Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.

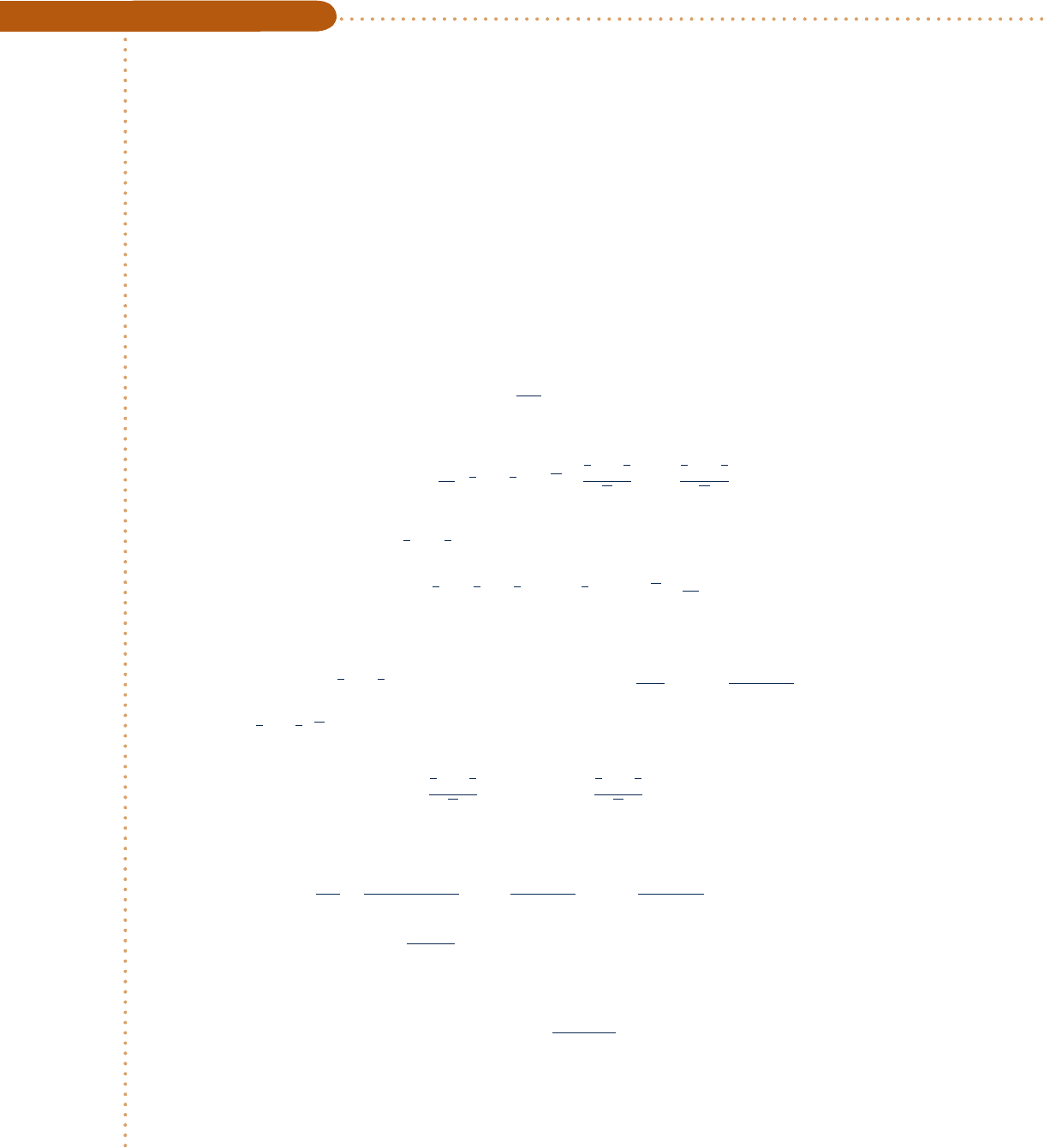
can be evaluated from Fig. A-5 using T
R2
and p
R2
. The use of Eq. 11.92 is illustrated
in the next example.
Using the Generalized Entropy Departure Chart
c c c c EXAMPLE 11.9 c
For the case of Example 11.8, determine (a) the rate of entropy production, in kJ/kg ? K, and (b) the isentropic
turbine efficiency.
SOLUTION
Known:
A turbine operating at steady state has nitrogen entering at 100 bar and 300 K and exiting at 40 bar
and 245 K.
Find: Determine the rate of entropy production, in kJ/kg ? K, and the isentropic turbine efficiency.
Schematic and Given Data: See Fig. E11.8.
Engineering Model: See Example 11.8.
Analysis:
(a)
At steady state, the control volume form of the entropy rate equation reduces to give
s
#
cv
m
#
5 s
2
2 s
1
The change in specific entropy required by this expression can be written as
s
2
2 s
1
5
1
M
es
2
*
2 s
1
*
2 R ca
s
*
2 s
R
b
2
2 a
s
*
2 s
R
b
1
df
where M is the molecular weight of nitrogen and the other terms have the same significance as in Eq. 11.92.
