Ojima I. (ed.) Fluorine in Medicinal Chemistry and Chemical Biology
Подождите немного. Документ загружается.

Fluorinated Moieties for Replacement of Amide and Peptide Bonds 277
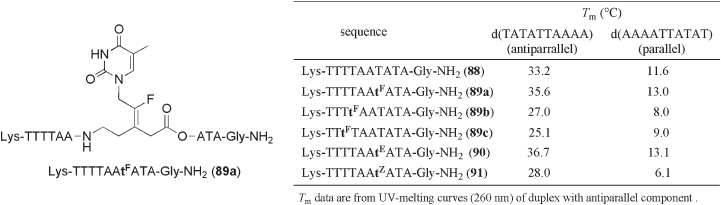
sponding PNA sequence, which was equipped with lysine units at the N - termini to enhance
water solubility and for easy purifi cation.
The T
m
value of fl uoroalkene isostere 89a with antiparallel DNA component was
found to be slightly higher than that of the normal PNA oligomer 88 ( ∆ T
m
= +2.4 ° C) (see
Figure 10.8 ). This result indicates better stabilization of the PNA/DNA duplex through
introduction of a fl uoroalkene unit at the central part of the PNA backbone. Nonfl uorinated
( E ) - alkene isostere 90 [ note: The ( E ) - alkene isostere has the same geometric confi guration
as the ( Z ) - fl uoroalkene isostere] at the same location in the PNA sequence provided better
stabilization of the PNA/DNA duplex with antiparallel DNA component than that of 88
( ∆ T
m
= +3.5 ° C). In contrast, nonfl uorinated ( Z ) - alkene isostere 91 resulted in signifi cant
destabilization of the duplex ( ∆ T
m
= − 5.2 ° C). Thus, it is suggested that the Z - confi guration
of the double bond may cause a structural mismatch. Interestingly, the stability of the
PNA/DNA duplexes of decamers 89 , containing single fl uoroalkene modifi cation with
antiparallel DNA, was found to be strongly dependent on its position in the PNA sequence,
with ∆ T
m
values ranging from +2.4 to − 8.1 ° C. Additionally, a similar tendency was
observed for the PNA/DNA duplexes with parallel DNA.
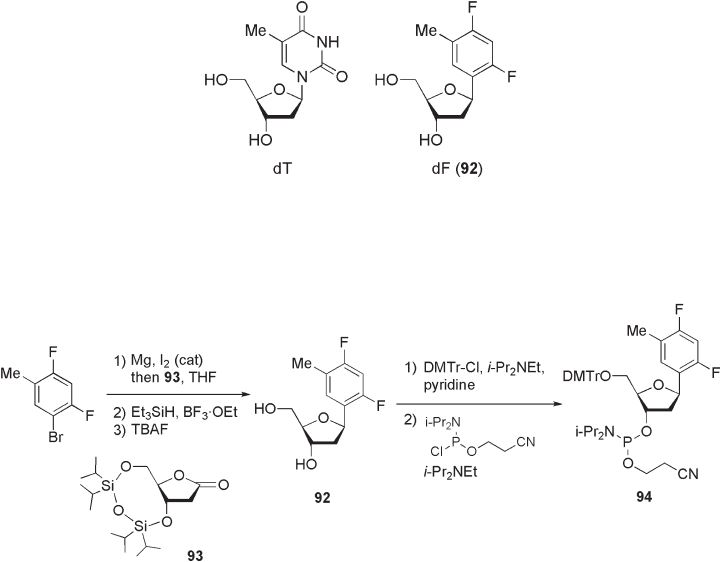
10.2.2.4 Fluoroarenes as Nucleoside Base Mimics
The use of fl uoroarene derivatives as structural mimetics of nucleobases has been studied
extensively [47, 48] . In pioneering work in this fi eld, Kool and co - workers reported the
structural similarity between natural 2 ′ - deoxythymidine (dT) and 1 ′ ,2 ′ - dideoxy - 1 ′ - (2,4 -
difl uoro - 5 - methylphenyl) - β - d - ribofuranoside 92 (dF) (see Figure 10.9 ) [49, 50] .
