Ojima I. (ed.) Fluorine in Medicinal Chemistry and Chemical Biology
Подождите немного. Документ загружается.

Perfl uorinated Heteroaromatic Systems as Scaffolds for Drug Discovery 297
(e.g. O - , N - , C - , S - centred) makes the theoretical number of highly functionalized pyridine
ring systems that could be accessed by this methodology very large indeed if the sequence
of nucleophilic aromatic substitution processes can be achieved in a controllable manner.
Surprisingly, however, the number of sequential nucleophilic substitution processes using
pentafl uoropyridine as the starting material is very small despite the potential reactivity
of these systems, but the few examples reported so far demonstrate the viability of the
potential use of this substrate as a scaffold in the manner indicated in Figure 11.4 . For
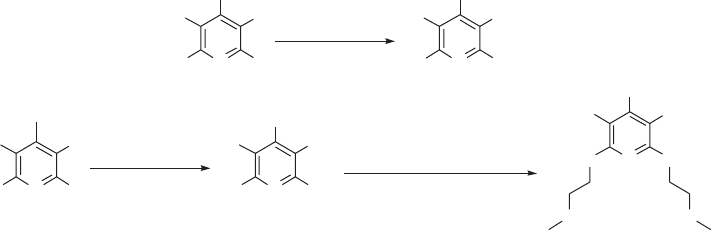
example, as shown in Figure 11.5 , heating 4 - methoxytetrafl uoropyridine 5 with an excess
of sodium methoxide in methanol gave the 2,4,6 - trimethoxypyridine derivative 6 [30] . In
a controlled stepwise process, perfl uoro - 4 - isopropylpyridine 7 , prepared by nucleophilic
substitution of the 4 - fl uorine atom of pentafl uoropyridine by perfl uoroisopropyl anion
(i.e. Nuc
1
= (CF
3
)
2
CF
−
, Figure 11.4 ), generated in situ from hexafl uoropropene and
TDAE [31] , subsequently gave trisubstituted products 8 (Nuc
2
and Nuc
3
, Figure 11.4 ) by
reaction with a range of oxygen - , nitrogen - and carbon - centred nucleophiles (see Figure
11.5 ) [32] .
The reactivity profi le established for pentafl uoropyridine, where the 4, 2 - and 6 -
positions are sequentially, regiospecifi cally substituted by a succession of oxygen - centred
nucleophiles, has allowed medicinal chemists to use pentafl uoropyridine as a core scaffold
for the synthesis of small arrays of biologically active pyridine systems that fall within
the Lipinski parameters (see Table 11.3 ).
The factor VIIa/TF (tissue factor) complex and Xa are proteins known to be involved
in the blood coagulation cascade [33] and, as such, are validated targets in the search
for novel antithrombotic drugs [34, 35] . Having established that a series of 2,6 -
diphenoxypyridines, including several 3,5 - difl uoro - 4 - methyldiaryloxypyridines derived
from 4 - methyltetrafl uoropyridine, to be modest inhibitors of factor Xa, medicinal chemists
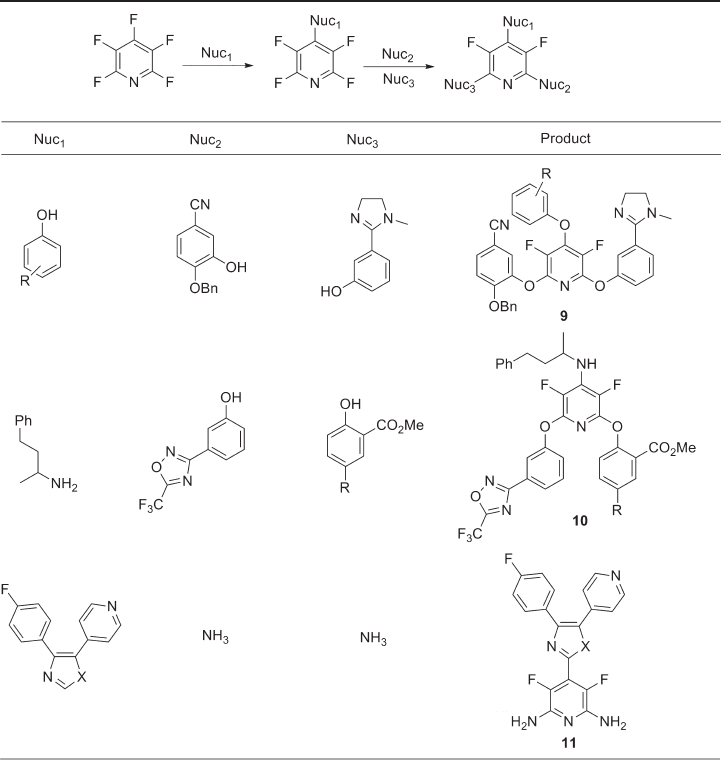
synthesized a small library of 3,5 - difl uorotriaryloxypyridines 9 from pentafl uoropyridine
[36] . The 2,4,6 - substitution pattern was obtained through sequential nucleophilic aromatic
substitution by substituted phenols in typically high yields and polysubstitution could
often be accomplished in a single reaction vessel. Systems derived from this series of
2,6 - diphenoxypyridines served as a basis for creating a more potent system, leading to the
potent FVIIa/TF inhibitor 10 , formed by reaction of a sequence of nitrogen, oxygen and
oxygen - centred nucleophiles.