The change in specific entropy s
2
*
2 s
1
*
can be evaluated using
s
2
*
2 s
1
*
5 s81T
2
22 s81T
1
22 R ln
p
2
p
1
With values from Table A-23
s
2
*
2 s
1
*
5 185.775 2 191.682 2 8.314 ln
40
1
00
5 1.711
kJ
kmol ? K
The terms
1
s
*
2 s
2
/
R at the inlet and exit can be determined from Fig. A-5. Using the reduced temperature
and reduced pressure values calculated in the solution to Example 11.8, inspection of Fig. A-5 gives
a
s
*
2 s
R
b
1
< 0.21,
a
s
*
2 s
R
b
2
< 0.14
Substituting values
s
#
cv
m
#
5
1
128 kg
/
kmol2
c1.711
kJ
kmol ? K
2 8.314
kJ
kmol ? K
10.14 2 0.212d
5 0.082
kJ
k
g
? K
(b) The isentropic turbine efficiency is defined in Sec. 6.12 as
h
t
5
1W
#
cv
/
m
#
2
1W
#
cv
/
m
#
2
s
11.7 Generalized Charts for Enthalpy and Entropy 673
c11ThermodynamicRelations.indd Page 673 6/21/10 9:36:08 PM user-s146 c11ThermodynamicRelations.indd Page 673 6/21/10 9:36:08 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

674 Chapter 11
Thermodynamic Relations
where the denominator is the work that would be developed by the turbine if the nitrogen expanded isentropi-
cally from the specified inlet state to the specified exit pressure. Thus, it is necessary to fix the state, call it 2s, at
the turbine exit for an expansion in which there is no change in specific entropy from inlet to exit. With
1
s
2
s
2 s
1
2
5 0 and procedures similar to those used in part (a)
0 5 s
2s
*
2 s
1
*
2 R ca
s
*
2 s
R
b
2s
2 a
s
*
2 s
R
b
1
d
0 5 cs81T
2s
22 s81T
1
22 R ln a
p
2
p
1
bd2 R ca
s
*
2 s
R
b
2s
2 a
s
*
2 s
R
b
1
d
Using values from part (a), the last equation becomes
0 5 s81T
2s
22 191.682 2 8.314 ln
40
100
2 R
a
s
*
2 s
R
b
2
s
1 1.746
or
s81T
2s
22 R
a
s
*
2 s
R
b
2
s
5 182.3
The temperature T
2s
can be determined in an iterative procedure using s8 data from Table A-23 and
1
s
*
2 s
2
/
R
from Fig. A-5 as follows: First, a value for the temperature T
2s
is assumed. The corresponding value of s8 can
then be obtained from Table A-23. The reduced temperature (T
R
)
2s
5 T
2s
/T
c
, together with p
R2
5 1.18, allows a
value for
1
s
*
2 s
2
/
R to be obtained from Fig. A-5. The procedure continues until agreement with the value on
the right side of the above equation is obtained. Using this procedure, T
2s
is found to be closely 228 K.
With the temperature T
2s
known, the work that would be developed by the turbine if the nitrogen expanded
isentropically from the specified inlet state to the specified exit pressure can be evaluated from
a
W
#
cv
m
#
b
s
5 h
1
2 h
2s
5
1
M
e1h
1
*
2 h
2s
*
22 RT
c
ca
h
*
2 h
RT
c
b
1
2 a
h
*
2 h
RT
c
b
2
s
df
From Table A-23, h
2
s
*
5 6654 kJ
/
kmol. From Fig. A-4 at p
R2
5 1.18 and (T
R
)
2s
5
228/126 5 1.81
a
h
*
2 h
RT
c
b
2s
< 0.36
Values for the other terms in the expression for 1W
#
cv
/
m
#
2
s
are obtained in the
solution to Example 11.8. Finally
a
W
#
cv
m
#
b
s
5
1
28
38723 2 6654 2 18.31421126210.5 2 0.36245 68.66 kJ
/
kg
With the work value from Example 11.8, the turbine efficiency is
➊ ht 5
1W
#
cv
y
m
#
2
1W
#
cv
y
m
#
2
s
5
50.1
68.66
5 0.73173%2
➊ We cannot expect extreme accuracy when reading data from a generalized
chart such as Fig. A-5, which affects the final calculated result.
Ability to…
❑
use data from the general-
ized entropy departure
chart to calculate the
entropy production.
❑
use data from the general-
ized enthalpy and entropy
departure charts to calcu-
late isentropic turbine
efficiency.
❑
use an iterative procedure
to calculate the tempera-
ture at the end of an isen-
tropic process using data
from the generalized
entropy departure chart.