As shown in Scheme 10.27 , fl uoroarene isostere 92 was prepared from 2,4 - difl uoro -
5 - bromotoluene and silyl - protected lactone 93 in three steps [51] . Thus, the coupling
reaction of lactone 93 with an aryl Grignard regent, which was prepared from 2,4 - difl uoro -
5 - bromotoluene and magnesium in the presence of catalytic amount of iodine, and the
subsequent reductive dehydroxylation of lactol intermediate by Et
3
SiH resulted in the β -
selective introduction of difl uorotoluyl group. Further treatment with TBAF deprotected
the silyl groups to give 92 in excellent yield. With the use of essentially the same proce-
dure, dichloro - and dibromo - analogues were also prepared. To incorporate 92 into the
DNA sequence, 5 ′ - OH selective protection of 92 with 4,4 ′ - dimethoxytrityl chloride (DMTr -
Cl) and subsequent phosphorylation with 2 - cyanoethyl N , N - diisopropylchlorophosphora-
midite gave the fully protected nucleotide precursor 94 , which was subjected to
Figure 10.8 T
m
data of PNA, alkenic PNA and fl uoroalkenic PNA.
278 Fluorine in Medicinal Chemistry and Chemical Biology
Figure 10.9 Natural 2 ′ - deoxythymidine and 1 ′ ,2 ′ - dideoxy - 1 ′ - (2,4 - difl uoro - 5 - methylphenyl) - β - D -
ribofuranoside.
Scheme 10.27
oligonucleotide synthesis using an automated synthesizer based on phosphoramidite chem-
istry [52] .
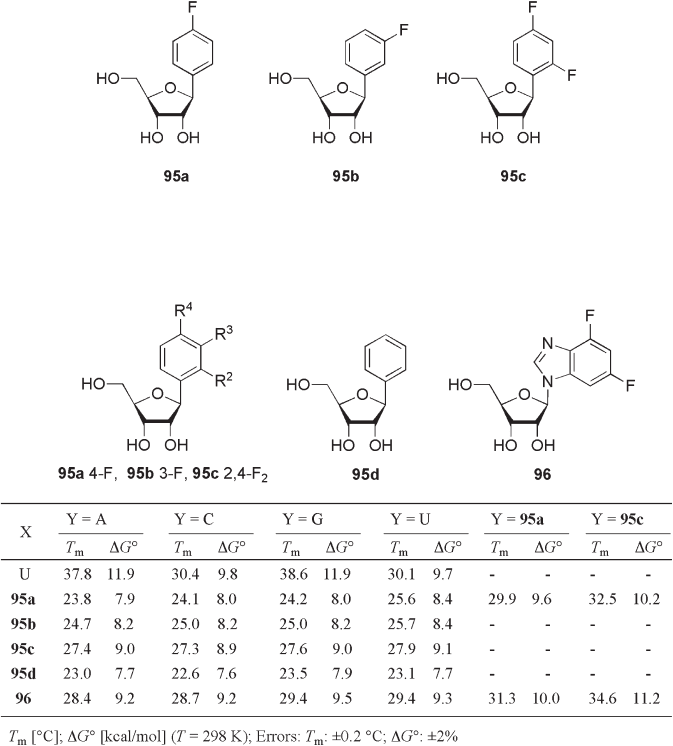
Fluoroarene isostere 92 was designed as a nonpolar nucleoside with the closest pos-
sible steric and structural similarity to the natural deoxythymidine, but without hydrogen -
bonding ability. Despite the proposed ability of C – F groups in fl uoroarene derivatives as
weak hydrogen - bonding acceptors and aromatic C – H groups as weak hydrogen - bonding
donors [53, 54] ,
1
H - NMR titration of 9 - ethyladenine with 2,4 - difl uorotoluene in aqueous
media did not show signifi cant change of chemical shifts in 9 - ethyladenine [50] . In general,
it is known that the hydrogen - bonds between molecules in aqueous solution are weak due
to the high dielectric constant of the medium as well as competitive hydrogen - bonding
with the solvent itself. Therefore, this observation indicates the lack of hydrogen - bonding
between the fl uoroarene nucleoside isostere and the complementary natural nucleoside in
aqueous media. On the other hand, Engeles and co - workers recently found intermolecular
C – H · · · F – C hydrogen bonding in the crystalline form of 1 ′ - deoxy - 1 ′ - (4 - fl uorophenyl) - β - d -
ribofuranose 95a (see Figure 10.10 ) [55] . The x - ray crystallographic analysis of 95a
(recrystallized from methanol) confi rmed that the distance between the fl uorine atom and
the arylic hydrogen at the 3 - position of another molecule (2.30 Å ) is signifi cantly shorter
than the sum of the van der Waals radii of fl uorine and hydrogen (2.55 Å ). Unfortunately,
Fluorinated Moieties for Replacement of Amide and Peptide Bonds 279
in the cases of 3 - fl uorophenyl - β - d - ribofuranose 95b and 2,4 - difl uorophenyl - β - d - ribofura-
