Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.

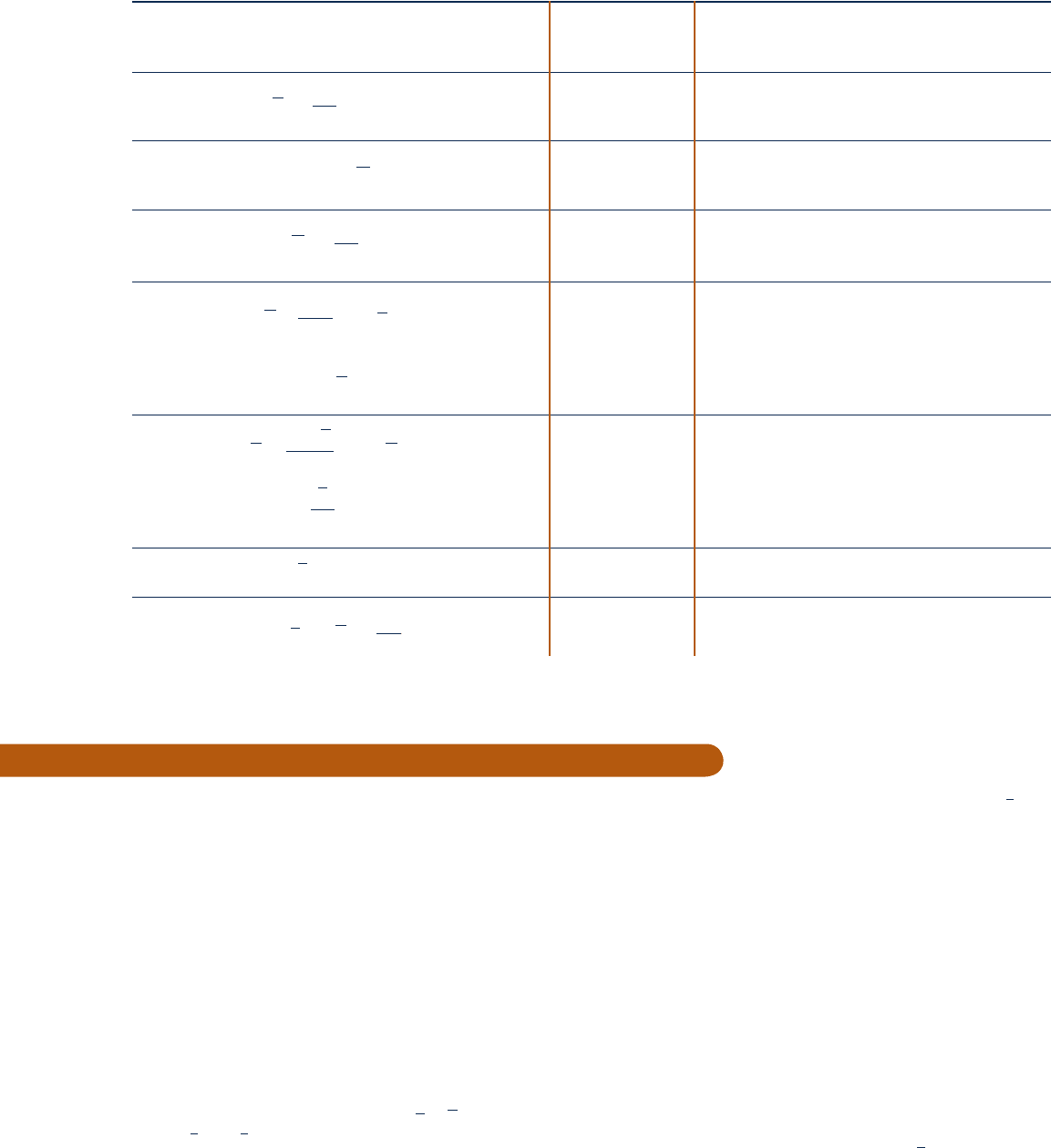
Properties of Multicomponent Mixtures
T
c
5
a
j
i
51
y
i
T
c, i
,
p
c
5
a
j
i
51
y
i
p
c, i
(11.97) p. 676
Kay’s rule for critical temperature and
pressure of mixtures
X
i
5
0X
0n
i
b
T,
p
, n
l
(11.102) p. 680
Partial molal property
—
X
i
and its relation to
extensive property X
X 5
a
j
i
51
n
i
X
i
(11.103) p. 680
X as a weighted sum of partial molal
properties
m
i
5 G
i
5
0G
0n
i
b
T,
p
, n
l
(11.107) p. 682 Chemical potential of species i in a mixture
RT a
0ln f
0p
b
T
5 y
(11.122) p. 685
Expressions for evaluating fugacity of a
single-component system
lim
p
S0
f
p
5 1
(11.123) p. 685
R
T a
0 l
n f
i
0p
b
T, n
5 V
i
(11.126a) p. 687
Expressions for evaluating fugacity of
mixture component i
lim
p
S0
a
f
i
y
i
p
b
5 1
(11.126b) p. 687
f
i
5
y
i
f
i
(11.134) p. 688 Lewis–Randall rule for ideal solutions
m
i
5 g
i
81RT ln
y
i
p
p
ref
(11.144) p. 690
Chemical potential of component i in an
ideal gas mixture
c EXERCISES: THINGS ENGINEERS THINK ABOUT
1. What is an advantage of using the Redlich–Kwong equation
of state in the generalized form given by Eq. 11.9 instead of
Eq. 11.7? A disadvantage?
