Ojima I. (ed.) Fluorine in Medicinal Chemistry and Chemical Biology
Подождите немного. Документ загружается.

318 Fluorine in Medicinal Chemistry and Chemical Biology
the basicity of the amino group due to the strong electron - withdrawing nature of
fl uorine.
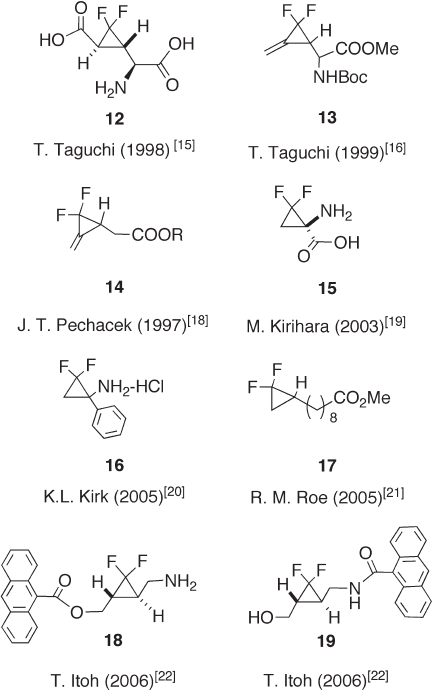
Taguchi and co - workers synthesized several biologically active gem -
difl uorocyclopropanes, such as metabotropic glutamate receptor agonist 12 [15] and
methylene - gem - difl uorocyclopropane 13 [16] . 2 - (2 - Carbohydroxy - 3,3 - difl uorocyclopropyl) -
glycines (F
2
CCGs) were synthesized by means of a chiral auxiliary method (for F
2
CCG - I
12 [15a, 15c] ) as well as a chiral pool method (for all eight possible stereoisomers [15c] ).
Enhancement of acidity of the ω - carboxyl group by fl uorine incorporation was confi rmed
by NMR titration experiments. Evaluation of the neuropharmacological activities of
F
2
CCGs in rat, compared with those of the corresponding cyclopropylglycine isomers,
revealed that the (2 S ,1 ′ S ,2 ′ S ) - isomer, l - F
2
CCG - I 12 , was a potent agonist for metabotropic
Figure 12.6 Examples of biologically active gem - difl uorocyclopropanes (simple
molecules).
gem-Difl uorocyclopropanes as Key Building Blocks for Novel Biologically Active Molecules 319
glutamate receptors, which induced a priming effect on α - aminopimelate and l - glutamate
[15c, 15d, 5e] . Methylenecyclopropylglycine (MCPG) and its metabolite, MCPF - CoA,
are known to have inhibitory activity against enoyl - CoA hydratase (crotonase), respon-
sible for fatty acid metabolism [17] . Four possible stereoisomers of methylene - gem -
difl uorocyclopropylglycines 13 (F
2
MCPG) were synthesized [16] , although their
biological activities were not reported.
Barger and co - workers also synthesized F
2
MCP derivatives 14 and evaluated their
activities against the nematode pest Meloidogyne incognite [18] . Unfortunately, com-
pounds 14 were inactive up to a concentration of 400 ppm, while the corresponding non-
fl uorinated compound (R = H) showed 100% inhibition of the microbe at only 7.8 ppm
[18] . Kirihara et al. reported a highly effi cient method for the synthesis of the optically
active gem - difl uorocyclopropane analogue 15 of 1 - aminocyclopropane - 1 - carboxylic acid
using a chemo - enzymatic strategy [19] . Kirk, Haufe, and their co - workers synthesized
2,2 - difl uoro - 1 - phenylcyclopropylamine ( 16 ) and evaluated its inhibitory activity for
fl avin - containing monoamine oxidase (MAO). Unfortunately, no inhibitory effect was
observed and it was concluded that monofl uorine substitution was essential to cause MAO
inhibition [20] . Recently, Roe et al. reported an interesting biologically active simple gem -
difl uorocyclopropane 17 , which acted as a competitive inhibitor of the juvenile hormone -
epoxide hydrolase [21] .