nose 95c , no H · · · F distance shorter than 2.55 Å was observed. This intermolecular C –
H · · · F – C hydrogen bond also showed nearly linear arrangement with dihedral angle of
158 ° . Interestingly, the H · · · F distance in a single crystal of 95a recrystallized from water
was found to be longer (2.38 Å ) than in that recrystallized from methanol (2.30 Å ) due to
the incorporation of a water molecule into the unit cell: that is, a water molecule is placed
between 2 ′ - OH and 5 ′ - OH, which makes the F · · · H distance longer.
Engeles and co - workers also reported the effect of these fl uoroarene isosteres on the
thermodynamic properties of the duplex RNA [55] (see Figure 10.11 ). In the 12 - mer oli-
gonucleotides (5 ′ - CUU UUC XUU CUU paired with 3 ′ - GAA AAG YAA GAA), the
Figure 10.10 Fluoroarene nucleoside isosteres.
Figure 10.11 Thermodynamic properties of modifi ed/unmodifi ed duplex RNA (5 ′ - CUU
UUC XUU CUU paired with 3 ′ - GAA AAG YAA GAA).
280 Fluorine in Medicinal Chemistry and Chemical Biology
wobble base pair U · G showed the highest T
m
(38.6 ° C). Single modifi cation of oligonucle-
otide sequence by the incorporation of nonfl uorinated phenyl isostere 95d signifi cantly
destabilized the RNA duplex structure, although fl uorinated analogues 95a , 95b and 95c
improved the thermal stability of the duplex RNA compared with 95d . In particular, the
T
m
values of singly modifi ed duplex RNAs with 2,4 - difl uorophenyl isostere 95c were
notably higher than those with monofl uorinated isosteres. 4,6 - Difl uorobenzoimidazole
isostere 96 was also found to be an effective mimic of purine bases such as inosine. Sur-
prisingly, the pairing of fl uorinated nucleobase isosteres with a purine or pyrimidine
nucleobase resulted in similar T
m
values. Thus, these fl uoroarene isosteres potentially act
as a new class of universal base, which can pair with all natural bases without energy loss.
The evaluation of doubly modifi ed duplex RNAs also suggested a signifi cant interaction
between 96 and 95c ( T
m
= 34.6 ° C). It was also demonstrated by the exhaustive thermal
analysis that the duplex RNA with 95c · 96 base pair is 0.6 kcal/mol more stable than that
of the calculated value. To explain this stabilization, Engeles and co - workers proposed a
weak F · · · H hydrogen - bond between the fl uorinated nucleotides [55] .
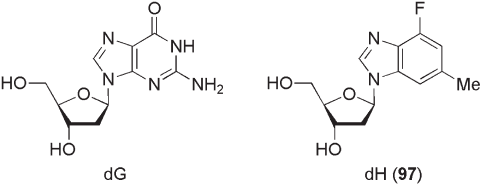
Kool and co - workers also reported 1 ′ ,2 ′ - dideoxy - 1 ′ - (4 - fl uoro - 6 - methylbenzoimid-
azolyl) - β - d - ribofuranose ( 97 , dH) as a highly effective nonpolar isostere of 2 ′ - deoxy-
guanosine (dG) (see Figure 10.12 ) [56] . In crystalline forms, the bond lengths and base
shapes in these compounds are quite close, although the orientation of the base with respect
to the furanose ring is different. That is, the natural dG adopts an anti conformation with
a torsion angle χ of − 122 ° , while the base part of 97 is twisted by 58 ° relative to this and
falls between typical anti and syn ranges with a measured torsion angle χ of − 65 ° .