2. To determine the specific volume of superheated water vapor
at a known pressure and temperature, when would you use each
of the following: the steam tables, the generalized compressibility
chart, an equation of state, the ideal gas model?
3. If the function p 5 p(T, y) is an equation of state, is
1
0p
/
0T
2
y
a property? What are the independent variables of
1
0p
/
0T
2
y
?
4. In the expression
1
0u
/
0T
2
y
, what does the subscript y signify?
5. Explain how a Mollier diagram provides a graphical
representation of the fundamental function h(s, p).
6. How is the Clapeyron equation used?
7. For a gas whose equation of state is
py 5 RT, are the specific
heats
c
p
and c
y
necessarily functions of temperature alone?
8. Referring to the phase diagram for water (Fig. 3.5), explain
why ice melts under the blade of an ice skate.
9. Can you devise a way to determine the specific heat
c
p
of a
gas by direct measurement? Indirectly, using other measured
data?
10. For an ideal gas, what is the value of the Joule–Thomson
coefficient?
11. At what states is the entropy departure negligible? The
fugacity coefficient, f/p, closely equal to unity?
12. In Eq. 11.107, what do the subscripts T, p, and n
l
signify?
What does i denote?
13. How does Eq. 11.108 reduce for a system consisting of a
pure substance? Repeat for an ideal gas mixture.
14. If two different liquids of known volumes are mixed, is the
final volume necessarily equal to the sum of the original
volumes?
15. For a binary solution at temperature T and pressure p, how
would you determine the specific heat
c
p
? Repeat for an
ideal solution and for an ideal gas mixture.
Exercises: Things Engineers Think About 693
c11ThermodynamicRelations.indd693 Page 693 6/21/10 9:37:24 PM user-s146 c11ThermodynamicRelations.indd693 Page 693 6/21/10 9:37:24 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

694 Chapter 11
Thermodynamic Relations
c PROBLEMS: DEVELOPING ENGINEERING SKILLS
Using Equations of State
11.1 Owing to safety requirements, the pressure within a
19.3 ft
3
cylinder should not exceed 52 atm. Check the pressure
within the cylinder if filled with 100 lb of CO
2
maintained
at 2128F using the
(a) van der Waals equation.
(b) compressibility chart.
(c) ideal gas equation of state.
11.2 Ten pounds mass of propane have a volume of 2 ft
3
and
a pressure of 600 lbf/in.
2
Determine the temperature, in 8R,
using the
(a) van der Waals equation.
(b) compressibility chart.
(c) ideal gas equation of state.
(d) propane tables.
11.3 The pressure within a 23.3-m
3
tank should not exceed
105 bar. Check the pressure within the tank if filled with
1000 kg of water vapor maintained at 3608C using the
(a) ideal gas equation of state.
(b) van der Waals equation.
(c) Redlich–Kwong equation.
(d) compressibility chart.
(e) steam tables.
11.4 Estimate the pressure of water vapor at a temperature of
5008C and a density of 24 kg/m
3
using the
(a) steam tables.
(b) compressibility chart.
(c) Redlich–Kwong equation.
(d) van der Waals equation.
(e) ideal gas equation of state.
11.5 Methane gas flows through a pipeline with a volumetric
flow rate of 11 ft
3
/s at a pressure of 183 atm and a
temperature of 568F. Determine the mass flow rate, in lb/s,
using the
(a) ideal gas equation of state.