✓
Skills Developed
Determine the rate of entropy production, in kJ/K per kg of
nitrogen flowing, assuming the ideal gas model. Ans. 0.061 kJ/kg ? K.
c11ThermodynamicRelations.indd Page 674 6/21/10 9:36:10 PM user-s146 c11ThermodynamicRelations.indd Page 674 6/21/10 9:36:10 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
11.8 p–y–T Relations for Gas Mixtures
Many systems of interest involve mixtures of two or more components. The principles
of thermodynamics introduced thus far are applicable to systems involving mixtures,
but to apply such principles requires that mixture properties be evaluated.
Since an unlimited variety of mixtures can be formed from a given set of pure
components by varying the relative amounts present, the properties of mixtures are
available in tabular, graphical, or equation forms only in particular cases such as air.
Generally, special means are required for determining mixture properties.
In this section, methods for evaluating the p–y–T relations for pure components
introduced in previous sections of the book are adapted to obtain plausible estimates
for gas mixtures. In Sec. 11.9 some general aspects of property evaluation for multi-
component systems are introduced.
To evaluate the properties of a mixture requires knowledge of the composition.
The composition can be described by giving the number of moles (kmol or lbmol) of
each component present. The total number of moles, n, is the sum of the number of
moles of each of the components
n 5 n
1
1 n
2
1
. . .
1 n
j
5
a
j
i
51
n
i
(11.93)
The relative amounts of the components present can be described in terms of mole
fractions. The mole fraction y
i
of component i is defined as
y
i
5
n
i
n
(11.94)
Dividing each term of Eq. 11.93 by the total number of moles and using Eq. 11.94
1 5
a
j
i
51
y
i
(11.95)
That is, the sum of the mole fractions of all components present is equal to unity.
Most techniques for estimating mixture properties are empirical in character and
are not derived from fundamental principles. The realm of validity of any particular
technique can be established only by comparing predicted property values with
empirical data. The brief discussion to follow is intended only to show how certain
of the procedures for evaluating the p–y–T relations of pure components introduced
previously can be extended to gas mixtures.
MIXTURE EQUATION OF STATE. One way the p–y–T relation for a gas mix-
ture can be estimated is by applying to the overall mixture an equation of state such
as introduced in Sec. 11.1. The constants appearing in the equation selected would be
mixture values determined with empirical combining rules developed for the equa-
tion. For example, mixture values of the constants a and b for use in the van der
Waals and Redlich–Kwong equations would be obtained using relations of the form
a 5 a
a
j
i
51
y
i
a
i
1
/
2
b
2
,
b 5 a
a
j
i
51
y
i
b
i
b
(11.96)
where a
i
and b
i
are the values of the constants for component i and y
i
is the mole
fraction. Combination rules for obtaining mixture values for the constants in other
equations of state also have been suggested.
KAY’S RULE. The principle of corresponding states method for single components
introduced in Sec. 3.11.3 can be extended to mixtures by regarding the mixture as if
it were a single pure component having critical properties calculated by one of several
mixture rules. Perhaps the simplest of these, requiring only the determination of a
mole fraction averaged critical temperature T
c
and critical pressure p
c
, is Kay’s rule
TAKE NOTE...
The special case of ideal gas
mixtures is considered in
Secs. 12.1–12.4, with
applications to psychromet-
rics in the second part of
Chap. 12 and reacting mix-
tures in Chaps. 13 and 14.
Kay’s rule
11.8 p–y–T Relations for Gas Mixtures 675
c11ThermodynamicRelations.indd Page 675 6/21/10 9:36:14 PM user-s146 c11ThermodynamicRelations.indd Page 675 6/21/10 9:36:14 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
676 Chapter 11 Thermodynamic Relations
T
c
5
a
j
i
51
y
i
T
c,i
,
p
c
5
a
j
i
51
y
i
p
c,i
(11.97)
where T
c,i
, p
c,i
, and y
i
are the critical temperature, critical pressure, and mole fraction
of component i, respectively. Using T
c
and p
c
, the mixture compressibility factor Z is
obtained as for a single pure component. The unknown quantity from among the
pressure p, volume V, temperature T, and total number of moles n of the gas mixture
can then be obtained by solving
Z 5
p
V
n
RT
(11.98)
Mixture values for T
c
and p
c
also can be used to enter the generalized enthalpy
departure and entropy departure charts introduced in Sec. 11.7.
ADDITIVE PRESSURE RULE. Additional means for estimating p–y–T relations
for mixtures are provided by empirical mixture rules, of which several are found in
the engineering literature. Among these are the additive pressure and additive volume
rules. According to the additive pressure rule, the pressure of a gas mixture occupying
volume V at temperature T is expressible as a sum of pressures exerted by the indi-
vidual components:
p 5 p
1
1 p
2
1 p
3
1
. . .
4
T,V
(11.99a)
where the pressures p
1
, p
2
, etc. are evaluated by considering the respective compo-
nents to be at the volume and temperature of the mixture. These pressures would be
determined using tabular or graphical p–y–T data or a suitable equation of state.
