Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.

c11ThermodynamicRelations.indd703 Page 703 6/21/10 9:37:38 PM user-s146 c11ThermodynamicRelations.indd703 Page 703 6/21/10 9:37:38 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

704
Library stacks require careful temperature and humidity control using air-conditioning
processes considered in Sec. 12.8.
© GreHinsdale Corbis RF/Age Fotostock America, Inc.
ENGINEERING CONTEXT Many systems of interest involve gas mixtures of two or more compo-
nents. To apply the principles of thermodynamics introduced thus far to these systems requires that we eval-
uate properties of the mixtures. Means are available for determining the properties of mixtures from the
mixture composition and the properties of the individual pure components from which the mixtures are
formed. Methods for this purpose are discussed both in Chap. 11 and in the present chapter.
The objective of the present chapter is to study mixtures where the overall mixture and each of its compo-
nents can be modeled as ideal gases. General ideal gas mixture considerations are provided in the first part
of the chapter. Understanding the behavior of ideal gas mixtures of dry air and water vapor is prerequisite
to considering air-conditioning processes in the second part of the chapter, which is identified by the head-
ing, Psychrometric Applications. In those processes, we sometimes must consider the presence of liquid
water as well. We will also need to know how to handle ideal gas mixtures when we study the subjects of
combustion and chemical equilibrium in Chaps. 13 and 14, respectively.
c12IdealGasMixtureandPsychrometr704 Page 704 6/29/10 11:53:45 AM user-s146c12IdealGasMixtureandPsychrometr704 Page 704 6/29/10 11:53:45 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

Ideal Gas Mixture
and Psychrometric
Applications
12
When you complete your study of this chapter, you will be able to...
c
describe ideal gas mixture composition in terms of mass fractions or mole fractions.
c
use the Dalton model to relate pressure, volume, and temperature and to calculate
changes in U, H, and S for ideal gas mixtures.
c
apply mass, energy, and entropy balances to systems involving ideal gas mixtures,
including mixing processes.
c
demonstrate understanding of psychrometric terminology, including humidity ratio,
relative humidity, mixture enthalpy, and dew point temperature.
c
use the psychrometric chart to represent common air-conditioning processes and to
retrieve data.
c
apply mass, energy, and entropy balances to analyze air-conditioning processes and
cooling towers.
LEARNING OUTCOMES
705
c12IdealGasMixtureandPsychrometr705 Page 705 6/29/10 11:53:54 AM user-s146c12IdealGasMixtureandPsychrometr705 Page 705 6/29/10 11:53:54 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

706 Chapter 12
Ideal Gas Mixture and Psychrometric Applications
Ideal Gas Mixtures: General
Considerations
12.1 Describing Mixture Composition
To specify the state of a mixture requires the composition and the values of two
independent intensive properties such as temperature and pressure. The object of the
present section is to consider ways for describing mixture composition. In subsequent
sections, we show how mixture properties other than composition can be evaluated.
Consider a closed system consisting of a gaseous mixture of two or more compo-
nents. The composition of the mixture can be described by giving the mass or the
number of moles of each component present. With Eq. 1.8, the mass, the number of
moles, and the molecular weight of a component i are related by
n
i
5
m
i
M
i
(12.1)
where m
i
is the mass, n
i
is the number of moles, and M
i
is the molecular weight of
component i, respectively. When m
i
is expressed in terms of the kilogram, n
i
is in
kmol. When m
i
is in terms of the pound mass, n
i
is in lbmol. However, any unit of
mass can be used in this relationship.
The total mass of the mixture, m, is the sum of the masses of its components
m 5 m
1
1 m
2
1
. . .
1 m
j
5
a
j
i51
m
i
(12.2)
The relative amounts of the components present in the mixture can be specified
in terms of mass fractions. The mass fraction mf
i
of component i is defined as
mf
i
5
m
i
m
(12.3)
A listing of the mass fractions of the components of a mixture is sometimes referred
to as a
gravimetric analysis.
Dividing each term of Eq. 12.2 by the total mass of mixture m and using Eq. 12.3
1 5
a
j
i51
mf
i
(12.4)
That is, the sum of the mass fractions of all the components in a mixture is equal to
unity.