Figure 11.5 Polysubstituted products from pentafl uoropyridine [30, 32] .
NF
F
CF(CF
3
)
2
F
F
NO
F
CF(CF
3
)
2
F
O
O O
NF
F
F
F
F
CF
2
=CF-CF
3
TDAE, MeCN
CH
3
OCH
2
CH
2
O
-
Na
+
THF, reflux, 24 h
NMeO
F
OMe
F
OMeNF
F
OMe
F
F
MeONa, MeOH
5
6, 74%
7, 76%
8, 76%
298 Fluorine in Medicinal Chemistry and Chemical Biology
It is thought that inhibition of the p38 kinase protein could treat the underlying cause
of chronic infl ammatory diseases, and it is in this context that chemists in Switzerland
prepared a diverse set of aryl - substituted pyridinylimidazoles 11 to achieve potentially
high - affi nity binding to the active site [37] . Deprotonation of the SEM (2 - [trimethylsilyl]
ethoxymethyl) - protected imidazole gave a carbanion that reacted as a nucleophile with
pentafl uoropyridine to give the expected 4 - substituted pyridine. Bromination at the 4 -
and 5 - positions of the imidazole was followed by regioselective Stille reaction to yield a
pyridinylimidazole derivative, while subsequent Suzuki or Stille coupling at the remaining
carbon – bromine bond was followed by deprotection of the imidazole. Finally, diamination
Table 11.3 Biologically active polysubstituted systems synthesized from
pentafl uoropyridine
Perfl uorinated Heteroaromatic Systems as Scaffolds for Drug Discovery 299
at the 2 - and 6 - positions of the tetrafl uoropyridine gave the desired biologically active
3,5 - difl uoropyridine system 11 .