We developed a synthetic methodology for preparing optically active gem -
difl uorocyclopropane building blocks using lipase technology [4] . Using this methodology,
gem - difl uorocyclopropanes 18 and 19 , bearing a 9 - anthracenecarbonyl group, were syn-
thesized. These compounds showed a strong DNA - cleavage property switched on by
photoirradiation [22] (see Figure 12.6 ).
Nucleoside analogues have been recognized as potential chemotherapeutic agents and
in fact several carbocyclic nucleosides exhibit potent anti - HIV activities. Accordingly,
gem - difl uorocyclopropane - containing nucleoside analogues and mimics have attracted
substantial interest and various compounds have been synthesized (see Figures 12.7
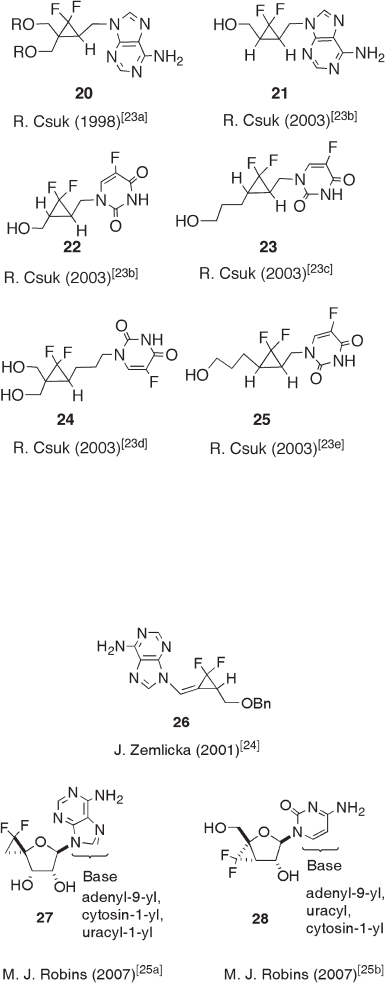
and 12.8 ). Csuk pioneered this fi eld and his group prepared a good number of gem -
difl uorocyclopropane - containing nucleoside mimics as represented by compounds 20 – 25
[23] , some which were found to possess antitumor activity and anti - HIV activity. Although
all compounds reported by Csuk ’ s group are racemic, optically active compounds could
be prepared using the enzymatic resolution technology developed by us [4] and by
Kirihara ’ s group [19] .
Zemlicka and co - workers synthesized methylene - gem - difl uorocyclopropane mimics
of nucleoside and found that the Z - isomer of adenine - 9 - yl analogue 26 exhibited anticancer
activity against leukemia and solid tumor cell lines in vitro (see Figure 12.8 ) [24] .
Adenin - 9 - yl, 2 - amino - 6 - chloropurin - 9 - yl, and guanin - 9 - yl analogues of 26 were synthe-
sized and evaluated for their activities against HCMV, HSV - 1, HSV - 2, EBV, VZV, and
HIV - 1. However, only the E - isomer of adeninyl analogue 26 showed moderate antiviral
activity against the Towne strain of HCMV propagated in human foreskin fi broblast (HFF)
cells (EC
50
21 µ M), which was noncyctotoxic at this concentration [24] . Recently, Robins
and co - workers reported the synthesis of the gem - difl uorocyclopropane analogues of
nucleosides such as 27 and 28 (see Figure 12.8 ) [25] , but no biological activities of these
compounds have yet been disclosed.
320 Fluorine in Medicinal Chemistry and Chemical Biology
Figure 12.8 Nucleoside - type biologically active gem - difl uorocyclopropanes prepared by the
Zemlick and Robins group.