The total number of moles in a mixture, n, is the sum of the number of moles of
each of its components
n 5 n
1
1 n
2
1
. . .
1 n
j
5
a
j
i51
n
i
(12.5)
The relative amounts of the components present in the mixture also can be described
in terms of mole fractions. The mole fraction y
i
of component i is de fined as
y
i
5
n
i
n
(12.6)
mass fractions
gravimetric analysis
mole fractions
TAKE NOTE...
c
In Secs. 12.1–12.3, we
introduce mixture concepts
required for study of psy-
chrometrics in the second
part of this chapter and
combustion in Chap. 13.
c
In Sec. 12.4, we extend
the discussion of mixtures
and provide several solved
examples illustrating
important types of mixture
applications. For economy
of effort, some readers may
elect to defer Sec. 12.4
and proceed directly to
content having more
immediate interest for
them: psychrometrics
beginning in Sec. 12.5 or
combustion beginning in
Sec. 13.1.
c12IdealGasMixtureandPsychrometr706 Page 706 6/29/10 11:53:55 AM user-s146c12IdealGasMixtureandPsychrometr706 Page 706 6/29/10 11:53:55 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
A listing of the mole fractions of the components of a mixture may be called a molar
analysis
. An analysis of a mixture in terms of mole fractions is also called a
volumetric analysis.
Dividing each term of Eq. 12.5 by the total number of moles of mixture n and
using Eq. 12.6
1 5
a
j
i51
y
i
(12.7)
That is, the sum of the mole fractions of all the components in a mixture is equal to unity.
The apparent (or average) molecular weight of the mixture, M, is defined as the ratio
of the total mass of the mixture, m, to the total number of moles of mixture, n
M 5
m
n
(12.8)
Equation 12.8 can be expressed in a convenient alternative form. With Eq. 12.2, it
becomes
M 5
m
1
1 m
2
1
. . .
1 m
j
n
Introducing m
i
5 n
i
M
i
from Eq. 12.1
M 5
n
1
M
1
1 n
2
M
2
1
. . .
1 n
j
M
j
n
Finally, with Eq. 12.6, the apparent molecular weight of the mixture can be calculated
as a mole-fraction average of the component molecular weights
M 5
a
j
i51
y
i
M
i
(12.9)
consider the case of air. A sample of atmospheric air contains sev-
eral gaseous components including water vapor and contaminants such as dust, pollen,
and pollutants. The term dry air refers only to the gaseous components when all water
vapor and contaminants have been removed. The molar analysis of a typical sample of
dry air is given in Table 12.1. Selecting molecular weights for nitrogen, oxygen, argon,
and carbon dioxide from Table A-1, and neglecting the trace substances neon, helium,
etc., the apparent molecular weight of dry air obtained from Eq. 12.9 is
M < 0.7808128.0221 0.2095132.0021 0.0093139.9421 0.0003144.012
5 28.97 kg
/
kmol 5 28.97 lb
/
lbmol
This value, which is the entry for air in Tables A-1, would not be altered significantly
if the trace substances were also included in the calculation. b b b b b
molar analysis
volumetric analysis
apparent molecular weight
dry air
Approximate Composition of Dry Air
Mole Fraction
Component (%)
Nitrogen 78.08
Oxygen 20.95
Argon 0.93
Carbon dioxide 0.03
Neon, helium, methane, and others 0.01
TABLE 12.1
12.1 Describing Mixture Composition 707
c12IdealGasMixtureandPsychrometr707 Page 707 6/29/10 11:53:56 AM user-s146c12IdealGasMixtureandPsychrometr707 Page 707 6/29/10 11:53:56 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
708 Chapter 12 Ideal Gas Mixture and Psychrometric Applications
Next, we consider two examples illustrating, respectively, the conversion from an analy-
sis in terms of mole fractions to an analysis in terms of mass fractions, and conversely.
Converting Mole Fractions to Mass Fractions
c c c c EXAMPLE 12.1 c
Determine the mass, in kg, of CO
2
in 0.5 kmol of mixture.