11.2.2 Tetrafl uoropyrimidine as a Core Scaffold
Perfl uorinated diazines (pyrimidine, pyrazine and pyridazine) are typically 1000 times
more reactive towards nucleophiles than is pentafl uoropyridine and application of the
sequential nucleophilic substitution methodology to reactions involving various diazine
systems with a range of nucleophiles would, in principle, lead to the synthesis of many
novel polyfunctional diazine derivatives. However, only a very limited number of reports
concerning reactions of perfl uorinated diazines with nucleophiles have been published [25,
26] and the use of tetrafl uorodiazines as scaffolds has not been developed to any great
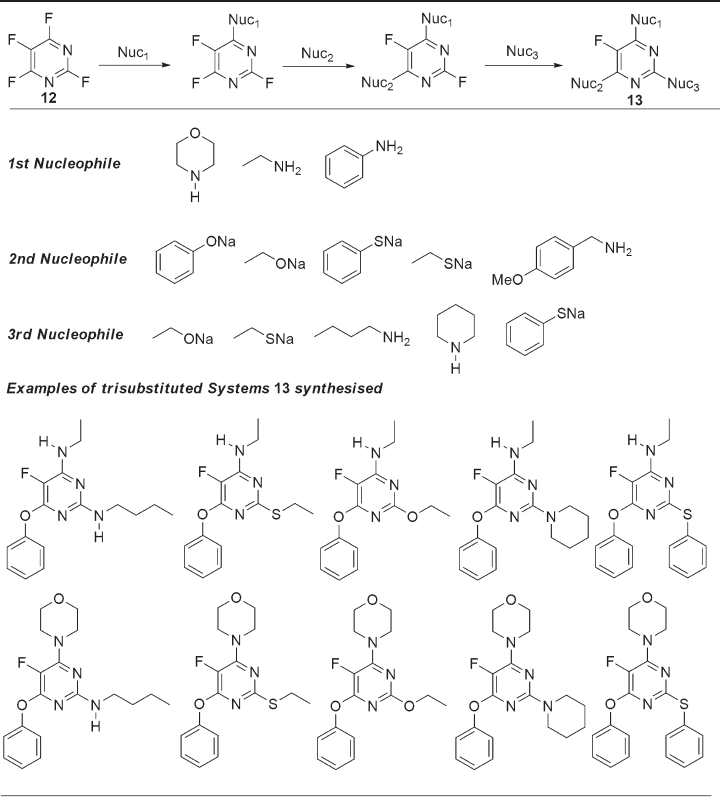
extent. Several instances of reactions of tetrafl uoropyrimidine 12 (Table 11.4 ) with a small
range of nucleophiles have been reported [38] and, in all cases, nucleophilic substitution
occurs selectively at the 4 - position. A recent systematic study of reactions of the 4 -
aminopyrimidine systems (see Table 11.4 ) found that second and third substitution
processes occurred selectively at the 6 - and 2 - positions, respectively, giving ready access
to a small array of 5 - fl uoro trisubstituted pyrimidine derivatives 13 [39] .
11.2.3 Perbromofl uoropyridine Scaffolds
Clearly, the range of nucleophiles that is available, the functionality that could be installed
(for example upon a pyridine or pyrimidine ring) and, of course, the functional groups on
pendant substituents may, in principle, allow access to a great variety of polyfunctional
pyridine analogues. However, despite this, the reactions outlined above are restricted to a
sequence of nucleophilic substitution processes, thereby limiting the variety of structural
arrays that can be synthesized from such perfl uorinated core scaffolds. Consequently,
related perhalogenated scaffolds that have more fl exible functionality may be advanta-
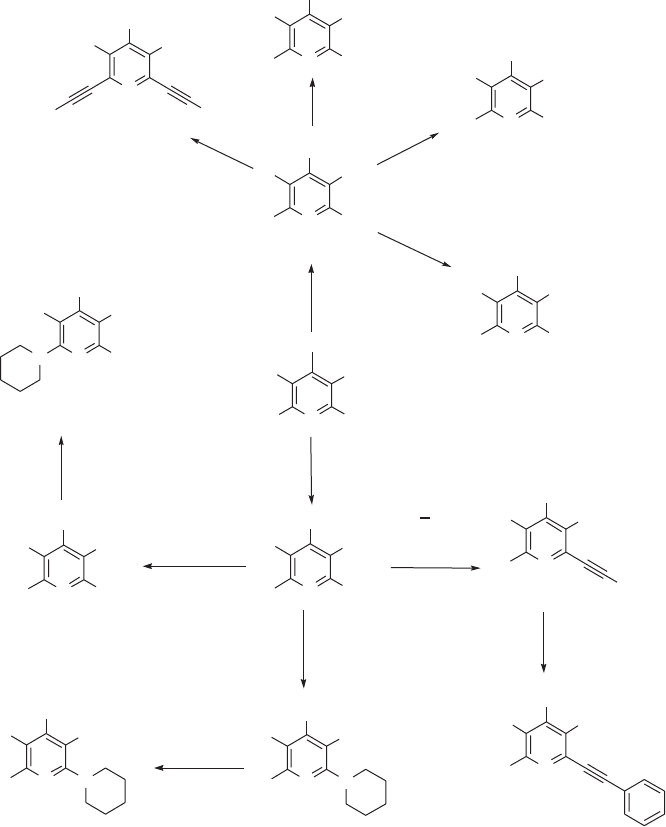
geous and, in this context, 2,4,6 - tribromo - 3,5 - difl uoropyridine 14 , synthesized by reaction
of pentafl uoropyridine with a mixture of hydrogen bromide and aluminium tribromide in
an autoclave at 140 ° C [40] was assessed as a potential polyfunctional scaffold system (see
Figure 11.6 ). In a series of model reactions it was established that the bromofl uoropyridine
system reacts with “ hard ” nucleophiles (e.g. oxygen - centred nucleophiles) to give products
15 arising from selective replacement of fl uorine, whereas “ soft ” nucleophiles (sulfur,
nitrogen, etc.) selectively replace bromine to give product 16 [40] . The presence of