However, NMR studies in D
2
O revealed that the conformation of 97 in solution was
essentially identical to that of dG. For instance, the conformations of the deoxyribose parts
of both nucleosides were classifi ed to be 70% S for dG and 66% S for 97 . In general, the
2 - deoxyribofuranoside structure preferentially occupies the S (2 ′ - endo ) conformation.
Additionally, nuclear Overhauser effect (NOE) data of 97 in D
2
O strongly indicate that
nucleoside 97 is favored as the anti - conformer.
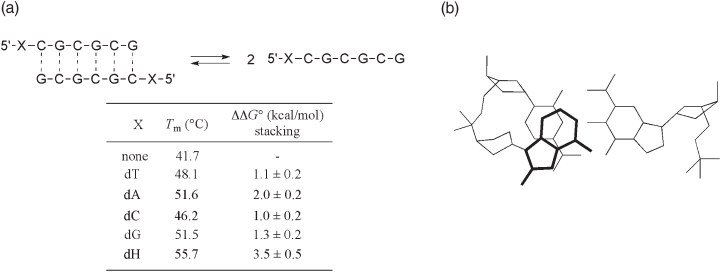
One of the most important aspects of 4 - fl uoro - 6 - methylbenzimidazole as a modifi ed
nucleobase is its strong stabilization of duplex DNA through a stacking effect. The stack-
ing properties of 97 and other modifi ed nucleosides can be measured by the dangling end
thermal denaturation studies of DNA duplexes with self - complementary stands (dXCGC-
Figure 10.12 2 ′ - Deoxyguanosine and 1 ′ ,2 ′ - dideoxy - 1 ′ - (4 - fl uoro - 6 - methylbenzoimidazolyl) - β - D - ribo -
furanose.
Fluorinated Moieties for Replacement of Amide and Peptide Bonds 281
GCG.) (see Figure 10.13 a) [57] . Regarding the free energy of stacking for the natural/
modifi ed nucleobases, this study showed that the nucleobase mimic 97 stacked consider-
ably more strongly than any of the four natural DNA bases. A possible stacking model
was proposed as shown in Figure 10.13 . In this model, the 4 - fl uoro - 6 - methylbenzimid-
azole ring nicely overlaps the cytosine base.
As an unique application of fl uoroarene isosteres, Kool and co - workers recently
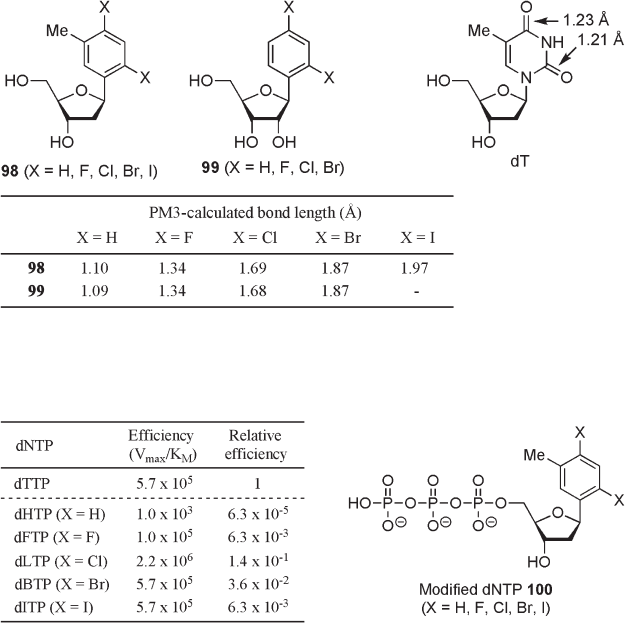
reported that sets of 1 ′ ,2 ′ - dideoxy - 1 ′ - (2,4 - dihalo - 5 - methylphenyl) - β - d - ribofuranose ( 98 )
and 1 ′ - deoxy - 1 ′ - (2,4 - dihalo - 5 - methylphenyl) - β - d - ribofuranose ( 99 ) as thymidine or
uridine analogues with gradually increasing size can be used as “ molecular rulers ” (see
Figure 10.14 ) [56] . X - ray crystallographic analysis and various NMR studies in D
2
O
showed that the conformations of these isosteres in both crystalline state and solution
closely resemble that of thymidine. Additionally, PM3 calculations of 98 and 99 suggested
that change of the substituent X from hydrogen to a series of halogen atoms would increase
the bond lengths at the 2,4 - positions, corresponding to oxygens of thymidine, ranging from
1.09 Å to 1.97 Å (see Figure 10.14 ). Therefore, enzymatic evaluation using a set of these
compounds should be able to elucidate the fi delity of substrate specifi city for the target
enzyme.