Ans. 1.76 kg.
The molar analysis of the gaseous products of combustion of a certain hydrocarbon fuel is CO
2
, 0.08; H
2
O, 0.11;
O
2
, 0.07; N
2
, 0.74. (a) Determine the apparent molecular weight of the mixture. (b) Determine the composition
in terms of mass fractions (gravimetric analysis).
SOLUTION
Known:
The molar analysis of the gaseous products of combustion of a hydrocarbon fuel is given.
Find: Determine (a) the apparent molecular weight of the mixture, (b) the composition in terms of mass fractions.
Analysis:
(a) Using Eq. 12.9 and molecular weights (rounded) from Table A-1
M 5 0.0814421 0.1111821 0.0713221 0.741282
5 28.46 kg
/
kmol 5 28.46 lb
/
lbmol
(b) Equations 12.1, 12.3, and 12.6 are the key relations required to determine the composition in terms of mass
fractions.
➊ Although the actual amount of mixture is not known, the calculations can be based on any convenient
amount. Let us base the solution on 1 kmol of mixture. Then, with Eq. 12.6 the amount n
i
of each component
present in kmol is numerically equal to the mole fraction, as listed in column (ii) of the accompanying table.
Column (iii) of the table gives the respective molecular weights of the components.
Column (iv) of the table gives the mass m
i
of each component, in kg per kmol of mixture, obtained with
Eq. 12.1 in the form m
i
5 M
i
n
i
. The values of column (iv) are obtained by multiplying each value of column
(ii) by the corresponding value of column (iii). The sum of the values in column (iv) is the mass of the mixture:
kg of mixture per kmol of mixture. Note that this sum is just the apparent mixture molecular weight determined
in part (a). Finally, using Eq. 12.3, column (v) gives the mass fractions as a percentage. The values of column
(v) are obtained by dividing the values of column (iv) by the column (iv) total and multiplying by 100.
➊ If the solution to part (b) were conducted on the basis of some other assumed amount of mixture—for exam-
ple, 100 kmol or 100 lbmol—the same result for the mass fractions would be obtained, as can be verified.
Ability to…
❑
calculate the apparent
molecular weight with known
mole fractions.
❑
determine the gravimetric
analysis given the molar analysis.
✓
Skills Developed
(i) (ii)* (iii) (iv)** (v)
Component n
i
3 M
i
5 m
i
mf
i
%
CO
2
0.08 3 44 5 3.52 12.37
H
2
O 0.11 3 18 5 1.98 6.96
O
2
0.07 3 32 5 2.24 7.87
N
2
0.74 3 28 5 20.72 72.80
1.00 28.46 100.00
*Entries in this column have units of kmol per kmol of mixture. For example, the first entry
is 0.08 kmol of CO
2
per kmol of mixture.
**Entries in this column have units of kg per kmol of mixture. For example, the first entry
is 3.52 kg of CO
2
per kmol of mixture. The column sum, 28.46, has units of kg of mixture
per kmol of mixture.
c12IdealGasMixtureandPsychrometr708 Page 708 7/29/10 4:51:48 PM user-s146c12IdealGasMixtureandPsychrometr708 Page 708 7/29/10 4:51:48 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
Converting Mass Fractions to Mole Fractions
c c c c EXAMPLE 12.2 c
A gas mixture has the following composition in terms of mass fractions: H
2
, 0.10; N
2
, 0.60; CO
2
, 0.30. Determine
(a) the composition in terms of mole fractions and (b) the apparent molecular weight of the mixture.
SOLUTION
Known:
The gravimetric analysis of a gas mixture is known.
Find: Determine the analysis of the mixture in terms of mole fractions (molar analysis) and the apparent molec-
ular weight of the mixture.
Analysis:
(a) Equations 12.1, 12.3, and 12.6 are the key relations required to determine the composition in terms of mole
fractions.
➊
Although the actual amount of mixture is not known, the calculation can be based on any convenient
amount. Let us base the solution on 100 kg. Then, with Eq. 12.3, the amount m
i
of each component present,
in kg, is equal to the mass fraction multiplied by 100 kg. The values are listed in column (ii) of the accom-
panying table. Column (iii) of the table gives the respective molecular weights of the components.
