Ojima I. (ed.) Fluorine in Medicinal Chemistry and Chemical Biology
Подождите немного. Документ загружается.

Fluorinated Moieties for Replacement of Amide and Peptide Bonds 267
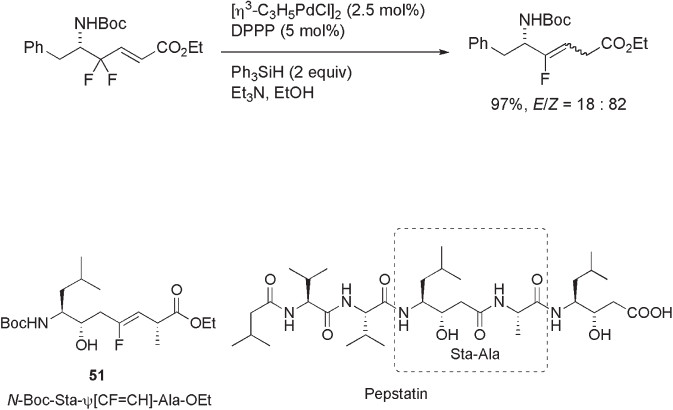
A facile synthesis of fl uoro - olefi ns through Pd(0) - catalyzed reductive defl uorination
of allylic gem - difl uorides, in particular γ , γ - difl uoro - α , β - enoates was recently reported (see
Scheme 10.16 ) [26] . Stereoselectivities were moderate to high ( E : Z = 1 : 1 – 1 : 9).
The reductive defl uorination – alkylation method shown in Scheme 10.12 was success-
fully applied to the synthesis of N - Boc - Sta - Ψ [CF = CH] - Ala ethyl ester 51 , which mimics
the central Sta - Ala unit of pepstatin, a naturally occurring inhibitor of aspartyl proteases
including renin, pepsin, HIV - 1 and HIV - 2 proteases (see Figure 10.3 ) [27] .
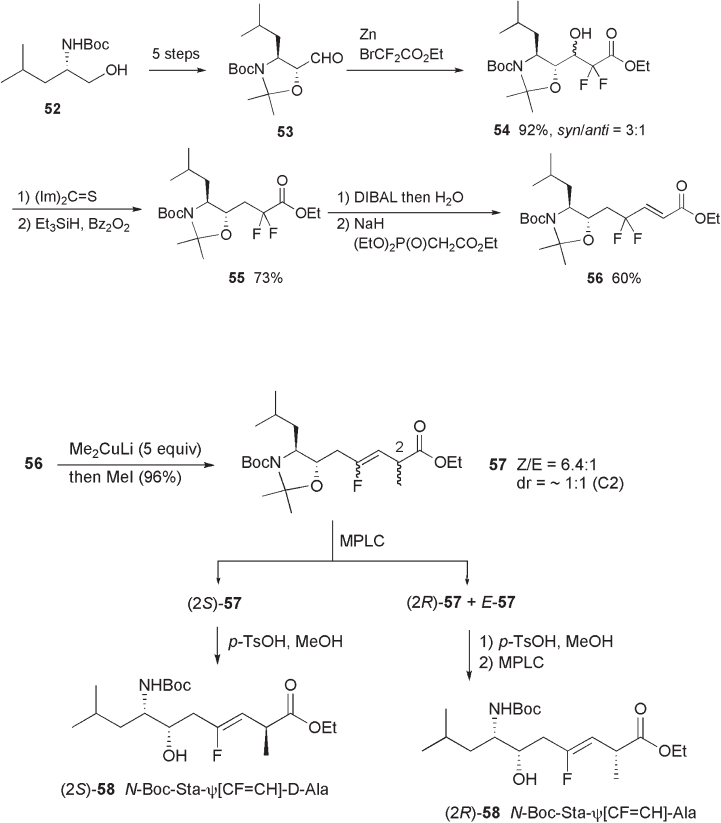
γ , γ - Difl uoro - α , β - enoate 56 , the key substrate for the reductive defl uorination – alkyla-
tion reaction, was synthesized in optically pure form using N - Boc leucinol 52 as the start-
ing material (see Scheme 10.17 ). The Reformatsky reaction of bromodifl uoroacetate with
aldehyde 53 , having natural statin confi guration, gave a 3 : 1 diastereomeric mixture of β -
hydroxy ester 54 , which was subjected to radical deoxygenation reaction to give difl uoro
ester 55 . DIBAL reduction of 55 , followed by the HWE reaction using phosphonoacetate
provided the key substrate 56 .