Figure 12.7 Nucleoside - type biologically active gem - difl uorocyclopropanes prepared by the
Csuk group.
gem-Difl uorocyclopropanes as Key Building Blocks for Novel Biologically Active Molecules 321
12.3 Synthesis of gem - Difl uorocyclopropanes
Three synthetic methods for gem - difl uorcyclopropane derivatives have been developed
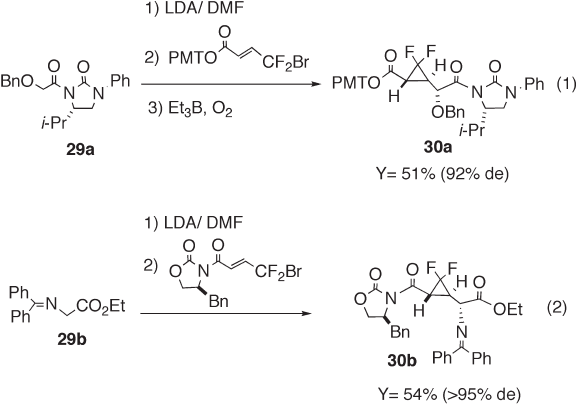
to date. Shibuya and Taguchi reported an effi cient route to optically active gem -
difl uorocyclopropane derivative 30a through diastereoselective Michael addition of Evans
enolate 29a to 4 - bromo - 4,4 - difl uorobut - 2 - enoate, followed by Et
3
B - mediated radical cou-
pling (see equation 1, Scheme 12.1 ) [15a] . Alternatively, for the synthesis of metabotropic
glutamate receptor agonist F
2
CCG - I, the Michael acceptor having the Evans oxazolidinone
as the chiral auxiliary was reacted with N - diphenylmethyleneglycinate 29b in DMF to
give difl uorocyclopropylglycine derivative 30b with an excellent diastereoselectivity
( > 95% de) (see equation 2, Scheme 12.1 ) [15c] .
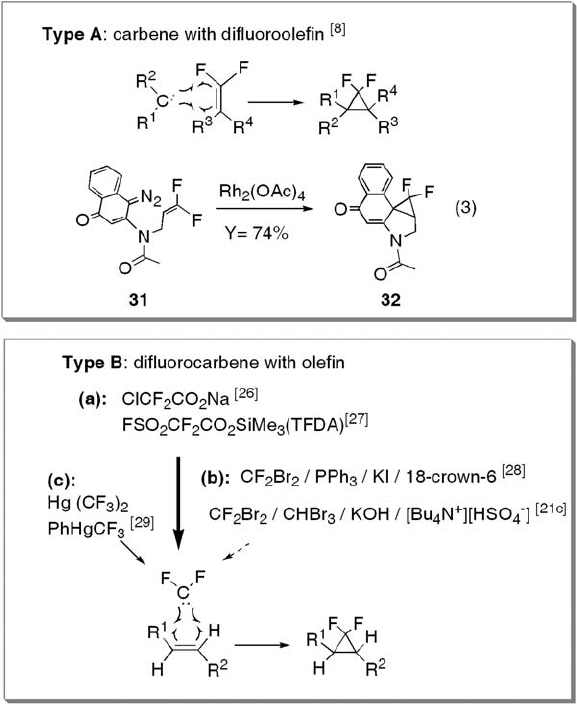
The most versatile method for constructing gem - difl uorocyclopropanes is the
carbine - addition reaction to olefi ns, which includes two types as shown in Scheme 12.2
(type A and type B). Boger and Jenkins synthesized the gem - difl uorocyclopropane moiety
through intramolecular metal - carbenoid addition to 1,1 - difl uoroalkene group (type A).
Thus, diazoquinone 31 was treated with 0.1 – 0.2 equivalents of Rh
2
(OAc)
4
in toluene at
110 ° C to give gem - difl uorocyclopropane 32 in good yield [8] (see equation 3 in Scheme
12.2 ). The most widely used method for preparing gem - difl uorocyclopropanes is the
difl uorocarbene addition to olefi ns (type B), which is applicable to large - scale preparation.
Although numerous reactions for generating difl uorocarbene have been reported, three
major methods are shown under type B in Scheme 12.2 [26 – 29] : (a) pyrolysis of chloro-
difl uoroacetate [26] or trimethylsilyl fl uorosulfonyldifl uoroacetate (TFDA), known as
Dolbier ’ s reagent [27] ; (b) generation from dibromodifl uoromethane following Barton ’ s
Scheme 12.1 Synthesis of gem - difl uorocyclopropane through diastereoselective Michael
reaction followed by radical coupling.