carbon – bromine bonds on this scaffold allows Pd - catalysed Sonogashira [41] and Suzuki
[42] coupling reactions to occur giving, for example, 17 , and selective debromo - lithiation
at the 4 - position followed by trapping of the lithiated pyridine species [43] by a variety
of electrophiles, giving access to a wide range of polyfunctional pyridine systems 18 that
could be utilised as scaffolds in their own right. Subsequently, a combination of nucleo-
philic aromatic substitution and Pd - catalysed Sonogashira reactions involving pentafl uo-
ropyridine as the core scaffold has enabled the synthesis of several pentasubstituted
pyridine systems such as 19 and 20 [44] (see Figure 11.6 ).
300 Fluorine in Medicinal Chemistry and Chemical Biology
11.2.4 Polyfunctional Fluorinated [5,6] - and [6,6] - Bicyclic
Heteroaromatic Scaffolds
Bicyclic nitrogen heterocyclic systems can have a range of very valuable biological
activity and there are several examples of [5,6] - and [6,6] - ring - fused systems in which a
ring - fused pyridine motif is a constituent part (see Figure 11.2 ). However, many bicyclic
nitrogen - containing heterocycles remain surprisingly inaccessible [23] despite the relative
simplicity of their molecular structures, and the chemistry of even the least complex
heterocycles of this class remains largely unexploited. Inevitably, this provides great
opportunities for the discovery of new small - molecule chemical entities that fall within
Table 11.4 Tetrafl uoropyrimidine as a core scaffold [39]
Perfl uorinated Heteroaromatic Systems as Scaffolds for Drug Discovery 301
the RO5, if suitable polyfunctional, bicyclic, nitrogenated heterocyclic scaffolds can be
reliably accessed.
In this context, a polyfl uorinated imidazopyridine system 21 has been prepared by a
multistep synthetic route (see Figure 11.7 ) beginning from 3 - chlorotetrafl uoropyridine 22 ,
via the formation and subsequent condensation of 3,4 - diamino - 2,5,6 - trifl uoropyridine with
diethoxymethyl acetate [45] . Subsequent reaction of the ring - fused system with ammonia
demonstrated the potential of such systems as polyfunctional scaffolds, if much shorter
Figure 11.6 Perfl uorobromopyridine derivatives as core scaffolds [40 – 44] .
NF
F
F
F
NBr
F
Br
F
Br
14, 91%
HBr, AlBr
3
140
o
C
MeONa
MeOH, r.t.
NBr
F
Br
OMe
Br
15, 70%
N
Br
F
SPh
F
Br
NBr
F
E
F
Br
16, 85%
N
F
Br
F
PhPh
17, 72%
18, E = H, SiMe
3
,
CO
2
H, PhCO
PhSNa, MeCN,
reflux, 24 h
(a) BuLi, Et
2
O, -78
o
C.
(b) E
+
, -78
o
C - r.t.
Ph-C=C-H, CuI,
Pd(Ph
3
P)
2
Cl
2
Et
3
N, r.t.