As expected, Me
2
CuLi - mediated reductive defl uorination of 56 and subsequent meth-
ylation with methyl iodide proceeded smoothly to give methylated product 57 in 96%
yield. Regarding the stereoselectivity, a moderate Z - selectivity ( Z : E = 6.4 : 1) was observed
for the olefi n moiety, but the C - 2 - methylation proceeded in nonselective manner. Fortu-
nately, two diastereomers were readily separable by medium - pressure liquid chromatog-
raphy (MPLC) as shown in Scheme 10.18 . Structural assignments were made based on
the x - ray crystallographic analysis of (2 S ) - 57 .
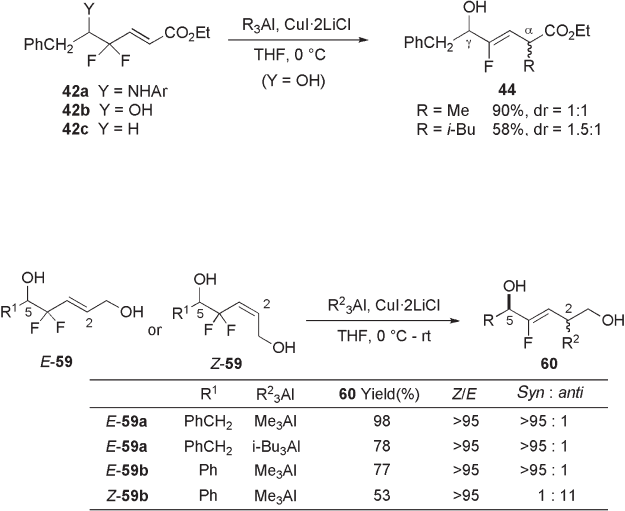
As described above, an organocopper reagent derived from alkyl lithium, such as
Me
2
CuLi, readily reacts with γ , γ - difl uoro - α , β - enoate 42 to form a reductive defl uorinated
metal species 43 , exclusively (see Scheme 10.12 ). To facilitate the reactivity of fl uorine
as a leaving group, we also investigated the effects of alkylaluminum on this reaction as
Scheme 10.16
Figure 10.3 Pepstatin and Sta - ψ [CF = CH] - Ala.
268 Fluorine in Medicinal Chemistry and Chemical Biology
Scheme 10.17
Scheme 10.18
an additive and/or alkylating reagent, since it is well documented that aluminum, in addi-
tion to its high oxygenophilicity (O - Al 511 ± 3 kJ/mol), has exceedingly high affi nity
toward fl uorine (F - Al 663 ± 3 kJ/mol) [28] . We found that, in the presence of Cu(I), the
reaction of γ , γ - difl uoro - α , β - enoate 42b having a free hydroxyl group at the δ - position with
trialkylaluminum (5 equivalents) gave allylic alkylation product 44 , whereas γ , γ - difl uoro -
α , β - enoate having amino group 42a or hydrogen derivative 42c did not give the alkylated
products (see Scheme 10.19 ) [22, 23] . With the hydroxyl substrate 44b , the reaction pro-
ceeded in a Z - selective manner but without or with very low diastereoselectivity.
Fluorinated Moieties for Replacement of Amide and Peptide Bonds 269
Scheme 10.19
Excellent diastereoselectivity was realized on using the substrate of allylic alcohol
substrates 59 instead of α , β - unsaturated ester substrate 42 (Y = OH) mentioned above.