322 Fluorine in Medicinal Chemistry and Chemical Biology
or Schlosser ’ s procedure [28] ; and (c) generation from trifl uoromethylmercury compounds,
known as Seyferth ’ s reagents [29] . Of these methods, (b) and (c) can generate difl uoro-
carbenes under mild conditions. Unfortunately, CF
2
Br
2
and Seyferth ’ s reagent are no
longer commercially available because of their inherently hazardous properties. Therefore,
ClCF
2
COONa is currently the sole commercial source for difl uorocarbene generation.
Since this reaction requires harsh conditions (180 – 190 ° C in diglyme), development of a
new and effi cient method is desirable for the generation of difl uorocarbene under mild
conditions.
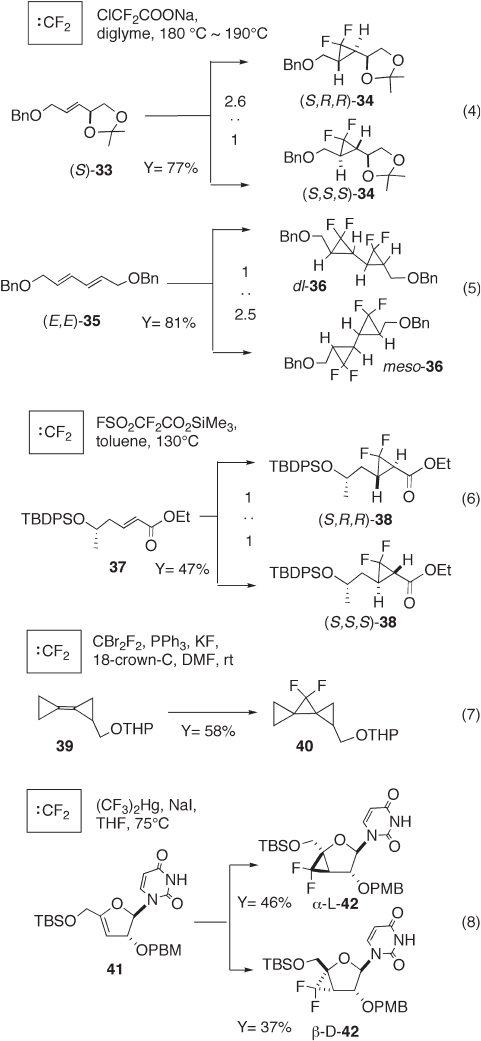
Scheme 12.3 summarizes typical examples for the synthesis of gem -
difl uorocyclopropanes via difl uorocarbene addition to olefi ns. Although high temperature
is necessary to generate difl uorocarbene by pyrolysis of ClCF
2
COONa, addition of
difl uorocarbene to carbon – carbon double bonds proceeds in a stereospecifi c cis manner
Scheme 12.2 Preparation of gem - difl uorocyclopropanes through addition of difl uorocarbene
to olefi n.
gem-Difl uorocyclopropanes as Key Building Blocks for Novel Biologically Active Molecules 323
Scheme 12.3 Typical examples of the synthesis of gem - difl uorocyclopropanes using difl uo-
rocarbene addition to olefi n.
324 Fluorine in Medicinal Chemistry and Chemical Biology
[19a] . Taguchi and co - workers prepared optically pure gem - difl uorocyclopropane 34
through addition of difl uorocarbene to optically active olefi n ( S ) - 33 , followed by
chromatographic separation of the resulting diastereomers, ( S,R,R ) - 34 and ( S,S,S ) - 34
(2.6 : 1, 77% yield) (see equation 4, Scheme 12.3 ) [15a] . We prepared novel bis - gem -
difl uorocyclopropanes 36 as a mixture of dl - 36 and meso - 36 (1 : 1.5, 81% yield) through
difl uorocarbene addition to ( E,E ) - diene 35 [4b] (see equation 5, Scheme 12.3 ). Generally,
pyrolysis of trimethylsilyl fl uorosulfonyldifl uoroacetate (TFDA) proceeds under milder
conditions (130 ° C) as shown in equation 6, Scheme 12.3 [14] . However, TFDA is avail-
able only in a few countries at present. Difl uorocarbene can be generated at room tem-
perature under the Barton – Schlosser conditions (CF
2
Br
2
, PPh
3
, KF, and 18 - crown - 6).