NBr
F
CF(CF
3
)
2
F
Br
MeONa
MeOH
N
Br
F
CF(CF
3
)
2
OMe
Br
Piperidine
MeCN, reflux
N
N
F
CF(CF
3
)
2
OMe
Br
62%
19a : 19b, 1:1, 64% combined
Ph-C=
C-H
Pd (0) catalyst
CuI, Et
3
N
NBr
F
CF(CF
3
)
2
F
63%
Ph
MeONa
MeOH
N
Br
MeO
CF(CF
3
)
2
F
20, 55%
NBr
F
CF(CF
3
)
2
OMe
N
Piperidine
MeCN
reflux
NBr
F
CF(CF
3
)
2
F
N
83%
MeONa
MeOH
19b, 80%
+ 19b
F
1) CF
2
=CF-CF, TDAE
2) HBr, AlBr
3
, 140
o
C
19a
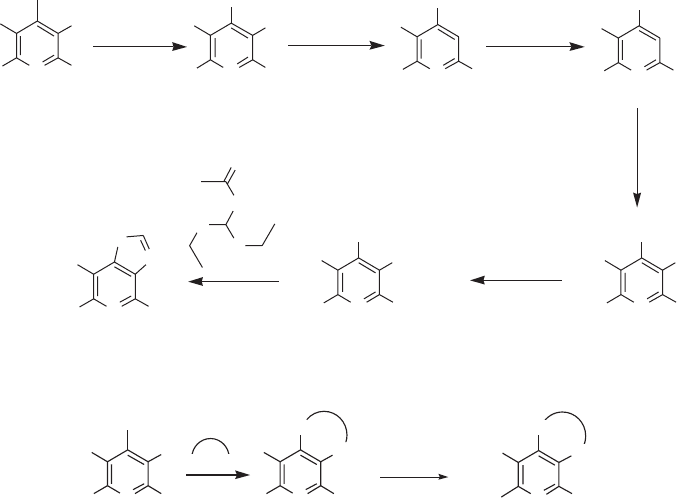
302 Fluorine in Medicinal Chemistry and Chemical Biology
synthetic sequences for the preparation of a range of these structural core scaffolds could
be developed.
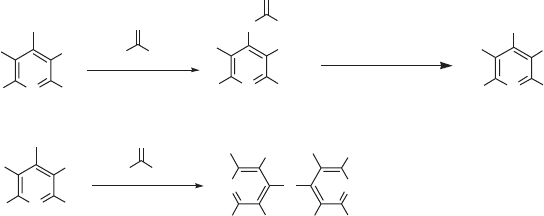
A convenient synthetic strategy for the synthesis of polyfl uorinated polycyclic ring
scaffolds involving reaction of pentafl uoropyridine with bifunctional nucleophiles could,
in principle, provide access to a wide range of polyfunctional systems (see Figure 11.8 ).
Here, for example, substitution of the 4 - position of the pentafl uoropyridine scaffold would,
in principle, be followed by attack at the adjacent 3 - position owing to the geometric con-
straints of the system to give appropriate ring - fused systems 23 . The bicyclic scaffold
possesses further sites that are activated towards nucleophilic attack and could, therefore,
provide approaches to the synthesis of a wide variety of functional fused ring systems 24 ,
if the orientation of subsequent nucleophilic substitution processes could be controlled.
However, initial attempts to use this strategy to prepare azapurine derivatives, by
reaction of pentafl uoropyridine and either guanidine or thiourea, led to 4 - aminopyridine
25 and bispyridyl derivatives 26 respectively via base - induced elimination processes [46]
(see Figure 11.9 ).
In these cases, while nucleophilic substitution at the 4 - position of pentafl uoropyridine
by guanidine could be achieved readily [46] , attack at the less activated 3 - position by the
weak NH
2
nucleophile present on the guanidine moiety made cyclization a less favoured
process than elimination. Consequently, in order to achieve cyclization by nucleophilic
substitution in the second step, a more reactive second nucleophile is necessary.
Figure 11.7 Synthesis of imidazopyridine systems 21 from 3 - chlorotetrafl uoropyridine 22 .
N
F
ClF
F F
N
NH
2
ClF
F F
N
NH
2
F
F F
10 % Pd / C
NEt
3
, H
2
N
NHNO
2
F
F F
NH
4
OH
N
NH
2
F
F F
NO
2
N
NH
2
F
F F
NH
2
N
F
F F
N
HN
KNO
3
H
2
SO
4
H
2
SO
4
Raney Ni
H
2
, EtOH
O
O
O
O
21
22
Figure 11.8 Strategy for the synthesis of ring - fused heterocycles from pentafl uoropyridine.