(b) van der Waals equation.
(c) compressibility chart.
11.6 Determine the specific volume of water vapor at 20 MPa
and 4008C, in m
3
/kg, using the
(a) steam tables.
(b) compressibility chart.
(c) Redlich–Kwong equation.
(d) van der Waals equation.
(e) ideal gas equation of state.
11.7 A vessel whose volume is 1 m
3
contains 4 kmol of methane
at 1008C. Owing to safety requirements, the pressure of the
methane should not exceed 12 MPa. Check the pressure
using the
(a) ideal gas equation of state.
(b) Redlich–Kwong equation.
(c) Benedict–Webb–Rubin equation.
11.8 Methane gas at 100 atm and 2188C is stored in a 10-m
3
tank. Determine the mass of methane contained in the tank,
in kg, using the
(a) ideal gas equation of state.
(b) van der Waals equation.
(c) Benedict–Webb–Rubin equation.
11.9 Using the Benedict–Webb–Rubin equation of state,
determine the volume, in m
3
, occupied by 165 kg of methane
at a pressure of 200 atm and temperature of 400 K. Compare
with the results obtained using the ideal gas equation of state
and the generalized compressibility chart.
11.10 A rigid tank contains 1 kg of oxygen (O
2
) at p
1
5 40 bar,
T
1
5 180 K. The gas is cooled until the temperature drops
to 150 K. Determine the volume of the tank, in m
3
, and the
final pressure, in bar, using the
(a) ideal gas equation of state.
(b) Redlich–Kwong equation.
(c) compressibility chart.
11.11 One pound mass of air initially occupying a volume of
0.4 ft
3
at a pressure of 1000 lbf/in.
2
expands in a piston–cylinder
assembly isothermally and without irreversibilities until the
volume is 2 ft
3
. Using the Redlich–Kwong equation of state,
determine the
(a) temperature, in 8R.
(b) final pressure, in lbf/in.
2
(c) work developed in the process, in Btu.
11.12 Water vapor initially at 2408C, 1 MPa expands in a
piston–cylinder assembly isothermally and without internal
irreversibilities to a final pressure of 0.1 MPa. Evaluate the
work done, in kJ/kg. Use a truncated virial equation of state
with the form
Z 5 1
1
B
y
1
C
y
2
where B and C are evaluated from steam table data at 2408C
and pressures ranging from 0 to 1 MPa.
11.13 Referring to the virial series, Eqs. 3.30 and 3.31, show
that B
ˆ
5
B
/
RT, C
ˆ
5
1
C 2 B
2
2
/
R
2
T
2
.
11.14 Express Eq. 11.5, the van der Waals equation, in terms
of the compressibility factor Z
(a) as a virial series in y
¿
R
. [Hint: Expand the
1
y¿
R
2 1
/
8
2
21
term of Eq. 11.5 in a series.]
(b) as a virial series in p
R
.
(c) Dropping terms involving (p
R
)
2
and higher in the virial
series of part (b), obtain the following approximate form:
Z 5 1 1 a
1
8
2
27
/
64
T
R
b
p
R
T
R
(d) Compare the compressibility factors determined from
the equation of part (c) with tabulated compressibility
factors from the literature for 0 , p
R
, 0.6 and each of
T
R
5 1.0, 1.2, 1.4, 1.6, 1.8, 2.0. Comment on the realm of
validity of the approximate form.
c11ThermodynamicRelations.indd694 Page 694 6/22/10 7:20:57 PM user-s146 c11ThermodynamicRelations.indd694 Page 694 6/22/10 7:20:57 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

11.15 The Berthelot equation of state has the form
p 5
RT
y 2 b
2
a
T
y
2
(a) Using Eqs. 11.3, show that
a 5
27
64
R
2
T
3
c
p
c
,
b 5
1
8
RT
c
p
c
(b) Express the equation in terms of the compressibility factor
Z, the reduced temperature T
R
, and the pseudoreduced specific
volume, y
¿
R
.
11.16 The Beattie–Bridgeman equation of state can be
expressed as
p 5
RT 11 2 e21y 1 B2
y
2
2
A
y
2
where
A
5 A
0
a1 2
a
y
b,
B 5 B
0
a1 2
b
y
b
e 5
c
yT
3
and A
0
, B
0
, a, b, and c are constants. Express this equation
of state in terms of the reduced pressure, p
R
, reduced
temperature, T
R
, pseudoreduced specific volume, y
¿
R
, and
appropriate dimensionless constants.