According to this concept, in 2005 Kool and Essingmann reported the utility of
“ molecular rulers ” as probes for the active - site tightness of DNA polymerase [58, 59] . In
kinetic studies of single nucleotide insertion in the steady state using a gel electorophore-
sis - based analysis, it was found that the effi ciency of the replication of 28 - mer/23 - mer
template - primer duplexes, having the sequence (5 ′ - ACT G A T CTC CCT ATA GTG AGT
CGT ATT A · 5 ′ - T AAT ACG ACT CAC TAT AGG GAG A) with variably sized nucleoside
triphosphates 100 promoted by DNA polymerase I from E. coli (Klenow fragment, exo-
nuclease defi cient), was highly dependent on the size of compounds (see Figure 10.15 ).
A comparison of the effi ciency for nucleotide insertions, which was measured by V
max
/ K
M
,
shows that the Cl - substituted analogue dLTP, whose chloroarene ring is larger than natural
thymine by 0.5 Å , is the most effi cient compound in this series. In addition, the relative
effi ciency of dLTP is almost the same as that of natural dTTP within experimental error,
Figure 10.13 (a) The helix – coil equilibrium of 5 ′ - XCGCGCG and dangling end thermal
denaturation studies. (b) Possible 5 ′ - end stacking geometry for the aromatic rings.
282 Fluorine in Medicinal Chemistry and Chemical Biology
Figure 10.14 PM3 - calculated bond length of arene isosteres.
Figure 10.15 Steady - state kinetic effi ciencies for single - nucleotide insertion by DNA poly-
merase I with variably sized nucleoside triphosphate.
despite the nonpolar nature of dLTP. When the molecule size exceeded that of the chlori-
nated analogue, the relative effi ciency dropped markedly by a factor of 164 for the largest
dITP in response to a subtle size increase of 0.35 Å over the optimum. This result suggests
that the active site of DNA polymerase strictly recognizes the steric bulkiness and the
shape of the substrate.
The “ molecular ruler ” methodology for determining the size of the active site of DNA
polymerase was also applied to the investigations of other DNA polymerases, such as T7
DNA polymerase and Dpo4 polymerase [60, 61] .