1) Nuc
3
2) Nuc
4
N
F
F
FF
F
1
N
Nuc
1
Nuc
2
FF
F
2
N
1
2
Nuc
4
Nuc
3
F
4232
Nuc
Nuc
Nuc Nuc
Perfl uorinated Heteroaromatic Systems as Scaffolds for Drug Discovery 303
Pentafl uoropyridine and several nucleophilic model difunctional secondary diamines,
such as N,N ′ - dimethylethylene diamine 27a , gave pyrido[3,4 - b ]pyrazine systems 28 in a
two - step one - pot annelation reaction, upon refl ux or microwave heating [47] (see Table
11.5 ). The chemistry of tetrahydropyrido[3,4 - b ]pyrazine systems is relatively undeveloped
because of the diffi cult, low - yielding multistep syntheses, either from diaminopyridine
[48] or chloroaminopyridine [49] precursors or by reduction of pyrido[3,4 - b ]pyrazine
derivatives by metal hydrides [50 – 55] , which are required to prepare even the simplest
member of this heterocyclic class. Indeed, for the synthesis of appropriate multisubstituted
scaffold systems based upon the tetrahydropyrido[3,4 - b ]pyrazine subunit, this diffi cult
situation is magnifi ed further. Less - nucleophilic primary diamine nucleophiles such as
27b gave only noncyclized products 30 arising from substitution of the 4 - position of
pentafl uoropyridine.
The pyrido[3,4 - b ]pyrazine scaffold reacted with a series of nucleophiles to give major
products arising from substitution at the 7 - position [47] . Although this process was not
regiospecifi c, the alkoxylated scaffold could be isolated and purifi ed by column chroma-
tography from the minor isomer that was formed by substitution of the fl uorine atom
located at the 5 - position.
The trifl uorinated scaffold reacts preferentially at the 7 - position with some product
arising from competing substitution at the 5 - position and this selectivity is due to the