Column (iv) of the table gives the amount n
i
of each component, in kmol per 100 kg of mixture, obtained
using Eq. 12.1. The values of column (iv) are obtained by dividing each value of column (ii) by the corre-
sponding value of column (iii). The sum of the values of column (iv) is the total amount of mixture, in kmol
per 100 kg of mixture. Finally, using Eq. 12.6, column (v) gives the mole fractions as a percentage. The values
of column (v) are obtained by dividing the values of column (iv) by the column (iv) total and multiplying
by 100.
(b) The apparent molecular weight of the mixture can be found by using Eq. 12.9 and the calculated mole frac-
tions. The value can be determined alternatively by using the column (iv) total giving the total amount of mixture
in kmol per 100 kg of mixture. Thus, with Eq. 12.8
M 5
m
n
5
100 k
g
7.82 kmol
5 12.79
kg
kmol
5 12.79
l
b
lbmol
➊ If the solution to part (a) were conducted on the basis of some other assumed
amount of mixture, the same result for the mass fractions would be obtained,
as can be verified.
➋ Although H
2
has the smallest mass fraction, its mole fraction is the largest.
Ability to…
❑
determine the molar analysis
given the gravimetric analysis.
✓
Skills Developed
How many kmol of H
2
would be present in 200 kg of mixture?
Ans. 10 kmol.
(i) (ii)* (iii) (iv)** (v)
Component m
i
4 M
i
5 n
i
y
i
%
H
2
10 4 2 5 5.00 63.9
N
2
60 4 28 5 2.14 27.4
CO
2
30 4 44 5 0.68 8.7