An alternative expression of the additive pressure rule in terms of compressibility
factors can be obtained. Since component i is considered to be at the volume and
temperature of the mixture, the compressibility factor Z
i
for this component is
Z
i
5
p
i
V
/
n
i
RT, so the pressure p
i
is
p
i
5
Z
i
n
i
R
T
V
Similarly, for the mixture
p 5
ZnR
T
V
Substituting these expressions into Eq. 11.99a and reducing gives the following rela-
tionship between the compressibility factors for the mixture Z and the mixture com-
ponents Z
i
Z 5
a
j
i
51
y
i
Z
i
4
T,V
(11.99b)
The compressibility factors Z
i
are determined assuming that component i occupies
the entire volume of the mixture at the temperature T.
ADDITIVE VOLUME RULE. The underlying assumption of the additive volume
rule
is that the volume V of a gas mixture at temperature T and pressure p is express-
ible as the sum of volumes occupied by the individual components:
V 5 V
1
1 V
2
1 V
3
1
. . .
4
p
,
T
(11.100a)
where the volumes V
1
, V
2
, etc. are evaluated by considering the respective compo-
nents to be at the pressure and temperature of the mixture. These volumes would be
determined from tabular or graphical p–y–T data or a suitable equation of state.
additive pressure rule
additive volume rule
c11ThermodynamicRelations.indd Page 676 6/21/10 9:36:15 PM user-s146 c11ThermodynamicRelations.indd Page 676 6/21/10 9:36:15 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
An alternative expression of the additive volume rule in terms of compressibility
factors can be obtained. Since component i is considered to be at the pressure and
temperature of the mixture, the compressibility factor Z
i
for this component is
Z
i
5
p
V
i
/
n
i
RT, so the volume V
i
is
V
i
5
Z
i
n
i
R
T
p
Similarly, for the mixture
V 5
ZnR
T
p
Substituting these expressions into Eq. 11.100a and reducing gives
Z 5
a
j
i
51
y
i
Z
i
4
p,
T
(11.100b)
The compressibility factors Z
i
are determined assuming that component i exists at
the temperature T and pressure p of the mixture.
The next example illustrates alternative means for estimating the pressure of a gas
mixture.
Estimating Mixture Pressure by Alternative Means
c c c c EXAMPLE 11.10 c
A mixture consisting of 0.18 kmol of methane (CH
4
) and 0.274 kmol of butane (C
4
H
10
) occupies a volume of
0.241 m
3
at a temperature of 2388C. The experimental value for the pressure is 68.9 bar. Calculate the pressure,