Thus, in the presence of CuI · 2LiCl, the reaction of the E - isomer of 5 - hydroxy - 4,4 - difl uoro -
2 - alken - 1 - ol ( E ) - 59 with trialkylaluminum proceeded smoothly to give the allylic substitu-
tion product 60 with complete 2,5 - syn - and Z - selectivity (see Scheme 10.20 ) [29, 30] . With
the Z - isomer substrate Z - 59 , reaction proceeded in a manner that favored the 2,5 - anti -
product, although the reactivity of the Z - isomer was clearly low compared with that of the
E - isomer. It should be noted that the C - 5 free hydroxyl functionality is crucial for the
reaction to occur, since the lack of this OH group (i.e., protection as its ether or amine
derivative in place of the OH group), resulted in no reaction under similar conditions,
recovering the starting material.
This highly stereoselective reaction would provide an effi cient method for the prepa-
ration of fl uoroalkene dipeptide isosteres 62 and related molecules such as depsipeptide
isosteres 61 (see Scheme 10.21 ) [29,30] .
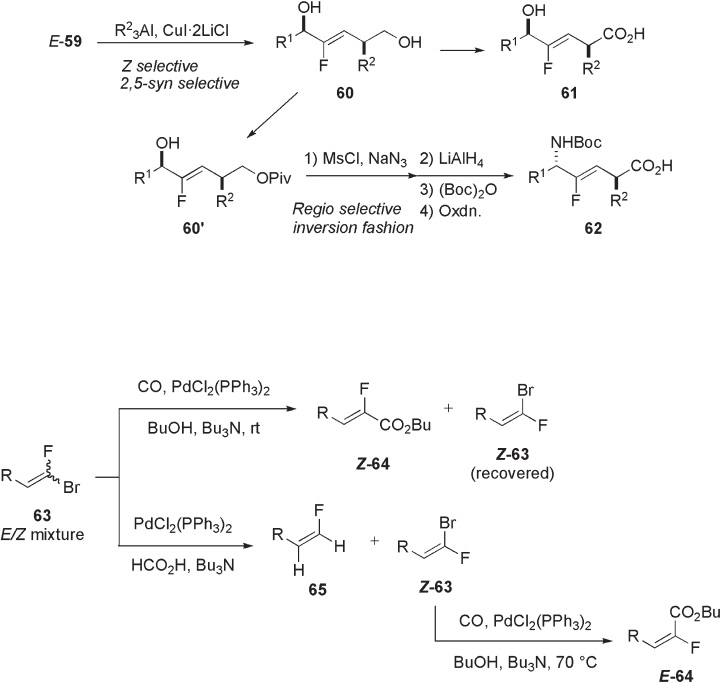
10.2.1.7 Cross - coupling Reaction of Fluorinated Alkenyl Bromides
In 2002, Burton and co - workers demonstrated that the Z - and E - isomers of bromofl uoro-
methylene compound 63 , easily obtained by the Wittig reaction of carbonyl compound
with Ph
3
P - CFBr
3
- Zn, were obtained through effi cient kinetic separation using the palla-
dium - catalyzed carboalkoxylation reaction (see Scheme 10.22 ) [31] . Since the E - isomer
Scheme 10.20
270 Fluorine in Medicinal Chemistry and Chemical Biology
Scheme 10.21
Scheme 10.22
of bromide 63 reacted much faster than the Z isomer, ( Z ) - α - fl uoroenoate ( Z ) - 64 was
preferentially obtained at low temperature. After a complete consumption of the E - isomer
by Pd - catalyzed reduction with HCO
2
H, the Z - isomer of bromide ( Z ) - 63 remaining was
subjected to carboalkoxylation at 70 ° C to give ( E ) - 64 .