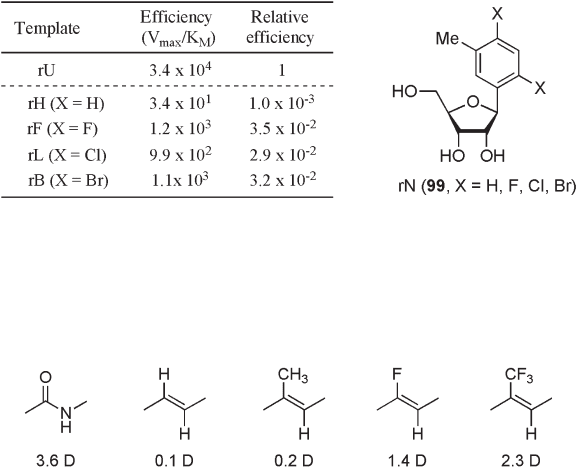
On the other hand, a set of 1 ′ - deoxy - 1 ′ - (2,4 - dihalo - 5 - methylphenyl) - β - d - ribofura-
noses ( 99 , rN) was used for the investigation of fl exible active site of reverse transcriptase
of HIV - 1 virus (HIV - RT) (see Figure 10.16 ) [62] . Since HIV - RT is well known as a highly
mutagenic polymerase, the understanding of its mutagenicity potentially provides a solu-
tion to the rapid development of drug resistance during treatment of AIDS. The effi ciencies
of the dATP incorporation to singly modifi ed RNA templates by HIV - RT are shown in
Figure 10.16 . All uracil analogues 99 directed preferential incorporation of dATP. Regard-
Fluorinated Moieties for Replacement of Amide and Peptide Bonds 283
ing the effect of size on the effi ciency, the smallest analogue (X = H) was less tolerated
than other analogues in the template, but there was otherwise little or no difference in
dATP incorporation effi ciencies. The results are remarkably different from those for the
DNA polymerases, and clearly suggest the high structural fl exibility of HIV - RT.
10.3 Trifl uoromethylated Alkenes Ψ [CH(CF
3
) = CH] as Dipeptide Isosteres
In 1998, Wipf and co - workers demonstrated that the replacement of a peptidic amide bond
with a trifl uoromethylated alkene mimics well the β - turn structure of the original peptide
[7] . AM1 - calculated dipolar moments of the amide bond and various alkene isosteres sug-
gested the electrostatic similarity between the amide and the trifl uoromethylalkene func-
tionalities (see Figure 10.17 ) [7] .
10.3.1 Synthetic Method
The synthesis of trifl uoromethylalkene dipeptide isosteres was reported by Wipf and co -
workers [7] . As shown in Scheme 10.28 , the multistep synthesis of Ala - Ψ [C(CF
3
) = CH] -
Xaa type isosteres was achieved from 1,1,1 - trichloro - 2,2,2 - trifl uoroethane. The
carboxylation of trifl uorotrichloroethane via trifl uorodichloroethylzinc intermediate, ester-
ifi cation with benzyl alcohol, and Reformatsky addition – elimination with acetaldehyde
Figure 10.16 Steady - state kinetic effi ciencies for single - nucleotide insertion by HIV - 1 reverse
transcriptase to duplex RNA templates [5 ′ - r(ACU GXU CUC CCU AUA GUG AGU CGU
AUU A), · 55 ′ - d(T AAT ACG ACT CAC TAT AGG GAG A)] with dATP.
Figure 10.17 Dipolar moments (debye) of amide and various alkene isosteres.
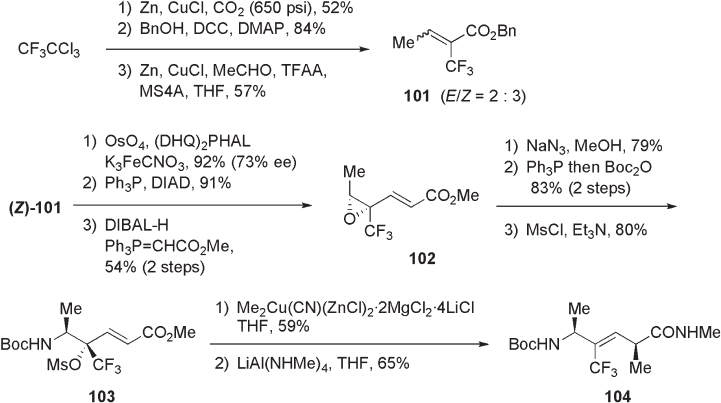
284 Fluorine in Medicinal Chemistry and Chemical Biology
yielded α - trifl uoromethylated crotonate 101 as a 2 : 3 mixture of E - and Z - isomers. The
Sharpless asymmetric dihydroxylation of ( Z ) - 101 , which was isolated by column chroma-
tography on silica gel, afforded chiral diol in 73% ee. Conversion of this diol to an epoxide
under Mitsunobu conditions, half - reduction of the ester moiety with DIBAL - H, and Wittig
olefi nation gave trifl uoromethylated epoxide 102 . Introduction of amino group through
ring opening of epoxide 102 , followed by allylic methylation of the resultant γ - mesyloxy -
α , β - enoate 103 with methylcuprate, and the fi nal amidation gave Ala - Ψ [C(CF
3
) = CH] - Ala
104 .