11.17 The Dieterici equation of state is
p 5 a
RT
y 2 b
b exp a
2a
RTy
b
(a) Using Eqs. 11.3, show that
a 5
4R
2
T
2
c
p
c
e
2
,
b 5
RT
c
p
c
e
2
(b) Show that the equation of state can be expressed in
terms of compressibility chart variables as
Z 5 a
y¿
R
y¿
R
2 1
/
e
2
b exp a
24
T
R
y¿
R
e
2
b
(c) Convert the result of part (b) to a virial series in y
¿
R
.
(Hint: Expand the
1
y¿
R
2 1
/
e
2
2
21
term in a series. Also expand
the exponential term in a series.)
11.18 The Peng–Robinson equation of state has the form
p 5
R
T
y 2 b
2
a
y
2
2
c
2
Using Eqs. 11.3, evaluate the constants a, b, c in terms of the
critical pressure p
c
, critical temperature T
c
, and critical
compressibility factor Z
c
.
11.19 The p–y–T relation for chlorofluorinated hydrocarbons
can be described by the Carnahan–Starling–DeSantis equation
of state
py
RT
5
1 1 b 1
b
2
2 b
3
1
1 1 b
2
3
2
a
RT1y 1 b2
where
b
5 b
/
4y, a 5 a
0
exp (a
1
T 1 a
2
T
2
), and b 5 b
0
1 b
1
T
1 b
2
T
2
. For Refrigerants 12 and 13, the required coefficients
for T in K, a in J ? L/(mol)
2
, and b in L/mol are given in
Table P11.19. Specify which of the two refrigerants would
allow the smaller amount of mass to be stored in a 10-m
3
vessel at 0.2 MPa, 808C.
Using Relations from Exact Differentials
11.20 The differential of pressure obtained from a certain
equation of state is given by one of the following expressions.
Determine the equation of state.
dp 5
21y 2 b
2
RT
d
y 1
1y 2 b2
2
RT
2
dT
dp 52
R
T
1
y 2 b
2
2
dy 1
R
y 2 b
dT
11.21 Introducing
d
Q
int
re
v
5 T dS into Eq. 6.8 gives
d
Q
int
r
ev
5 dU 1 p dV
Using this expression together with the test for exactness,
demonstrate that
Q
int
re
v
is not a property.
11.22 Show that Eq. 11.16 is satisfied by an equation of state
with the form p 5 [RT/(y 2 b)] 1 a.
11.23 For the functions x 5 x(y, w), y 5 y(z, w), z 5 z(x, w),
demonstrate that
0x
0y
b
w
0
y
0z
b
w
0z
0x
b
w
5 1
11.24 Using Eq. 11.35, check the consistency of
(a) the steam tables at 2 MPa, 4008C.
(b) the Refrigerant 134a tables at 2 bar, 508C.
11.25 Using Eq. 11.35, check the consistency of
(a) the steam tables at 100 lbf/in.
2
, 6008F.
(b) the Refrigerant 134a tables at 40 lbf/in.
2
, 1008F.
11.26 At a pressure of 1 atm, liquid water has a state of maxi-
mum density at about 48C. What can be concluded about
1
0s
/
0p
2
T
at
(a) 38C?
(b) 48C?
(c) 58C?
11.27 A gas enters a compressor operating at steady state and is
compressed isentropically. Does the specific enthalpy increase
or decrease as the gas passes from the inlet to the exit?
11.28 Show that T, p, h, c, and g can each be determined from
a fundamental thermodynamic function of the form u 5
u(s, y).
a
0
3 10
23
a
1
3 10
3
a
2
3 10
6
b
0
b
1
3 10
4
b
2
3 10
8
R-12 3.52412 22.77230 20.67318 0.15376 21.84195 25.03644
R-13 2.29813 23.41828 21.52430 0.12814 21.84474 210.7951
Table P11.19
Problems: Developing Engineering Skills 695
c11ThermodynamicRelations.indd695 Page 695 6/22/10 7:20:59 PM user-s146 c11ThermodynamicRelations.indd695 Page 695 6/22/10 7:20:59 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

696 Chapter 11
Thermodynamic Relations
11.29 Evaluate p, s, u, h, c
y
, and c
p
for a substance for which
the Helmholtz function has the form
c 52RT
ln
y
y¿
2 cT¿ c1 2
T
T¿
1
T
T¿
ln
T
T¿
d
where y9 and T9 denote specific volume and temperature,
respectively, at a reference state, and c is a constant.