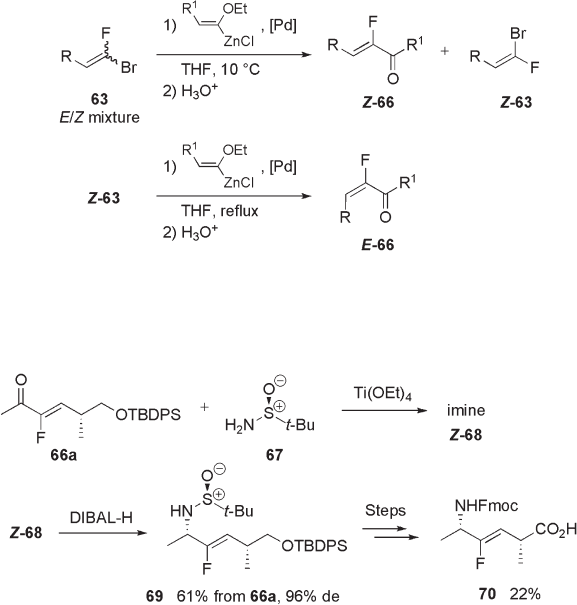
Pannecoucke and co - workers developed a highly stereospecifi c synthesis of ( Z ) - and
( E ) - α - fl uoroenones 66 through a kinetically controlled Negishi coupling reaction catalyzed
by palladium (see Scheme 10.23 ) [32] .
Further elaboration of both ( Z ) - and ( E ) - 66 led to the formation of fl uoro - olefi n
ψ [CF = CH] dipeptide isosteres in enantiomerically pure form. For example, chiral imine
( Z ) - 68 prepared by Ti(IV) - promoted condensation of α - fl uoroenone 66a with Ellman ’ s
sulfonamide 67 was reduced in a highly diastereoselective manner to give the reductive
amination product 69 , which was converted to the dipeptide isostere 70 via several steps
(see Scheme 10.24 ) [33] .
Fluorinated Moieties for Replacement of Amide and Peptide Bonds 271
Scheme 10.23
Scheme 10.24
10.2.2 Applications to Biologically Active Peptides and Related Studies
10.2.2.1 Substance P and Thermolysin
As an early example of the application of fl uoro - olefi n Ψ [CF = CH] dipeptide isosteres,
Allmendinger et al. reported the preparation of neuropeptide substance P (SP) analogues
containing the Phe - Ψ [(Z) - CF = CH] - Gly dipeptide unit [16] . In a receptor binding assay
(see Table 10.1 ), SP analogue ( S ) - 30a having the natural S - confi guration in the Phe moiety
was almost as active as SP itself (entry 1 vs. entry 6), while its diastereomer ( R ) - 30a , the
unnatural R - confi gured analogue, was 10 times less active than ( S ) - 30a (entry 2). A similar
difference was also observed in the binding affi nities of the pyro - Glu - containing hexapep-
tide analogues ( S ) - 30b and ( R ) - 30b (entries 3 and 4). Furthermore, compared to the non-
fl uorinated olefi nic analogue ( S ) - 30c , the fl uorinated analogue ( S ) - 30b bound 10 times
more strongly to the receptor (entry 3 vs. entry 5).
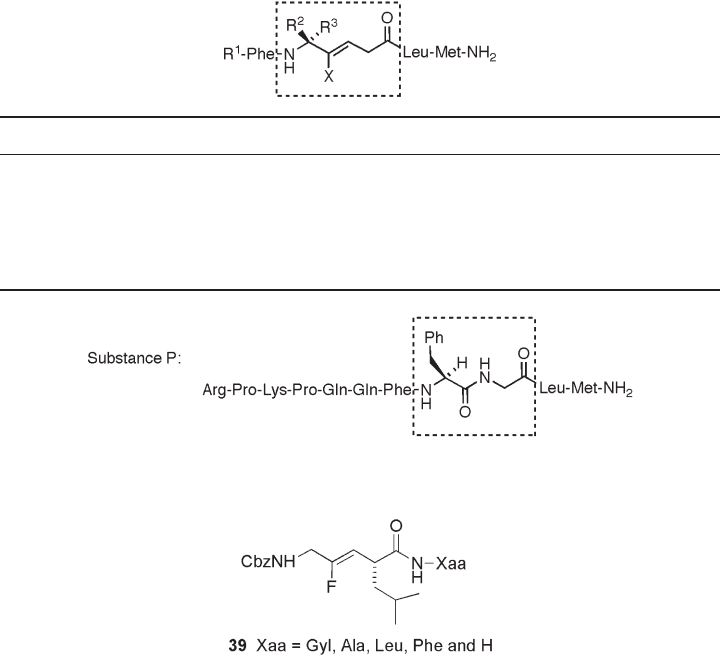
Bartlett and Otake experimentally verifi ed the applicability of tripeptide analogues
with the Cbz - Gly - Ψ [( Z ) - CF = CH] - Leu - Xaa structure as ground - state analogue inhibitors
of the zinc endopeptidase thermolysin (see Figure 10.4 ) [19] . These fl uoro - olefi n tripeptide
272 Fluorine in Medicinal Chemistry and Chemical Biology
Table 10.1 Receptor binding of Substance P and fl uoroalkene dipeptide analogues
entry X R
1
R
2
R
3
lC
50
1
( S ) - 30a
F Arg - Pro - Lys - Pro - Gln - Gln - Phe PhCH
2
H 2 nM
2
( R ) - 30a