Interesting spiro - gem - difl uorocyclopropane 40 was synthesized by de Meijere and co -
workers using this method (see equation 7, Scheme 12.3 ) [30] . To the best of our knowl-
edge, this is the most effi cient method for preparation of gem - difl uorocyclopropanes.
However, as mentioned above, it is impossible to obtain hazardous CF
2
Br
2
commercially.
For experienced researchers using appropriate safety precautions, toxic Seyferth ’ s reagent
is a useful source for preparing gem - difl uorocyclopropanes. Robins and co - workers syn-
thesized gem - difl uorocyclopropane nucleoside 42 using this method because substrate 41
was not tolerant of high temperature (see equation 8, Scheme 12.3 ) [25] .
12.4 Synthesis of Optically Active gem - D i fl uorocyclopropanes via
Enzymatic Resolution
Enzymatic resolution has been successfully applied to the preparation of optically active
gem - difl uorocyclopropanes (see Scheme 12.4 ). We succeeded in the fi rst optical resolution
of racemic gem - difl uorocyclopropane diacetate, trans - 43 , through lipase - catalyzed
enantiomer - specifi c hydrolysis to give ( R,R ) - ( − ) - 44 with > 99% ee (see equation 9, Scheme
12.4 ) [4a] . We also applied lipase - catalyzed optical resolution to an effi cient preparation
of monoacetate cis - 46 from prochiral diacetate cis - 45 (see equation 10, Scheme 12.4 ) [4a] .
Kirihara et al. reported the successful desymmetrization of diacetate 47 by lipase - catalyzed
enantiomer - selective hydrolysis to afford monoacetate ( R ) - 48 , which was further trans-
formed to enantiopure amino acid 15 (see equation 11, Scheme 12.4 ) [19] . We demon-
strated that the lipase - catalyzed enantiomer - specifi c hydrolysis was useful for
bis - gem - difl uorocyclopropane 49 . Thus, optically pure diacetate ( R,S,S,R ) - 49 and ( S,R,R,S ) -
diol 50 , were obtained in good yields, while meso - 49 was converted to the single mono-
acetate enantiomer ( R,S,R,S ) - 51 via effi cient desymmetrization (see equation 12,
Scheme 12.4 ) [4b, 4e] . Since these mono - and bis - gem - difl uorocyclopropanes have two
hydroxymethyl groups to modify, a variety of compounds can be prepared using them as
building blocks [4, 22] .
de Meijere and co - workers reported the synthesis of enantiopure 7,7 - difl uorodispiro
[2.0.2.1]heptylmethanols ( 54 ) via difl uorocyclopropanation of methylenespirobiscyclo-
propane (1 S ,3 R ) - 53 ( > 99% ee), which was obtained by lipase - catalyzed enantiomer -
specifi c transesterifi cation of ( ± ) - 52 , followed by separation of two diastereomers (see
equation 13, Scheme 12.4 ) [30] .