11.30 The Mollier diagram provides a graphical representation
of the fundamental thermodynamic function h 5 h(s, p).
Show that at any state fixed by s and p the properties T, y,
u, c, and g can be evaluated using data obtained from the
diagram.
11.31 Derive the relation c
p
52T10
2
g
y
0T
2
2
p
.
Evaluating Ds, Du, and Dh
11.32 Using p–y–T data for saturated ammonia from Table
A-13E, calculate at 208F
(a) h
g
2 h
f
.
(b) u
g
2 u
f
.
(c) s
g
2 s
f
.
Compare with values obtained from Table A-13E.
11.33 Using p–y–T data for saturated water from the steam
tables, calculate at 508C
(a) h
g
2 h
f
.
(b) u
g
2 u
f
.
(c) s
g
2 s
f
.
Compare with values obtained from the steam tables.
11.34 Using h
fg
, y
fg
, and p
sat
at 108F from the Refrigerant 134a
tables, estimate the saturation pressure at 208F. Comment on
the accuracy of your estimate.
11.35 Using h
fg
, y
fg
, and p
sat
at 268C from the ammonia tables,
estimate the saturation pressure at 308C. Comment on the
accuracy of your estimate.
11.36 Using triple-point data for water from Table A-6E,
estimate the saturation pressure at 2408F. Compare with the
value listed in Table A-6E.
11.37 At 08C, the specific volumes of saturated solid water (ice)
and saturated liquid water are, respectively, y
i
5 1.0911 3
10
23
m
3
/kg and y
f
5 1.0002 3 10
23
m
3
/kg, and the change in
specific enthalpy on melting is h
if
5 333.4 kJ/kg. Calculate the
melting temperature of ice at (a) 250 bar, (b) 500 bar. Locate
your answers on a sketch of the p–T diagram for water.
11.38 The line representing the two-phase solid–liquid region
on the phase diagram slopes to the left for substances that
expand on freezing and to the right for substances that
contract on freezing (Sec. 3.2.2). Verify this for the cases of
lead that contracts on freezing and bismuth that expands on
freezing.
11.39 Consider a four-legged chair at rest on an ice rink. The
total mass of the chair and a person sitting on it is 80 kg. If
the ice temperature is 228C, determine the minimum total
area, in cm
2
, the tips of the chair legs can have before the
ice in contact with the legs would melt. Use data from
Problem 11.37 and let the local acceleration of gravity be
9.8 m/s
2
.
11.40 Over a certain temperature interval, the saturation
pressure–temperature curve of a substance is represented by
an equation of the form ln p
sat
5 A 2 B/T, where A and B
are empirically determined constants.
(a) Obtain expressions for h
g
2 h
f
and s
g
2 s
f
in terms of
p–y–T data and the constant B.
(b) Using the results of part (a), calculate h
g
2 h
f
and s
g
2 s
f
for water vapor at 258C and compare with steam table data.
11.41 Using data for water from Table A-2, determine the
constants A and B to give the best fit in a least-squares sense
to the saturation pressure in the interval from 20 to 308C by
the equation ln p
sat
5 A 2 B/T. Using this equation, determine
dp
sat
/dT at 258C. Calculate h
g
2
h
f
at 258C and compare with
the steam table value.
11.42 Over limited intervals of temperature, the saturation
pressure–temperature curve for two-phase liquid–vapor states
can be represented by an equation of the form ln p
sat
5
A 2 B/T, where A and B are constants. Derive the following
expression relating any three states on such a portion of the
curve:
p
sat, 3
p
sat, 1
5 a
p
sat, 2
p
sat,1
b
t
where t = T
2
(T
3
2 T
1
)/T
3
(T
2
2 T
1
).
11.43 Use the result of Problem 11.42 to determine
(a) the saturation pressure at 308C using saturation pressure–
temperature data at 20 and 408C from Table A-2. Compare
with the table value for saturation pressure at 308C.
(b) the saturation temperature at 0.006 MPa using saturation
pressure–temperature data at 20 to 408C from Table A-2.
Compare with the saturation temperature at 0.006 MPa
given in Table A-3.
11.44 Complete the following exercises dealing with slopes:
(a) At the triple point of water, evaluate the ratio of the
slope of the vaporization line to the slope of the sublimation
line. Use steam table data to obtain a numerical value for
the ratio.
(b) Consider the superheated vapor region of a temperature–
entropy diagram. Show that the slope of a constant specific
volume line is greater than the slope of a constant pressure
line through the same state.