in bar, exerted by the mixture by using (a) the ideal gas equation of state, (b) Kay’s rule together with the gen-
eralized compressibility chart, (c) the van der Waals equation, and (d) the rule of additive pressures employing
the generalized compressibility chart. Compare the calculated values with the known experimental value.
SOLUTION
Known:
A mixture of two specified hydrocarbons with known molar amounts occupies a known volume at a
specified temperature.
Find: Determine the pressure, in bar, using four alternative methods, and compare the results with the experi-
mental value.
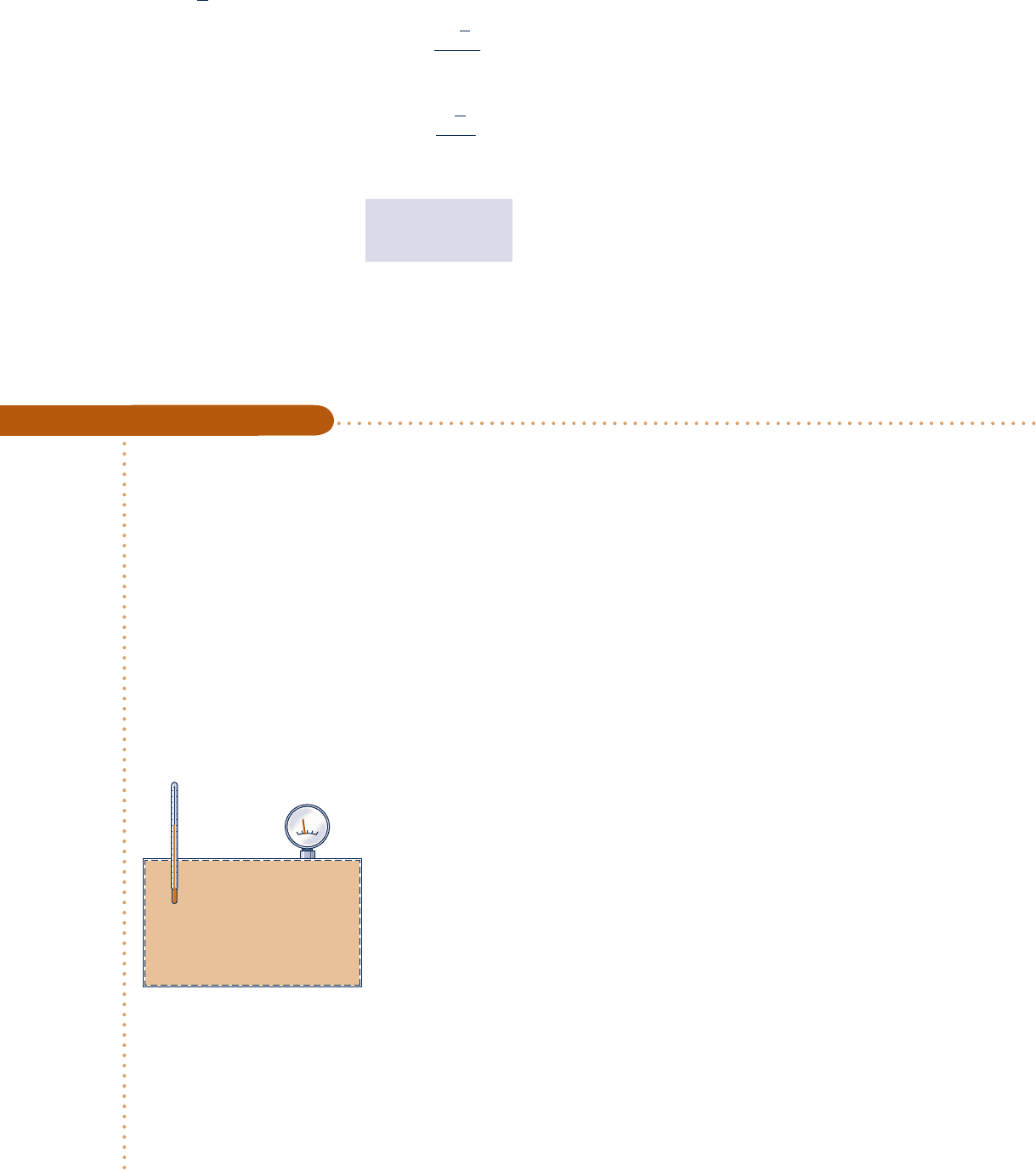
Schematic and Given Data:
Engineering Model:
As shown in the accompanying figure, the system is the
mixture.
Analysis: The total number of moles of mixture n is
n
5
0.
1
8
1
0.
2
7
4 5
0.
4
5
4 km
o
l
p = ?
T = 238°C
0.18 kmol CH
4
0.274 kmol C
4
H
10
V = 0.241 m
3
Fig. E11.10
11.8 p–y–T Relations for Gas Mixtures 677
c11ThermodynamicRelations.indd Page 677 6/21/10 9:36:16 PM user-s146 c11ThermodynamicRelations.indd Page 677 6/21/10 9:36:16 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
678 Chapter 11
Thermodynamic Relations
Thus, the mole fractions of the methane and butane are, respectively
y
1
5 0.396
and
y
2
5 0.604
The specific volume of the mixture on a molar basis is
y 5
0.241 m
3
1
0.18 1 0.274
2
kmol
5 0.531
m
3
kmol
(a) Substituting values into the ideal gas equation of state
p
5
RT
y
5
1
8314 N ? m
/
kmol ? K
21
511 K
2
1
0.531 m
3
/
kmol
2
`
1 bar
10
5
N
/
m
2
`
5
80.0
1
ba
r
(b) To apply Kay’s rule, the critical temperature and pressure for each component are required. From Table A-1,
for methane
T
c1
5 191 K,
p
c1
5 46.4 bar
and for butane
T
c2
5 425 K,
p
c2
5 38.0 bar
Thus, with Eqs. 11.97
T
c
5 y
1
T
c1
1 y
2
T
c2
5
1
0.396
21
191
2
1
1
0.604
21
425
2
5 332.3 K
p
c
5 y
1
p
c1
1 y
2
p
c2
5
1
0.396
21
46.4
2
1
1
0.604
21
38.0
2
5 41.33 bar
Treating the mixture as a pure component having the above values for the critical temperature and pressure,
the following reduced properties are determined for the mixture:
T
R
5
T
T
c
5
511
332.3
5 1.54
y¿
R
5
yp
c
RT
c
5
10.5312141.332
Z
10
5
Z
1
8314
21
332.3
2
5
0.794
Turning to Fig. A-2, Z <
0.88
. The mixture pressure is then found from
p
5
ZnRT
V
5 Z
RT
y
5 0.88
1
8314
21
511
2
10.5312
Z
10
5
Z
5
70.