activating infl uence of ring nitrogen and maximizing of the number of activating fl uorine
atoms that are ortho to the site of attack (see Figure 11.10 ). The difl uorinated scaffolds
are still activated towards nucleophilic attack and a short series of fl uoropyrido[3,4 -
b ]pyrazines 29 has been synthesized in which the remaining fl uorine atoms that are located
ortho to pyridine nitrogen were displaced [47] .
By a similar strategy, an imidazopyridine scaffold 32 was synthesized by reaction of
pentafl uoropyridine and benzamidine 31 [56] and, in this case, subsequent nucleophilic
substitution occurs at the 5 position to give 33 , presumably because of interaction of the
nucleophile and the imidazo ring NH bond which directs the incoming nucleophile to the
less activated site adjacent to pyridine ring nitrogen.
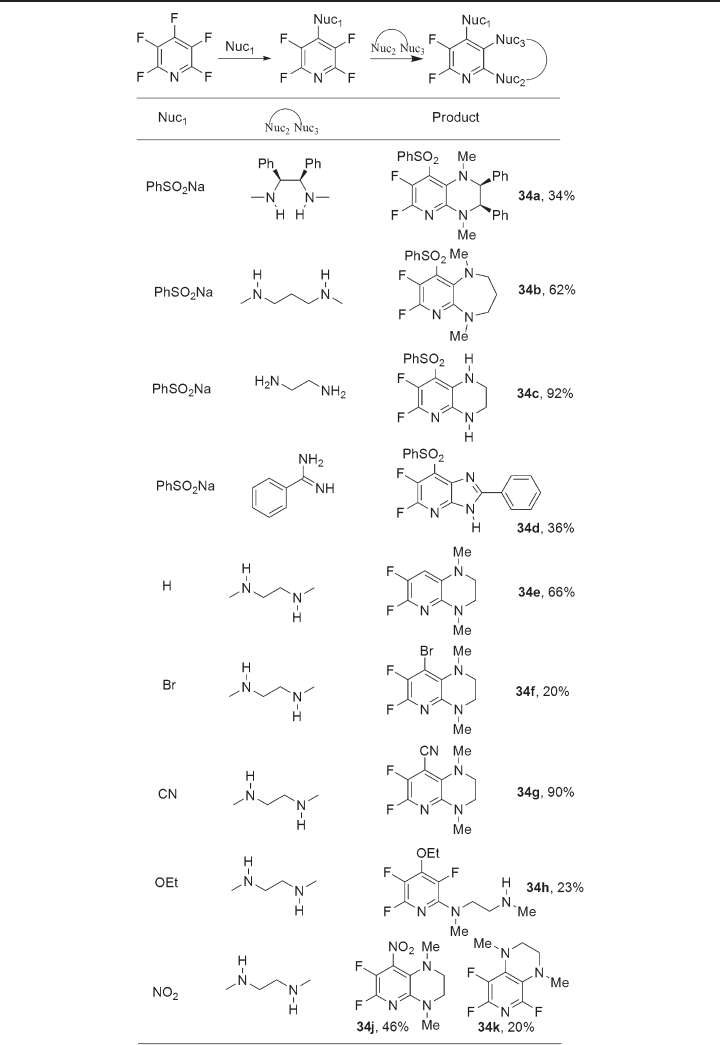
This overall synthetic strategy (see Figure 11.8 ) was adapted to give a range of
isomeric pyrido[2,3 - b ]pyrazines structures 34 by simply varying the order of reaction
with appropriate mono - and difunctional nucleophiles [57, 58] (see Table 11.6 ).
For example, reaction of sodium phenylsulfi nate with pentafl uoropyridine gives the
Figure 11.9 Reactions of pentafl uoropyridine with urea and guanidine systems [46] .
N
F
F
F
FF
NH
NH
2
H
2
N
N
F
F F
NaH
N
F
HN
F
FF
NH
2
NH
64 %
S
NH
2
H
2
N
NaH
F
NH
2
Sulpholane
NEt
3
, 80 - 200
o
C
N
F
F
F
FF
N S N
F F
FF F F
FF
25, 38%
26, 71%
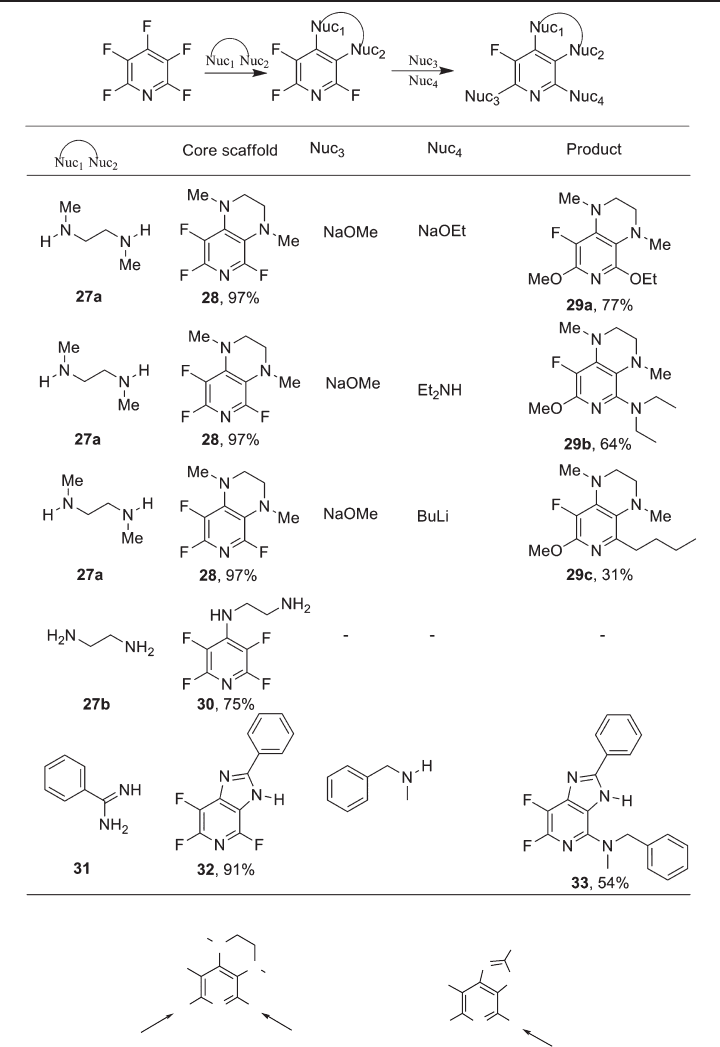
Table 11.5 Synthesis of pyrido[3,4 - b ]pyrazine and imidazopyridine scaffolds [47, 56]
Figure 11.10 Regioselectivity of nucleophilic substitution of pyrido[3,4 - b ]pyrazine and
imidazopyridine scaffolds [56] .