(c) An enthalpy–entropy diagram (Mollier diagram) is often
used in analyzing steam turbines. Obtain an expression for
the slope of a constant-pressure line on such a diagram in
terms of p–y–T data only.
(d) A pressure–enthalpy diagram is often used in the
refrigeration industry. Obtain an expression for the slope of
an isentropic line on such a diagram in terms of p–y–T data
only.
11.45 Using only p–y–T data from the ammonia tables, evaluate
the changes in specific enthalpy and entropy for a process
from 70 lbf/in.
2
, 408F to 14 lbf/in.
2
, 408F. Compare with the
table values.
11.46 One kmol of argon at 300 K is initially confined to one
side of a rigid, insulated container divided into equal volumes
of 0.2 m
3
by a partition. The other side is initially evacuated.
The partition is removed and the argon expands to fill the
c11ThermodynamicRelations.indd696 Page 696 6/22/10 7:21:02 PM user-s146 c11ThermodynamicRelations.indd696 Page 696 6/22/10 7:21:02 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

entire container. Using the van der Waals equation of state,
determine the final temperature of the argon, in K. Repeat
using the ideal gas equation of state.
11.47 Obtain the relationship between c
p
and c
y
for a gas that
obeys the equation of state p(y 2 b) 5 RT.
11.48 The p–y–T relation for a certain gas is represented closely
by y = RT/p 1 B 2 A/RT, where R is the gas constant and
A and B are constants. Determine expressions for the changes
in specific enthalpy, internal energy, and entropy, [h(p
2
, T) 2
h(p
1
, T)], [u(p
2
, T) 2 u(p
1
, T)], and [s(p
2
, T) 2 s(p
1
, T)],
respectively.
11.49 Develop expressions for the specific enthalpy, internal
energy, and entropy changes [h(y
2
, T) 2 h(y
1
, T)], [u(y
2
, T) 2
u(y
1
, T)], [s(y
2
, T) 2 s(y
1
, T)], using the
(a) van der Waals equation of state.
(b) Redlich–Kwong equation of state.
11.50 At certain states, the p–y–T data of a gas can be
expressed as Z 5 1 2 Ap/T
4
, where Z is the compressibility
factor and A is a constant.
(a) Obtain an expression for (0p
/
0T)
y
in terms of p, T, A, and
the gas constant R.
(b) Obtain an expression for the change in specific entropy,
[s(p
2
, T) 2 s (p
1
, T)].
(c) Obtain an expression for the change in specific enthalpy,
[h(p
2
, T) 2 h (p
1
, T)].
11.51 For a gas whose p–y–T behavior is described by Z 5
1 1 Bp/RT, where B is a function of temperature, derive
expressions for the specific enthalpy, internal energy, and
entropy changes, [h(p
2
, T) 2 h(p
1
, T)], [u(p
2
, T) 2 u(p
1
, T)],
and [s(p
2
, T) 2 s(p
1
, T)].
11.52 For a gas whose p–y–T behavior is described by Z 5
1 1 B/y 1 C/y
2
, where B and C are functions of temperature,
derive an expression for the specific entropy change, [s(y
2
, T) 2
s(y
1
, T)].
Using Other Thermodynamic Relations
11.53 The volume of a 1-kg copper sphere is not allowed to
vary by more than 0.1%. If the pressure exerted on the
sphere is increased from 10 bar while the temperature
remains constant at 300 K, determine the maximum allowed
pressure, in bar. Average values of r, b, and k are 8888 kg/m
3
,
49.2 3 10
26
(K)
21
, and 0.776 3 10
211
m
2
/N respectively.
11.54 The volume of a 1-lb copper sphere is not allowed to
vary by more than 0.1%. If the pressure exerted on the
sphere is increased from 1 atm while the temperature remains
constant at 808F, determine the maximum allowed pressure,
in atm. Average values of r, b, and k are 555 lb/ft
3
, 2.75 3
10
25
(8R)
21
, and 3.72 3 10
210
ft
2
/lbf, respectively.
11.55 Develop expressions for the volume expansivity b and
the isothermal compressibility k for
(a) an ideal gas.
(b) a gas whose equation of state is p(y 2 b) 5 RT.
(c) a gas obeying the van der Waals equation.
11.56 Derive expressions for the volume expansivity b and the
isothermal compressibility k in terms of T, p, Z, and the first
partial derivatives of Z. For gas states with p
R
, 3, T
R
, 2,
determine the sign of k. Discuss.