100 7.82 100.0
*Entries in this column have units of kg per 100 kg of mixture. For example, the first entry
is 10 kg of H
2
per 100 kg of mixture.
**Entries in this column have units of kmol per 100 kg of mixture. For example, the first
entry is 5.00 kmol of H
2
per 100 kg of mixture. The column sum, 7.82, has units of kmol
of mixture per 100 kg of mixture.
➋
12.1 Describing Mixture Composition 709
c12IdealGasMixtureandPsychrometr709 Page 709 6/29/10 11:53:59 AM user-s146c12IdealGasMixtureandPsychrometr709 Page 709 6/29/10 11:53:59 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
710 Chapter 12
Ideal Gas Mixture and Psychrometric Applications
12.2 Relating p, V, and T for Ideal
Gas Mixtures
1
The definitions given in Sec. 12.1 apply generally to mixtures. In the present sec-
tion we are concerned only with ideal gas mixtures and introduce a model com-
monly used in conjunction with this idealization: the Dalton model.
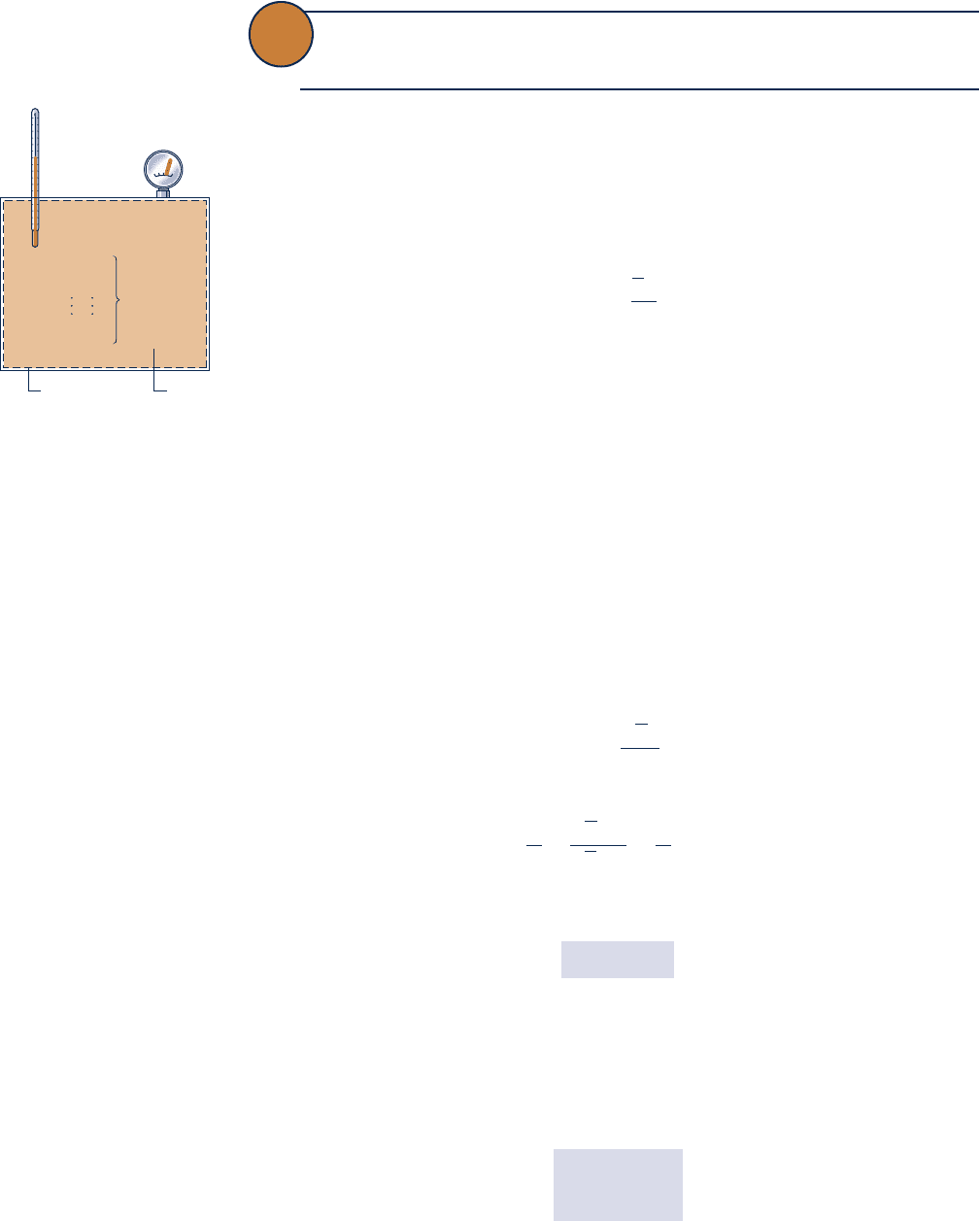
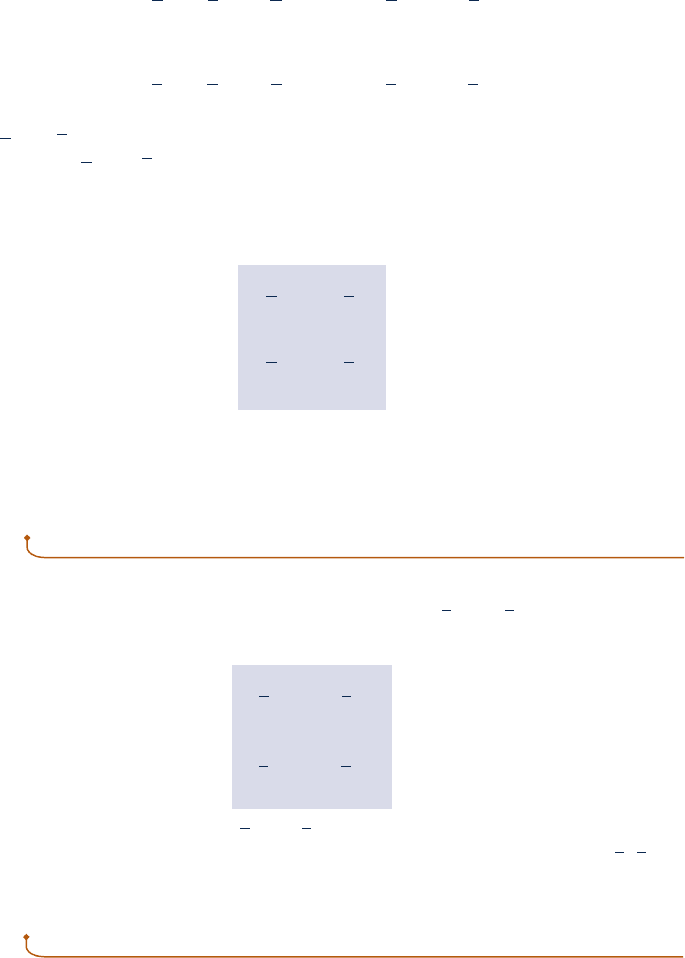
Consider a system consisting of a number of gases contained within a closed ves-
sel of volume V as shown in Fig. 12.1. The temperature of the gas mixture is T and
the pressure is p. The overall mixture is considered an ideal gas, so p, V, T, and the
total number of moles of mixture n are related by the ideal gas equation of state
p 5 n
R
T
V
(12.10)
With reference to this system let us consider the Dalton model.
