
Scheme 10.9
Fluorinated Moieties for Replacement of Amide and Peptide Bonds 265
Scheme 10.10
Scheme 10.11
Scheme 10.12
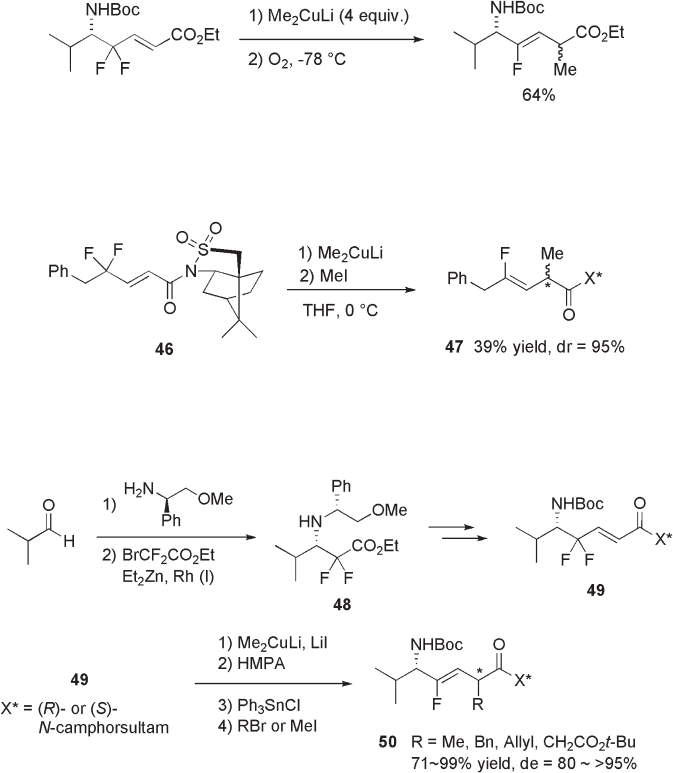
266 Fluorine in Medicinal Chemistry and Chemical Biology
Although Me
2
CuLi - mediated reductive defl uorination of 42 , followed by α - alkylation
with alkyl halide, gave product 45 in high yield and in Z - selective manner, the relative
stereochemistry at C - 5 and C - 2 was not controlled (see Scheme 10.12 ). For the stereo -
controlled alkylation at C - 2, a chiral auxiliary at the carboxyl moiety was used, and rela-
tively high diastereoselectivity was realized in the case of camphorsultam derivative 46
(see Scheme 10.14 ) [23] .
For the preparation of chiral nonracemic dipeptide isosteres, Otaka and Fujii also
reported the use of camphorsultam derivative 49 , which was prepared in optically pure
form through the Reformatsky reaction of BrCF
2
CO
2
Et with a chiral imine (see Scheme
10.15 ) [25] .
Scheme 10.13
Scheme 10.14
Scheme 10.15