11.57 Show that the isothermal compressibility k is always
greater than or equal to the isentropic compressibility a.
11.58 Prove that 10b
/
0p2
T
5210k
/
0T2
p
.
11.59 For aluminum at 08C, r 5 2700 kg/m
3
, b 5 71.4 3 10
28
(K)
21
, k 5 1.34 3 10
213
m
2
/N, and c
p
5 0.9211 kJ/kg ? K.
Determine the percent error in c
y
that would result if it were
assumed that c
p
5 c
y
.
11.60 Estimate the temperature rise, in 8C, of mercury, initially
at 08C and 1 bar if its pressure were raised to 1000 bar
isentropically. For mercury at 08C, c
p
5 28.0 kJ/kmol ? K,
y 5 0.0147 m
3
/
kmol, and b 5 17.8 3 10
25
(K)
21
.
11.61 At certain states, the p–y–T data for a particular gas
can be represented as Z 5 1 2 Ap/T
4
, where Z is the
compressibility factor and A is a constant. Obtain an
expression for the specific heat c
p
in terms of the gas constant
R, specific heat ratio k, and Z. Verify that your expression
reduces to Eq. 3.47a when Z 5 1.
11.62 For a gas obeying the van der Waals equation of state,
(a) show that
1
0c
y
/
0y
2
T
5 0.
(b) develop an expression for c
p
2 c
y
.
(c) develop expressions for [u(T
2
, y
2
) 2 u(T
1
, y
1
)] and [s(T
2
, y
2
)
2 s(T
1
, y
1
)].
(d) complete the Du and Ds evaluations if c
y
5 a 1 bT,
where a and b are constants.
11.63 If the value of the specific heat c
y
of air is 0.1965 Btu/
lb ? 8R at T
1
5 10008F, y
1
5 36.8ft
3
/lb, determine the value
of c
y
at T
2
5 10008F, y
2
5 0.0555 ft
3
/lb. Assume that air obeys
the Berthelot equation of state
p 5
RT
y 2 b
2
a
Ty
2
where
a 5
2
7
64
R
2
T
3
c
p
c
,
b 5
1
8
RT
c
p
c
11.64 Show that the specific heat ratio k can be expressed as
k 5 c
p
k/(c
p
k 2 Tyb
2
). Using this expression together with
data from the steam tables, evaluate k for water vapor at
200 lbf/in.
2
, 5008F.
11.65 For liquid water at 408C, 1 atm estimate
(a) c
y
, in kJ/kg ? K.
(b) the velocity of sound, in m/s.
Use Data from Table 11.2, as required.
11.66 Using steam table data, estimate the velocity of sound
in liquid water at (a) 208C, 50 bar, (b) 508F, 1500 lbf/in.
2
11.67 At a certain location in a wind tunnel, a stream of air is
at 5008F, 1 atm and has a velocity of 2115 ft/s. Determine the
Mach number at this location.
11.68 For a gas obeying the equation of state p(y 2 b) 5 RT,
where b is a positive constant, can the temperature be
reduced in a Joule–Thomson expansion?
Problems: Developing Engineering Skills 697
c11ThermodynamicRelations.indd697 Page 697 6/21/10 9:37:30 PM user-s146 c11ThermodynamicRelations.indd697 Page 697 6/21/10 9:37:30 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
698 Chapter 11
Thermodynamic Relations
11.69 A gas is described by y 5 RT/p 2 A/T 1 B, where A
and B are constants. For the gas
(a) obtain an expression for the temperatures at the Joule–
Thomson inversion states.
(b) obtain an expression for c
p
2 c
y
.
11.70 Determine the maximum Joule–Thomson inversion
temperature in terms of the critical temperature T
c
predicted
by the
(a) van der Waals equation.
(b) Redlich–Kwong equation.
(c) Dieterici equation given in Problem 11.17.
11.71 Derive an equation for the Joule–Thomson coefficient
as a function of T and y for a gas that obeys the van der
Waals equation of state and whose specific heat c
y
is given
by c
y
5 A 1 BT 1 CT
2
, where A, B, C are constants.
Evaluate the temperatures at the inversion states in terms of
R, y, and the van der Waals constants a and b.
11.72 Show that Eq. 11.77 can be written as
m
J
5
T
2
c
p
a
01y
/
T2
0T
b
p
(a) Using this result, obtain an expression for the Joule–
Thomson coefficient for a gas obeying the equation of state
y 5
R
T
p
2
Ap
T
2
where A is a constant.