The Dalton model is consistent with the concept of an ideal gas as being made
up of molecules that exert negligible forces on one another and whose volume is
negligible relative to the volume occupied by the gas (Sec. 3.12.3). In the absence
of significant intermolecular forces, the behavior of each component is unaffected
by the presence of the other components. Moreover, if the volume occupied by
the molecules is a very small fraction of the total volume, the molecules of each gas
present may be regarded as free to roam throughout the full volume. In keeping with
this simple picture, the Dalton model assumes that each mixture component behaves
as an ideal gas as if it were alone at the temperature T and volume V of the mixture.
It follows from the Dalton model that the individual components would not exert
the mixture pressure p but rather a partial pressure. As shown below, the sum of the
partial pressures equals the mixture pressure. The partial pressure of component i, p
i
,
is the pressure that n
i
moles of component i would exert if the component were alone
in the volume V at the mixture temperature T. The partial pressure can be evaluated
using the ideal gas equation of state
p
i
5
n
i
R
T
V
(12.11)
Dividing Eq. 12.11 by Eq. 12.10
p
i
p
5
n
i
RT
/
V
n
RT
/
V
5
n
i
n
5 y
i
Thus, the partial pressure of component i can be evaluated in terms of its mole frac-
tion y
i
and the mixture pressure p
p
i
5 y
i
p (12.12)
To show that the sum of partial pressures equals the mixture pressure, sum both
sides of Eq. 12.12 to obtain
a
j
i51
p
i
5
a
j
i51
y
i
p 5 p
a
j
i51
y
i
Since the sum of the mole fractions is unity (Eq. 12.7), this becomes
p 5
a
j
i51
p
i
(12.13)
The Dalton model is a special case of the additive pressure rule for relating the
pressure, specific volume, and temperature of gas mixtures introduced in Sec. 11.8.
1
The concept of an ideal gas mixture is a special case of the ideal solution concept introduced in Sec. 11.9.5.
Dalton model
Gas 1 : n
1
Gas 2 : n
2
n moles
mixture
Pressure = p
Temperature = T
Gas j : n
j
Volume = VBoundary
Fig. 12.1 Mixture of several
gases.
partial pressure
c12IdealGasMixtureandPsychrometr710 Page 710 6/29/10 11:53:59 AM user-s146c12IdealGasMixtureandPsychrometr710 Page 710 6/29/10 11:53:59 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

Among numerous other mixture rules found in the engineering literature is the
Amagat model considered in the box that follows.
Introducing the Amagat Model
The underlying assumption of the Amagat model is that each mixture component
behaves as an ideal gas as if it existed separately at the pressure p and temperature T
of the mixture.
The volume that n
i
moles of component i would occupy if the component existed at p
and T is called the partial volume, V
i
, of component i. As shown below, the sum of the
partial volumes equals the total volume. The partial volume can be evaluated using the
ideal gas equation of state
V
i
5
n
i
RT
p
(12.14)
Dividing Eq. 12.14 by the total volume V
V
i
V
5
n
i
RT
/
p
n
RT
/
p
5
n
i
n
5 y
i
Thus, the partial volume of component i also can be evaluated in terms of its mole
fraction y
i
and the total volume
V
i
5 y
i
V (12.15)
This relationship between volume fraction and mole fraction underlies the use of the term
volumetric analysis as signifying an analysis of a mixture in terms of mole fractions.