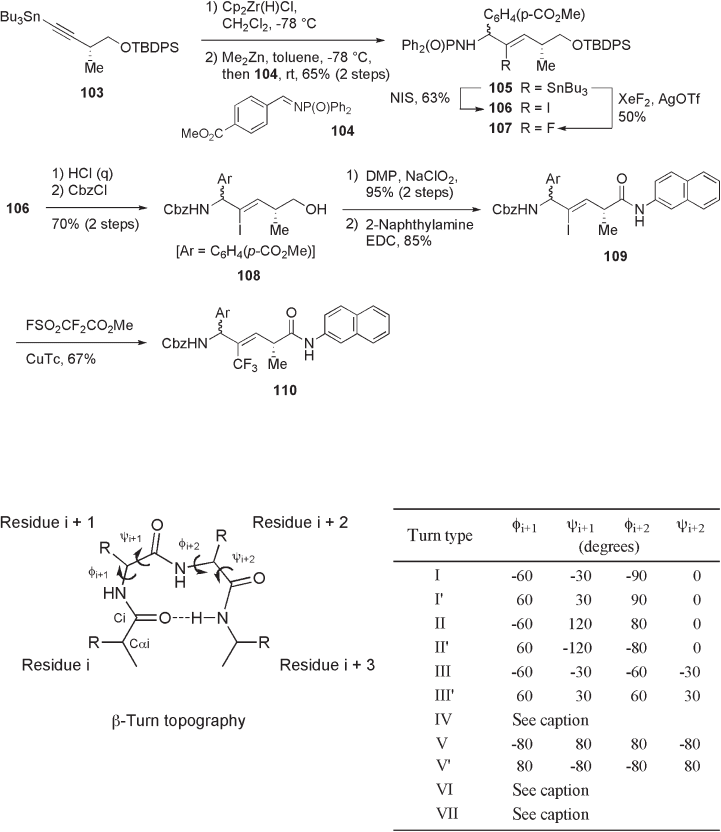
Later, Wipf and co - workers also reported the improved synthesis of trifl uoromethyl-
alkene isostere 110 through addition reaction of internal alkynes to N - phosphonylimines
(see Scheme 10.29 ) [63] . As shown in Scheme 10.29 , the reaction of N - phosphonylimine
104 with vinylzinc species, derived from alkynylstannane 103 through hydrozirconation,
followed by transmetallation with Me
2
Zn, gave vinylstannane 105 in 65% yield. The reac-
tion of 105 with NIS ( N - iodosuccinimide) afforded iodoalkene 106 in 63% yield. It should
be noted that vinylstannane 105 was also converted to fl uoroalkene 107 in moderate yield
through reaction with XeF
2
and AgOTf [64] . The resulting iodoalkene 106 was trans-
formed to naphthylamide 109 in a further four steps. Finally, the stereoselective introduc-
tion of a CF
3
group through reaction of 109 with FSO
2
CF
2
CO
2
Me [65] in the presence of
Cu(I) thiophenecarboxylate (CuTc) gave trifl uoromethylalkene isostere 110 in reasonable
yield.
10.3.2 Conformational Analysis and Biological Aspects
One of the most important structural properties of trifl uoromethylalkene isosteres is the
reproduction of β - turn topography. As shown in Figure 10.18 , natural β - turn structure can
Scheme 10.28
Fluorinated Moieties for Replacement of Amide and Peptide Bonds 285
Scheme 10.29
Figure 10.18 Structure and classifi cation of β - turns. Type IV β - turns are defi ned as those
having two or more angles which differ by at least 40 ° from the defi nitions of β - turn types I,
I ′ , II, II ′ , III and III ′ . Type VI β - turns have a cis - Pro at the position i +2. Type VII β - turns form
a kink in the protein chain created by ψ
i
+1
≈ 180 ° and | φ
i
+2
| < 60 ° or | ψ
i
+1
| < 60 ° and
φ
i
+2
≈ 180 ° .
be classifi ed broadly into 11 types [66] . Compared with nonfl uorinated alkene and meth-
ylalkene isosteres, the corresponding trifl uoromethylalkene isosteres act as more effective
β - turn promoters. Thus, the combination of A
1,3
and A
1,2
strains at the trifl uoromethylated
alkyne moiety leads to considerable restrictions in φ , ψ - dihedral angles in these peptides.