F Arg - Pro - Lys - Pro - Gln - Gln - Phe H PhCH
2
20 nM
3
( S ) - 30b
F Pyro - Glu - PhCH
2
H
0.8 µ M
4
( R ) - 30b
F Pyro - Glu - H PhCH
2
10 µ M
5
( S ) - 30c
H Pyro - Glu - PhCH
2
H
> 10 µ M
6 Substance P 1.3 nM
Figure 10.4 Tripeptide analogues of the Cbz - Gly - Ψ [CF = CH] - Leu - Xaa structure.
analogues 39 bind to thermolysin about one order of magnitude more tightly than the
substrates, in which the binding mode of the fl uoro - olefi n inhibitor and substrate are not
identical on the basis of K
i
vs. K
m
correlation,. Although these analogues are not particu-
larly potent as enzyme inhibitors, they are useful as substrate models in structural
studies.
10.2.2.2 Dipeptidyl Peptidase Inhibitors
Dipeptidyl peptidase IV (DPP IV, EC 3.4.14.5, CD26) is a serine protease cleaving off
dipeptides from the amino terminus of peptides or proteins having proline or alanine at
the penultimate position. Since prolylamides are known to play a critical role in peptide
structure and function, and because of their high resistance toward nonspecifi c enzymatic
hydrolysis, a few enzymes capable of cleaving this structural motif have attracted consid-
Fluorinated Moieties for Replacement of Amide and Peptide Bonds 273
erable attention. It has been shown that DPP IV inhibition can be used as a new tool for
controlling type II diabetes [34] . Typically, these inhibitors possess a dipeptide skeleton
with a free amine terminus and a pyrrolidine ring attached to an electrophilic site for Ser -
OH scavenging such as a nitrile, a boronic acid, or a hydroxamate (see Figure 10.5 ).
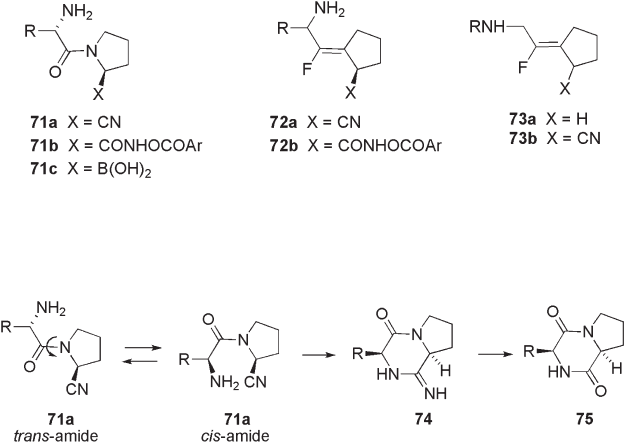
Since the discovery of Xaa - (2 - cyano)pyrrolidines ( 71a , X = CN, Figure 10.5 ) as
potent and reversible inhibitors of DPP IV, the optimization of these “ lead ” structures in
terms of activity and selectivity have been extensively investigated [35] . It was suggested
that for strong enzyme inhibition, reversible interaction of the electrophilic nitrile group
in the inhibitors and the Ser - OH group in the active site of the enzyme would play an
important role. However, these nitrile - containing inhibitors 71a suffer from the inactiva-
tion process to form cyclic amidines 74 and/or hydrolyzed product, diketopiperadine
derivative 75 , due to a facile intramolecular cyclization of N - terminal amine to the nitrile
group through conformational change from trans - amide to cis - amide as shown in Scheme
10.25 .