N
N
N
Me
Me
F
F F
A
ctivated by
ortho ring N
ortho F
meta F
Activated by:
ortho ring N
meta F
Deactivated by:
para F
N
F
F F
Activated by:
ortho ring N
Directed by NH
NH
N
Ph
Perfl uorinated Heteroaromatic Systems as Scaffolds for Drug Discovery 305
Table 11.6 Synthesis of pyrido[2,3 - b ]pyrazine scaffolds [57, 58]
306 Fluorine in Medicinal Chemistry and Chemical Biology
Figure 11.11 Reaction of 4 - cyanotetrafl uoropyridine with amidines [56] .
NF
F
CN
F
F
+
NH
NH
2
Ph
NaHCO
3
MeCN
NF
F
CN
F
N NH
2
Ph
+
N
N
N
H
2
N Ph
F
F F
.HCl
36, 20% 37, 5%
N
F
F
CN
F
F
N
N
N
H
2
N Ph
F
F F
NF
F N
F
N
NH
··
2
Ph
N
N
N
Ph
F
F F
H H
N
reflux, 4 d
NF
F
CN
F
F
+
NH
NH
2
Ph
NaHCO
3
MeCN
N
N
N
H
2
N Ph
F
N F
.HCl
38, 58%
reflux, 4 d
H
2
N
Ph
35
4 - phenylsulfonylpyridine derivative and, since the phenylsulfonyl group is strongly
electron withdrawing, annelation by reaction with appropriate diamines proceeded
readily to give pyrido[2,3 - b ]pyrazine scaffolds [57, 58] . In addition, 4 - bromo - and 4 -
cyanotetrafl uoropyridine systems gave the corresponding pyrido[2,3 - b ]pyrazines, 34f and
34g respectively, upon reaction with N,N ′ - dimethylethylene diamine [58] .
However, not all tetrafl uoropyridine derivatives were suitable substrates for the
synthesis of pyrido[2,3 - b ]pyrazine scaffolds by analogous annelation reactions [58] . The
4 - ethoxy and 4 - dimethylaminotetrafl uoropyridine systems gave only noncyclized products
34h and 34i , respectively, arising from substitution of the 2 - position. However, the 4 - nitro
derivative led to the formation of a mixture of products 34j, k arising from substitution
of both the 2 - fl uorine and 4 - nitro group, which is itself a very labile leaving group in
nucleophilic aromatic substitution processes [58] .
Additionally, 4 - cyanotetrafl uoropyridine 35 reacts with amidines [56] to give a
mixture of products 36 and 37 arising from substitution of fl uorine at both the 2 - and
3 - positions in a 1 : 1 ratio (see Figure 11.11 ). The product derived from 3 - substitution and
subsequent cyclization onto the cyano group, refl ects the activating infl uence of the
strongly electron - withdrawing cyano group on adjacent heteroaromatic carbon sites and
the electrophilicity of the cyano group itself. An excess of the amidine leads to high yields
of 38 [56] .
Preliminary reactions involving scaffold 39 derived from 1,2 - diaminoethane with
nitrogen and sulfur nucleophiles led to products 40 arising from substitution of the phe-
nylsulfonyl group, a very good leaving group that is located at an activated site para
to pyridine nitrogen, and reaction of acetic anhydride gave selective acylation yielding
41 (see Figure 11.12 ), indicating some of the possibilities for functionalization of these
bicyclic scaffolds [57] .
In an application of this annelation strategy to a medicinal chemistry project, tricyclic
2 - pyridone 42 was synthesized using pentafl uoropyridine as the core scaffold and all but
one of the fl uorine atoms were displaced in a multistep process in which a carbon – oxygen
centred difunctional nucleophile is used as the annelating reagent (see Figure 11.13 ).