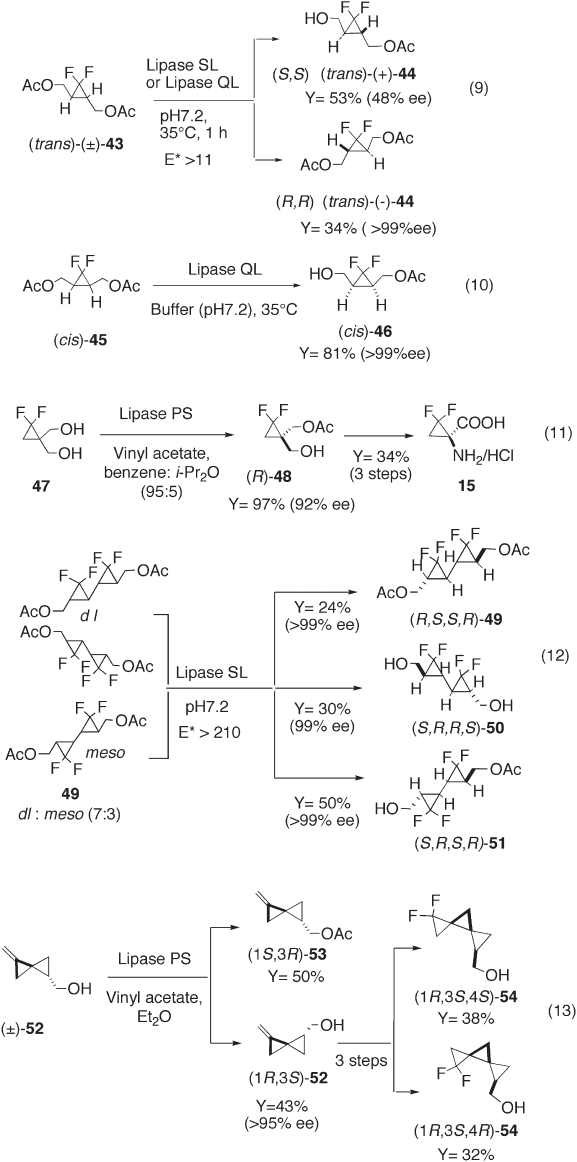
Scheme 12.4 Preparation of optically active gem - difl uorocyclopropanes using enzymatic
resolution.
326 Fluorine in Medicinal Chemistry and Chemical Biology
12.5 Biologically Active gem - Difl uorocyclopropanes:
Anthracene – gem - Difl uorocyclopropane Hybrids as DNA Cleavage
Agents Switched on by Photoirradiation
As described above, gem - difl uorocyclopropane analogues of structurally complex
biologically active natural products reported to date have not shown enhanced activities
[8, 14] , while rather simple synthetic gem - difl uorocyclopropanes have shown interesting
biological activities [15, 16, 18 – 22] . We recently reported the synthesis of gem -
difl uorocyclopropanes that showed a strong DNA - cleaving property switched on by
photoirradiation [22] .
During the course of determining the stereochemistry of 1,3 - bishydroxymethyl - 2,2 -
difl uorocyclopropane on the basis of CD (circular dischroism) spectroscopic analysis of
the corresponding 9 - anthracenecarboxylic acid diester 55 , we recognized the interesting
fact that diester 55 was so unstable in dichloromethane solution under sunlight that it
formed unidentifi ed complex compounds by opening its gem - difl uorocyclopropane ring
[4g] . We also found that, similar to this mono - difl uorocyclopropane 55 , the anthracenecar-
boxylic acid diester of bis - gem - difl uorocyclopropane was rapidly decomposed by expo-
sure to sunlight [4c] . It has been shown that anthracene or anthraquinone derivatives serve
as potent DNA - cleavage agents [31 – 34] . For example, Schuster and co - workers reported
that anthraquinonecarboxamide caused GG - selective cleavage of duplex DNA [32] , while
Kumar and Punzalan found that anthracene - substituted alkylamine derivatives possessed
potent DNA - cleaving activity [33] . Toshima et al. reported that quinoxaline - carbohydrate
hybrids acted as GG - selective DNA - cleaving or DNA - binding agents, depending on the
structure of the sugar moiety [34] . From these results, we anticipated that our anthracene -
gem - difl uorocyclopropane hybrids would possess DNA - cleaving activity on photoirradia-
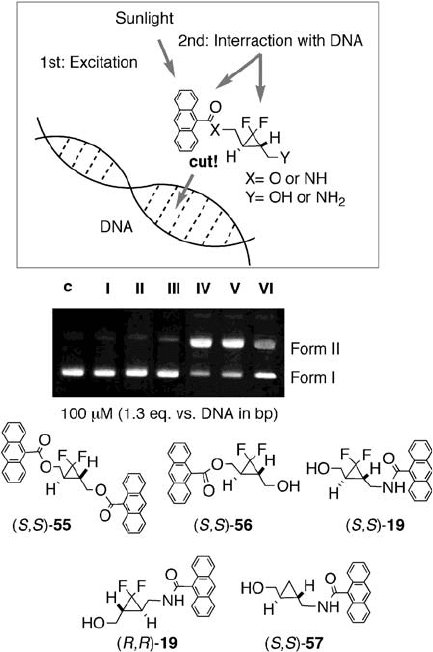
tion (see Figure 12.9 ). It has been shown that decomposition of a gem - difl uorocyclopropane
ring proceeds via a radical species [35] , which may cause DNA - damage [36] . We
prepared optically active anthracenecarbonyl derivatives of gem - difl uorocyclopropane,
19, 55 , and 56 , and evaluated their DNA - cleaving activities. As shown in Figure 12.9 ,