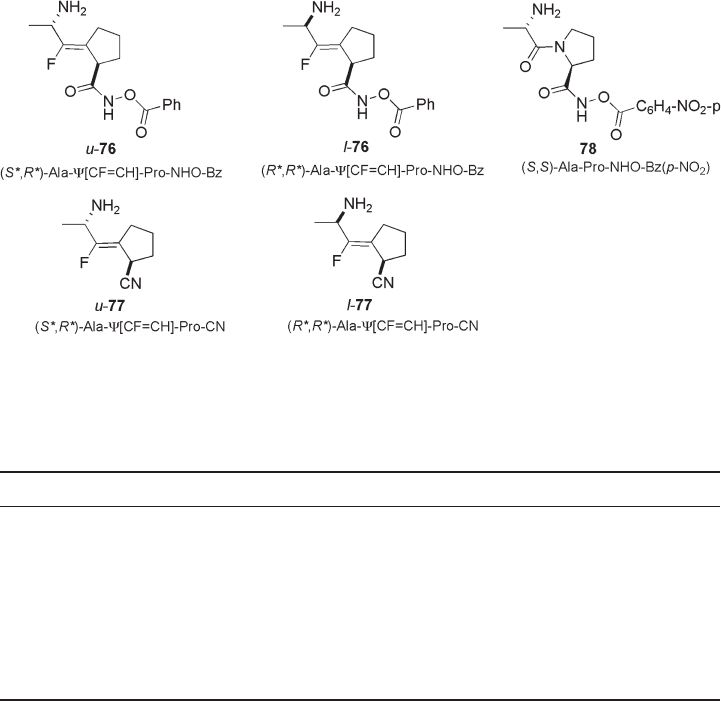
To solve the inactivation issues mentioned above, the concept of conformationally
restricted ( Z ) - fl uoro - olefi n dipeptide isosteres that mimic the active trans conformation of
the DPP IV inhibitors was applied by Welch and co - workers to the preparation of inhibi-
tors having Ala - Ψ [CF = CH] - Pro structure ( 76 and 78 ) and their inhibitory activities were
evaluated (see Figure 10.6 ) [36, 37] . DPP IV inhibitory activities and the stability of the
inhibitors 76 and 77 , in comparison with those of the model dipeptide Ala - Pro derivative
78 are summarized in Table 10.2 . These fl uoro - olefi n analogues, 76 and 77 , showed better
DPP IV inhibitory activity than that of 78 . In particular, u - 76 having the same relative
stereochemistry as the natural dipeptide (L - Xaa - L - X ′ aa) confi gurations exhibited potent
Figure 10.5 DDP IV inhibitory dipeptides and fl uoro - olefi n analogues.
Scheme 10.25
274 Fluorine in Medicinal Chemistry and Chemical Biology
Figure 10.6 Ala - Ψ [CF = CH] - Pro analogues as DDP IV inhibitors.
Table 10.2 Inhibition of DPP IV and stability of inhibitors
inhibitor Inhibition, %
([l], µ M) K
i
, µ M K
d
× 10
4
, min
− 1
a)
t
1/2
, h
u - 76
42
100
(10)
(250)
0.19 1.1 103
l - 76
4
17
(10)
(500)
14.4 ND ND
u - 77
16
50
(1)
(10)
7.69 –
b)
l - 77
14
47
(1)
(10)
6.03 –
b)
78 29 (1,100) 30.0 13.0 8.8
a)
K
d
: Decomposition rate constant
b)
No detectable degradation at pH 7.6 under buffered conditions.
activity ( K
i
= 0.19 µ M). Moreover, the stability of these fl uoro - olefi n - containing peptide
mimetics was remarkable. For example, no detectable degradation of the cyano derivatives
77 was observed at pH 7.6 under buffered conditions. Although the effects of the replace-
ment of the parent amide bond with the fl uoro - olefi n moiety in these molecules on inhibi-
tory potency and stability might not be fully deduced from these limited examples, good
affi nity of these fl uorine - modifi ed peptide mimetics to the enzyme was experimentally
verifi ed.