4
ba
r
(c) Mixture values for the van der Waals constants can be obtained using Eqs. 11.96. This requires values of the van
der Waals constants for each of the two mixture components. Table A-24 gives the following values for methane:
a
1
5 2.293 bar
a
m
3
kmol
b
2
,
b
1
5 0.0428
m
3
kmol
Similarly, from Table A-24 for butane
a
2
5 13.86 bar
a
m
3
kmol
b
2
,
b
2
5 0.1162
m
3
kmol
Then, the first of Eqs. 11.96 gives a mixture value for the constant a as
a 5
1
y
1
a
1
1
/
2
1 y
2
a
1
/
2
2
2
2
5
3
0.396
1
2.293
2
1
/
2
1 0.604
1
13.86
2
1
/
2
4
2
5 8.113 bar
a
m
3
kmol
b
2
Substituting into the second of Eqs. 11.96 gives a mixture value for the constant b
b 5
y
1
b
1
1
y
2
b
2
5
1
0.396
21
0.0428
2
1
1
0.604
21
0.1162
2
5 0.087
m
3
km
o
l
c11ThermodynamicRelations.indd Page 678 6/21/10 9:36:18 PM user-s146 c11ThermodynamicRelations.indd Page 678 6/21/10 9:36:18 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
11.9 Analyzing Multicomponent Systems
In the preceding section we considered means for evaluating the p–y–T relation of gas
mixtures by extending methods developed for pure components. The current section is
devoted to the development of some general aspects of the properties of systems with
two or more components. Primary emphasis is on the case of gas mixtures, but the
methods developed also apply to solutions. When liquids and solids are under consid-
eration, the term solution is sometimes used in place of mixture. The present discussion
is limited to nonreacting mixtures or solutions in a single phase. The effects of chemical
reactions and equilibrium between different phases are taken up in Chaps. 13 and 14.
To describe multicomponent systems, composition must be included in our thermo-
dynamic relations. This leads to the definition and development of several new con-
cepts, including the partial molal property, the chemical potential, and the fugacity.
Inserting the mixture values for a and b into the van der Waals equation together with known data
p
5
RT
y 2 b
2
a
y
2
5
18314 N ? m
/
kmol ? K21511 K2
10.531 2 0.08721m
3
/
kmol2
`
1 bar
10
5
N
/
m
2
`2
8.113 bar 1m
3
/
kmol2
2
10.531 m
3
/
kmol2
2
5 66.91 bar
(d) To apply the additive pressure rule with the generalized compressibility chart requires that the compress-
ibility factor for each component be determined assuming that the component occupies the entire volume at the
mixture temperature. With this assumption, the following reduced properties are obtained for methane
T
R1
5
T
T
c1
5
511
191
5 2.69
y¿
R1
5
y
1
p
c1
RT
c1
5
10.241 m
3
/
0.18 kmol2146.4 bar2
18314 N ? m
/
kmol ? K21191 K2
`
10
5
N
/
m
2
1 bar
`5 3.91
With these reduced properties, Fig. A-2 gives Z
1
<
1
.
0
.
Similarly, for butane
T
R2
5
T
T
c2
5
511
425
5 1.2
y¿
R2
5
y
2
p
c2
RT
c2
5
10.8821382
Z
10
5
Z
18314214252
5 0.95
From Fig. A-2, Z
2
<
0
.
8
.
The compressibility factor for the mixture determined from Eq. 11.99b is
Z 5 y
1
Z
1
1 y
2
Z
2
5 10.396211.021 10.604210.825 0.88.
Accordingly, the same value for pressure as determined in part (b) using Kay’s
rule results: p 5 70.4 bar.
In this particular example, the ideal gas equation of state gives a value for
pressure that exceeds the experimental value by nearly 16%. Kay’s rule and the
rule of additive pressures give pressure values about 3% greater than the exper-
imental value. The van der Waals equation with mixture values for the constants
gives a pressure value about 3% less than the experimental value.
Ability to…
❑
calculate the pressure of
a gas mixture using four
alternative methods.
✓
Skills Developed
Convert the mixture analysis from a molar basis to a mass
fraction basis. Ans. Methane: 0.153, Butane: 0.847.
solution
TAKE NOTE...
Section 11.9 may be deferred
until Secs. 12.1–12.4 have
been studied.
11.9 Analyzing Multicomponent Systems 679
c11ThermodynamicRelations.indd Page 679 6/21/10 9:36:22 PM user-s146 c11ThermodynamicRelations.indd Page 679 6/21/10 9:36:22 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
680 Chapter 11
Thermodynamic Relations
11.9.1
Partial Molal Properties
In the present discussion we introduce the concept of a partial molal property and
illustrate its use. This concept plays an important role in subsequent discussions of
multicomponent systems.
DEFINING PARTIAL MOLAL PROPERTIES. Any extensive thermodynamic
property X of a single-phase, single-component system is a function of two indepen-
dent intensive properties and the size of the system. Selecting temperature and pres-
sure as the independent properties and the number of moles n as the measure of size,
we have X 5 X(T, p, n). For a single-phase, multicomponent system, the extensive
property X must then be a function of temperature, pressure, and the number of
moles of each component present, X 5 X(T, p, n
1
, n
2
, . . . , n
j
).
If each mole number is increased by a factor a, the size of the system increases
by the same factor, and so does the value of the extensive property X. That is
aX1T, p, n
1
, n
2
, . . . , n
j
25 X1T, p, an
1
, an
2
, . . . , an
j
2
Differentiating with respect to a while holding temperature, pressure, and the mole
numbers fixed and using the chain rule on the right side gives
X 5
0X
01an
1
2
n
1
1
0X
01an
2
2
n
2
1
. . .
1
0X
01an
j
2
n
j
This equation holds for all values of a. In particular, it holds for a 5 1. Setting a 5 1
X 5
a
j
i51
n
i
0X
0n
i
b
T, p, n
l
(11.101)
where the subscript n
l
denotes that all n’s except n
i
are held fixed during differentia-
tion.