286 Fluorine in Medicinal Chemistry and Chemical Biology
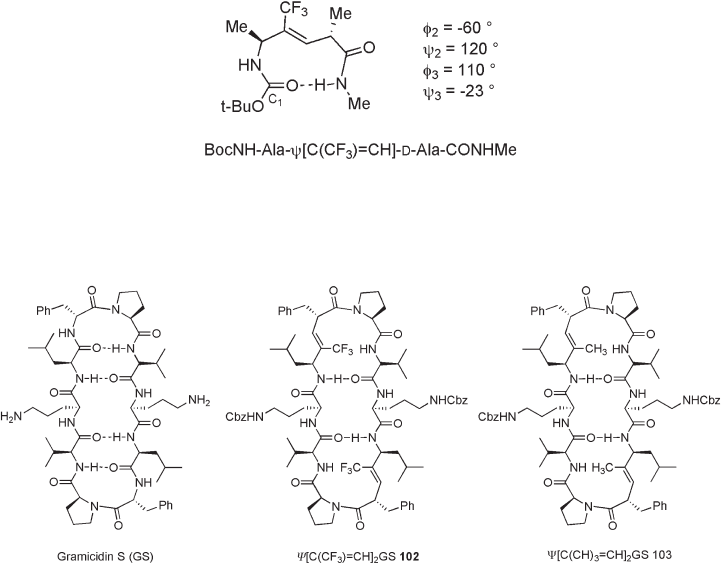
For instance, BocNH - l - Ala - Ψ [C(CF
3
) = CH] - d - Ala - CONHMe takes solely type - II β -
turn structure ( φ
2
= − 60 ° , ψ
2
= 120 ° , φ
3
= 110 ° , ψ
3
= − 23 ° ) in crystalline form (see Figure
10.19 ). In this case, the corresponding methylated alkene isostere also exists as a similar
conformer in crystalline form, although the simple alkene isostere does not have a hydro-
gen - bond between the carbonyl oxygen at residue 1 and the amide proton at residue 4.
A similar observation was reported for the trifl uoromethylalkene isostere of gramici-
din S (GS), which is an antibiotic cyclodecapeptide (see Figure 10.20 ) [67] . In natural GS,
the rigid and amphipathic antiparallel β - pleated sheet is held in place by two type - II ′ β -
turns at both d - p he - Pro positions as well as four intramolecular hydrogen - bonds between
the valine and leucine residues. Base on the x - ray crystallographic analysis, it is confi rmed
that trifl uoromethylalkene isostere 102 adopts the pleated antiparallel β - sheet structure
with two hydrogen bonds in solid state. The d - p he - Pro unit has an ideal set of dihedral
angles for the type II ′ β - turn ( φ
2
= 137 ° , ψ
2
= − 95 ° , φ
3
= − 82 ° , ψ
3
= − 5.7 ° ). Comparison
of natural GS and Ψ [C(CF
3
) = CH]
2
GS in solution by variable temperature NMR and CD
(circular dischroism) analyses also indicates the structural similarity of these two com-
pounds. In contrast, CD spectra of nonfl uorinated isostere Ψ [C(CH
3
) = CH]
2
GS 103 suggest
the presence of disordered peptide conformation. Antibacterial activities of des - Cbz - 102
and des - Cbz - 103 also mimic that of the natural GS (MIC = 5 – 15 µ g/mL).
Figure 10.19 β - Turn structure of BocNH - L - Ala - ψ [C(CF
3
) = CH] - D - Ala - CONHMe.
Figure 10.20 Structures of gramicidin S, Ψ [C(CF
3
) = CH]
2
GS, and Ψ [C(CH
3
) = CH]
2
GS.