Augustyns and co - workers reported a structure – activity relationship (SAR) study of
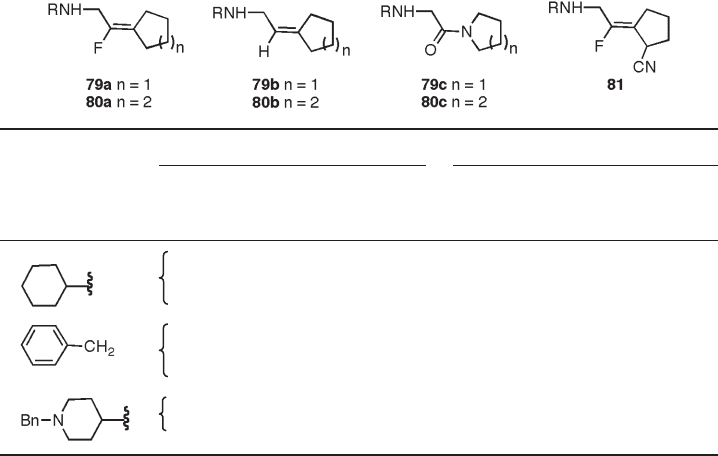
fl uoro - olefi n analogues of N - substituted glycylpyrrolidines 79a , glycylpiperidines 80a , and
glycyl - (2 - cyano)pyrrolidines 81 as well as the corresponding olefi n analogues 79b and
80b for their activities as DPP IV or DPP II inhibitors (Table 10.3 ) [38] . Except for 79c - 1 ,
most of these compounds exhibited a strong preferential binding to DPP II. Recent crystal-
lographic analyses of peptidic inhibitor – DPP IV complexes revealed that the P
2
– P
1
amide
Fluorinated Moieties for Replacement of Amide and Peptide Bonds 275
oxygen atom of inhibitors is involved in hydrogen bonding with the Asp710 and Arg125
residues of the enzyme. Assuming the importance of such a hydrogen bond, the observed
low affi nity of the fl uoro - olefi n isosteres, 79a and 80a , for DPP IV could be attributed to
the fact that they are much weaker hydrogen - bond acceptors than an amide functionality.
In contrast, the hydrogen - bond formation seems less critical for DPP II inhibition. More-
over, 81 , wherein a nitrile group was introduced to fl uoro - olefi n isostere 79a , did not result
in increased DPP IV or DPP II inhibitory activity, although these analogues showed sub-
stantially increased stability in solution over the parent amide compound.