To show that the sum of partial volumes equals the total volume, sum both sides of
Eq. 12.15 to obtain
a
j
i51
V
i
5
a
j
i51
y
i
V 5 V
a
j
i51
y
i
Since the sum of the mole fractions equals unity, this becomes
V 5
a
j
i51
V
i
(12.16)
Finally, note that the Amagat model is a special case of the additive volume model
introduced in Sec. 11.8.
12.3 Evaluating U, H, S, and Specific Heats
To apply the conservation of energy principle to a system involving an ideal gas
mixture requires evaluation of the internal energy, enthalpy, or specific heats of the
mixture at various states. Similarly, to conduct an analysis using the second law normally
requires the entropy of the mixture. The objective of the present section is to develop
means to evaluate these properties for ideal gas mixtures.
12.3.1 Evaluating U and H
Consider a closed system consisting of an ideal gas mixture. Extensive properties of
the mixture, such as U, H, or S, can be found by adding the contribution of each
component at the condition at which the component exists in the mixture. Let us apply
this model to internal energy and enthalpy.
12.3 Evaluating U, H, S, and Specific Heats 711
c12IdealGasMixtureandPsychrometr711 Page 711 6/29/10 11:54:02 AM user-s146c12IdealGasMixtureandPsychrometr711 Page 711 6/29/10 11:54:02 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
712 Chapter 12
Ideal Gas Mixture and Psychrometric Applications
Since the internal energy and enthalpy of ideal gases are functions of temperature
only, the values of these properties for each component present in the mixture are
determined by the mixture temperature alone. Accordingly
U 5 U
1
1 U
2
1
. . .
1 U
j
5
a
j
i51
U
i
(12.17)
H 5 H
1
1 H
2
1
. . .
1 H
j
5
a
j
i51
H
i
(12.18)
where U
i
and H
i
are the internal energy and enthalpy, respectively, of component i
evaluated at the mixture temperature.
Equations 12.17 and 12.18 can be rewritten on a molar basis as
nu 5 n
1
u
1
1 n
2
u
2
1
. . .
1 n
j
u
j
5
a
j
i51
n
i
u
i
(12.19)
and
nh 5 n
1
h
1
1 n
2
h
2
1
. . .
1 n
j
h
j
5
a
j
i51
n
i
h
i
(12.20)
where u and h are the specific internal energy and enthalpy of the mixture per mole
of mixture, and u
i
and h
i
are the specific internal energy and enthalpy of component
i per mole of i. Dividing by the total number of moles of mixture, n, gives expressions
for the specific internal energy and enthalpy of the mixture per mole of mixture,
respectively
u 5
a
j
i51
y
i
u
i
(12.21)
h 5
a
j
i51
y
i
h
i
(12.22)
Each of the molar internal energy and enthalpy terms appearing in Eqs. 12.19 through
12.22 is evaluated at the mixture temperature only.
12.3.2 Evaluating c
y
and c
p
Differentiation of Eqs. 12.21 and 12.22 with respect to temperature results, respec-
tively, in the following expressions for the specific heats c
y
and c
p
of the mixture on
a molar basis
c
y
5
a
j
i51
y
i
c
y,i
(12.23)
c
p
5
a
j
i51
y
i
c
p,i
(12.24)
That is, the mixture specific heats c
p
and c
y
are mole-fraction averages of the respec-
tive component specific heats. The specific heat ratio for the mixture is k 5 c
p
/
c
y
.
12.3.3 Evaluating S
The entropy of a mixture can be found, as for U and H, by adding the contribution
of each component at the condition at which the component exists in the mixture.
c12IdealGasMixtureandPsychrometr712 Page 712 6/29/10 11:54:04 AM user-s146c12IdealGasMixtureandPsychrometr712 Page 712 6/29/10 11:54:04 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