The partial molal property X
i
is by definition
X
i
5
0X
0n
i
b
T, p, n
l
(11.102)
The partial molal property X
i
is a property of the mixture and not simply a property
of component i, for X
i
depends in general on temperature, pressure, and mixture
composition: X
i
1T, p, n
1
, n
2
, . . . , n
j
2. Partial molal properties are intensive properties
of the mixture.
Introducing Eq. 11.102, Eq. 11.101 becomes
X 5
a
j
i51
n
i
X
i
(11.103)
This equation shows that the extensive property X can be expressed as a weighted
sum of the partial molal properties X
i
.
Selecting the extensive property X in Eq. 11.103 to be volume, internal energy,
enthalpy, and entropy, respectively, gives
V 5
a
j
i51
n
i
V
i
,
U 5
a
j
i51
n
i
U
i
,
H 5
a
j
i51
n
i
H
i
,
S 5
a
j
i51
n
i
S
i
(11.104)
where
V
i
,
U
i
,
H
i
,
S
i
denote the partial molal volume, internal energy, enthalpy, and
entropy. Similar expressions can be written for the Gibbs function G and the Helmholtz
function C. Moreover, the relations between these extensive properties: H 5 U 1 pV,
G 5 H 2 TS, C = U 2 TS can be differentiated with respect to n
i
while holding tem-
perature, pressure, and the remaining n’s constant to produce corresponding relations
partial molal property
c11ThermodynamicRelations.indd Page 680 6/21/10 9:36:25 PM user-s146 c11ThermodynamicRelations.indd Page 680 6/21/10 9:36:25 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
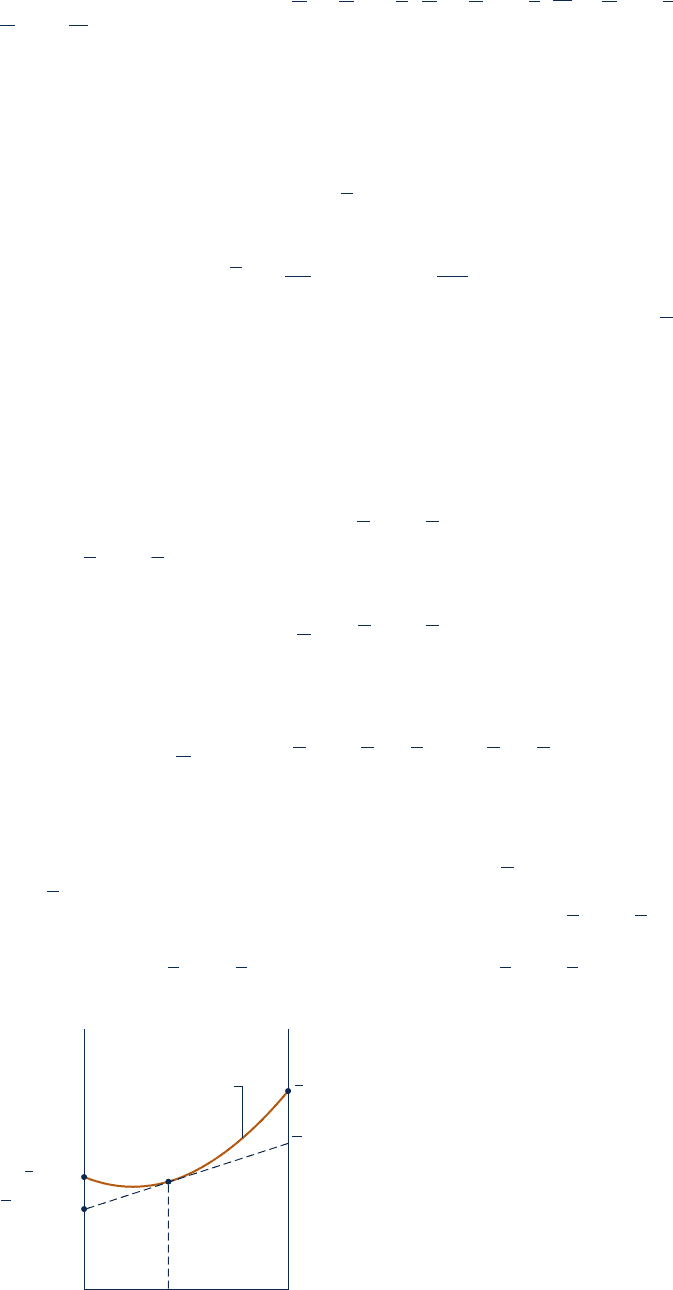
among partial molal properties: H
i
5 U
i
1 pV
i
, G
i
5 H
i
2 T S
i
, °
i
5 U
i
2 T S
i
, where
G
i
and
°
i
are the partial molal Gibbs function and Helmholtz function, respectively.
Several additional relations involving partial molal properties are developed later in
this section.