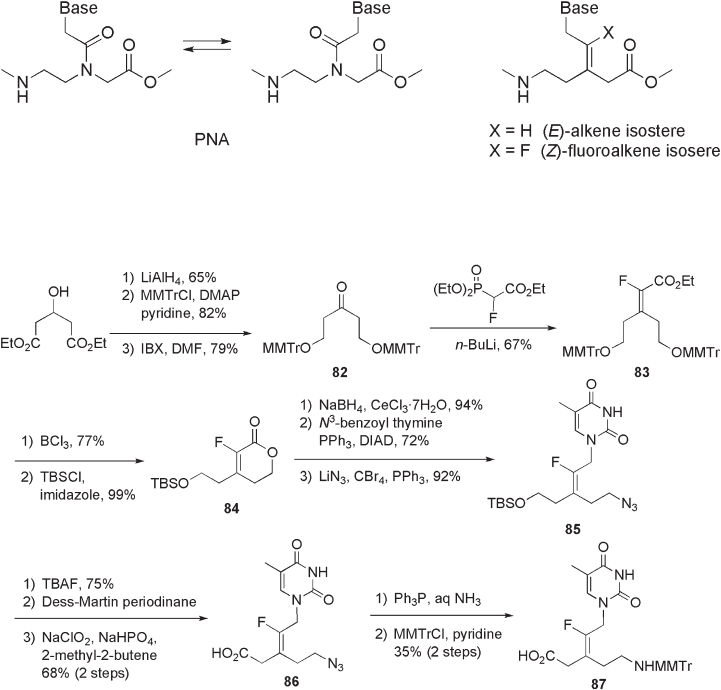
10.2.2.3 Peptide - Nucleic Acid
Peptide - nucleic acid (PNA) is a DNA analogue based on a polyamide backbone. It is
known that PNAs strongly bind to complementary DNA and RNA sequences, obeying the
Watson – Crick hydrogen - bonding rules, by taking advantage of the lack of electrostatic
repulsion between anionic ribose - phosphate moieties in the backbone [39] . The potential
utility of PNAs as gene - therapy agents has been attracting much attention from medicinal
chemists, although their poor cellular uptake, low solubility in water, and self - aggregation
are remaining problems for clinical applications [40, 41] . To realize more specifi c and
higher affi nity to DNA and RNA, modifi cation of PNA backbone has been extensively
studied. Leumann and co - workers reported the synthesis and evaluation of fl uoroalkene
and nonfl uorinated alkene isosteres of PNAs as conformationally locked mimics (see
Figure 10.7 ) [42 – 45] .
Table 10.3 Inhibition of DPP IV and DPP II
R n = 1 n = 2
compd.
lC
50
( µ M) lC
50
( µ M)
Compd.
lC
50
( µ M) lC
50
( µ M)
DPP lV DPP ll DPP lV DPP ll
1
79a - 1
> 1000 90 ± 1
80a - 1
> 500 500 ± 50
79b - 1
> 1000 62 ± 12
80b - 1
> 1000 136 ± 14
79c - 1
148 ± 26 276 ± 72
80c - 1
> 1000 177 ± 54
2
79a - 2
> 1000 38 ± 3
80a - 2
> 1000 34 ± 4
79b - 2
> 1000 26 ± 3
80b - 2
> 1000 55 ± 4
79c - 2
1000 ± 10 500 ± 50
80c - 2
> 1000 397 ± 25
3
79a - 3
> 250 1.3 ± 0.2
80a - 3
> 250 1.0 ± 0.1
79a - 3 ND ND 80c - 3
> 1000 3.1 ± 0.1
276 Fluorine in Medicinal Chemistry and Chemical Biology
The thymidyl - PNA monomer 87 having a fl uoroalkene structure was obtained by
multistep reactions using the Wittig reaction for the construction of the fl uoroalkene func-
tionality as shown in Scheme 10.26 . The Wittig reaction of bis - MMTr - protected symmetric
ketone 82 , derived from diethyl 3 - hydroxyglutarate with (EtO)
2
P(O)CHFCO
2
Et, gave
fl uoroalkene 83 in 67% yield. The reaction of 83 with BCl
3
and the subsequent silylation
gave fl uorinated lactone 84 . The introduction of thymidyl group and amino functionality
to 84 was achieved by the chemoselective Mitsunobu reaction of a diol, prepared by 1,2 -
reduction of 84 , with N
3
- benzoyl thymine and Ph
3
P/CBr
4
- mediated S
N
2 - type substitution
of the homoallylic alcohol moiety with LiN
3
to give azide 85 . Conversion of the azide
group of 85 to an MMTr - amino acid functionality in fi ve steps provided monomeric fl uo-
roalkene isostere unit 87 . With the use of essentially the same method, a fl uoroalkene
isostere of an adenyl - PNA was also synthesized. By means of MMTr/acyl solid - phase
peptide synthesis [46] , these fl uoroalkenic monomers were incorporated into the corre-
Figure 10.7 Rotameric forms of PNA and the structures of alkenic isosteres.
Scheme 10.26