( S,S ) - 55 (lane II) and ( S,S ) - 56 (lane IV) showed only weak DNA - cleaving activities,
while much stronger activity was exhibited by anthracenecarboxamidomethyl - gem -
difl uorocyclopropane 19 . In the presence of 100 µ M of ( S,S ) - 19 , which corresponds to 1.3
molar equivalents to a DNA base pair, DNA was cleaved by photoirradiation and super-
coiled φ X174 DNA (Form I) was converted to the relaxed form (Form II) (see Figure 12.9 ,
lane V). No signifi cant difference in activity was observed between the enantiomers of 19 ,
i.e., ( S,S ) - 19 (lane V) and ( R,R ) - 19 (lane IV). Although nonfl uorinated cyclopropane
counterpart ( S,S ) - 57 also caused substantial DNA - cleavage (lane VI), the activity of
gem - difl uorocyclopropane ( S,S ) - 19 was obviously superior to that of ( S,S ) - 57 at the same
concentration. However, no sequence specifi city was observed in these DNA - cleavage
experiments.
We also found an interesting difference in DNA - cleaving activity between the enan-
tiomers of aminomethyl - gem - difl uorocyclopropylmethyl anthracenecarboxylate, ( S,S ) - 18
and ( R,R ) - 18 . As Figure 12.10 shows, ( R,R ) - 18 exhibited ∼ 10 - fold stronger DNA - cleaving
activity than ( S,S ) - 18 for supercoiled φ X174 DNA. The result clearly indicates that the
gem-Difl uorocyclopropanes as Key Building Blocks for Novel Biologically Active Molecules 327
chirality of the gem - difl uorocyclopropane moiety controls the DNA - cleaving activity of
these anthracene – gem - difl uorocyclopropane hybrids.
These results possibly suggest that the DNA - cleavage by these anthracene – gem -
difl uorocyclopropane hybrids is caused mainly by intercalation or minor - groove binding
of the anthracenyl group to DNA and the gem - difl uorocyclopropane component plays an
important role in determining the DNA - binding mode. Although we found no differences
in sequence specifi city between ( S,S ) - 18 and ( R,R ) - 18 , we hypothesize that the terminal
amino group may bind to the phosphate moiety of DNA, and then direct the intercalation
Figure 12.9 Photocleavage of supercoiled φ X174 plasmid DNA by cyclopropane derivatives.
Lanes: c, control; I, ethyl 9 - anthracenecarboxylate; II, (S,S) - 55 ; III, (S,S) - 56 ; IV, (R,R) - 19 ;
V, (S,S) - 19 ; VI, (S,S) - 57 . Reaction buffer: 20 mM sodium phosphate pH 7.0 (20% DMSO)
Samples were irradiated at 25 ° C for 45 min at a distance of 6 cm using a xenon lamp with
a polystyrene fi lter (365 nm). Electrophoresis was run using 0.8% agarose gel with TAE
buffer at 100 V for 40 min. The gel was stained with EtBr and visualized by UV - B lamp
(transilluminator).