EVALUATING PARTIAL MOLAL PROPERTIES. Partial molal properties can
be evaluated by several methods, including the following:
c If the property X can be measured, X
i
can be found by extrapolating a plot giving
1
¢X
/
¢n
i
2
T,
p
, n
l
versus Dn
i
. That is
X
i
5 a
0X
0n
i
b
T, p, n
l
5 lim
¢n
i
S0
a
¢X
¢n
i
b
T, p, n
l
c If an expression for X in terms of its independent variables is known, X
i
can be
evaluated by differentiation. The derivative can be determined analytically if the func-
tion is expressed analytically or found numerically if the function is in tabular form.
c When suitable data are available, a simple graphical procedure known as the
method of intercepts can be used to evaluate partial molal properties. In principle,
the method can be applied for any extensive property. To introduce this method,
let us consider the volume of a system consisting of two components, A and B. For
this system, Eq. 11.103 takes the form
V 5 n
A
V
A
1 n
B
V
B
where V
A
and V
B
are the partial molal volumes of A and B, respectively. Dividing
by the number of moles of mixture n
V
n
5 y
A
V
A
1 y
B
V
B
where y
A
and y
B
denote the mole fractions of A and B, respectively. Since y
A
1 y
B
5
1,
this becomes
V
n
5 11 2 y
B
2V
A
1 y
B
V
B
5 V
A
1 y
B
1V
B
2 V
A
2
This equation provides the basis for the method of intercepts. For example, refer to
Fig. 11.5, in which V/n is plotted as a function of y
B
at constant T and p. At a
specified value for y
B
, a tangent to the curve is shown on the figure. When extrapo-
lated, the tangent line intersects the axis on the left at V
A
and the axis on the right
at V
B
. These values for the partial molal volumes correspond to the particular spec-
ifications for T, p, and y
B
. At fixed temperature and pressure, V
A
and V
B
vary with
y
B
and are not equal to the molar specific volumes of pure A and pure B, denoted
on the figure as y
A
and y
B
, respectively. The values of y
A
and y
B
are fixed by tem-
perature and pressure only.
method of intercepts
V
––
n
V
––
n
T and p constant
as a function of y
B
v
A
(T, p)
V
A
(T, p, y
B
)
0
(pure A)
y
B
1.0
(pure B)
Mole fraction of B
Tangent
line
v
B
(T, p)
V
B
(T, p, y
B
)
Fig. 11.5 Illustration of the evaluation of
partial molal volumes by the method of
intercepts.
11.9 Analyzing Multicomponent Systems 681
c11ThermodynamicRelations.indd Page 681 6/21/10 9:36:27 PM user-s146 c11ThermodynamicRelations.indd Page 681 6/21/10 9:36:27 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
682 Chapter 11
Thermodynamic Relations
EXTENSIVE PROPERTY CHANGES ON MIXING. Let us conclude the pres-
ent discussion by evaluating the change in volume on mixing of pure components at
the same temperature and pressure, a result for which an application is given in the
discussion of Eq. 11.135. The total volume of the pure components before mixing is
V
components
5
a
j
i51
n
i
y
i
where y
i
is the molar specific volume of pure component i. The volume of the mixture is
V
mixture
5
a
j
i51
n
i
V
i
where V
i
is the partial molal volume of component i in the mixture. The volume
change on mixing is
¢V
mixing
5 V
mixture
2 V
components
5
a
j
i51
n
i
V
i
2
a
j
i51
n
i
y
i
or
¢V
mixing
5
a
j
i51
n
i
1V
i
2 y
i
2
(11.105)
Similar results can be obtained for other extensive properties, for example,
¢U
mixing
5
a
j
i51
n
i
1U
i
2 u
i
2
¢H
mixing
5
a
j
i51
n
i
1H
i
2 h
i
2
(11.106)
¢S
mixing
5
a
j
i51
n
i
1S
i
2 s
i
2
In Eqs. 11.106, u
i
,
h
i
, and s
i
denote the molar internal energy, enthalpy, and entropy
of pure component i, respectively. The symbols U
i
, H
i
, and S
i
denote the respective
partial molal properties.
11.9.2
Chemical Potential
Of the partial molal properties, the partial molal Gibbs function is particularly useful
in describing the behavior of mixtures and solutions. This quantity plays a central role
in the criteria for both chemical and phase equilibrium (Chap. 14). Because of its
importance in the study of multicomponent systems, the partial molal Gibbs function
of component i is given a special name and symbol. It is called the chemical potential
of component i and symbolized by m
i
m
i
5 G
i
5
0G
0n
i
b
T, p, n
l
(11.107)
Like temperature and pressure, the chemical potential m
i
is an intensive property.
Applying Eq. 11.103 together with Eq. 11.107, the following expression can be
written:
G 5
a
j
i51
n
i
m
i
(11.108)
chemical potential
c11ThermodynamicRelations.indd Page 682 6/21/10 9:36:29 PM user-s146 c11ThermodynamicRelations.indd Page 682 6/21/10 9:36:29 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