(b) Using the result of part (a), determine c
p
, in kJ/kg ? K,
for CO
2
at 400 K, 1 atm, where m
J
5 0.57 K/atm. For CO
2
,
A 5 2.78 3 10
23
m
5
? K
2
/kg ? N.
Developing Property Data
11.73 If the specific heat c
y
of a gas obeying the van der Waals
equation is given at a particular pressure, p9, by c
y
5 A 1
BT, where A and B are constants, develop an expression for
the change in specific entropy between any two states 1 and
2: [s(T
2
, p
2
) 2 s(T
1
, p
1
)].
11.74 For air, write a computer program that evaluates the
change in specific enthalpy from a state where the temperature
is 258C and the pressure is 1 atm to a state where the
temperature is T and the pressure is p. Use the van der Waals
equation of state and account for the variation of the ideal
gas specific heat as in Table A-21.
11.75 Using the Redlich–Kwong equation of state, determine
the changes in specific enthalpy, in kJ/kmol, and entropy, in
kJ/kmol ? K, for ethylene between 400 K, 1 bar and 400 K,
100 bar.
11.76 Using the Benedict–Webb–Rubin equation of state
together with a specific heat relation from Table A-21,
determine the change in specific enthalpy, in kJ/kmol, for
methane between 300 K, 1 atm and 400 K, 200 atm.
11.77 A certain pure, simple compressible substance has the
following property relations. The p–y–T relationship in the
vapor phase is
y 5
R
T
p
2
Bp
T
2
where y is in ft
3
/lb, T is in 8R, p is in lbf/ft
2
, R 5 50 ft ? lbf/
lb ? 8R, and B 5 100 ft
5
? (8R)
2
/lb ? lbf. The saturation
pressure, in lbf/ft
2
, is described by
ln p
sat
5 12 2
2400
T
The Joule–Thomson coefficient at 10 lbf/in.
2
, 2008F is
0.0048R ? ft
2
/lbf. The ideal gas specific heat c
p0
is constant
over the temperature range 0 to 3008F.
(a) Complete the accompanying table of property values
T p v
f
v
g
h
f
h
g
s
f
s
g
08F 0.03 0 0.000
1008F 0.03
for p in lbf/in.
2
, y in ft
3
/lb, h in Btu/lb, and s in Btu/lb ? 8R.
(b) Evaluate y, h, s at the state fixed by 15 lbf/in.
2
, 3008F.
11.78 In Table A-2, at temperatures up to 508C, the values of
u
f
and h
f
differ in most cases by 0.01 kJ/kg. Yet at each of
these temperatures the product p
sat
y
f
is small enough to be
neglected and the table values of u
f
and h
f
should be the
same. In a memorandum, provide a plausible explanation.
Using Enthalpy and Entropy Departures
11.79 Beginning with Eq. 11.90, derive Eq. 11.91.
11.80 Derive an expression giving
(a) the internal energy of a substance relative to that of its
ideal gas model at the same temperature: [u(T, y) 2 u*(T)].
(b) the entropy of a substance relative to that of its ideal gas
model at the same temperature and specific volume: [s(T, y) 2
s*(T, y)].
11.81 Derive expressions for the enthalpy and entropy
departures using an equation of state with the form Z 5 1 1
Bp
R
, where B is a function of the reduced temperature, T
R
.
11.82 The following expression for the enthalpy departure is
convenient for use with equations of state that are explicit
in pressure:
h
*
1T22 h1T, y2
RT
c
5 T
R
c1 2 Z 2
1
RT
#
y
q
cT a
0
p
0T
b
y
2 p ddy d
(a) Derive this expression.
(b) Using the given expression, evaluate the enthalpy
departure for a gas obeying the Redlich–Kwong equation of
state.
(c) Using the result of part (b), determine the change in
specific enthalpy, in kJ/kmol, for CO
2
undergoing an isothermal
process at 300 K from 50 to 20 bar.
11.83 Using the equation of state of Problem 11.14 (c), evaluate
y and c
p
for water vapor at 5508C, 20 MPa and compare with
data from Table A-4 and Fig. 3.9, respectively. Discuss.
11.84 Ethylene at 678C, 10 bar enters a compressor operating
at steady state and is compressed isothermally without
internal irreversibilities to 100 bar. Kinetic and potential
energy changes are negligible. Evaluate in kJ per kg of
ethylene flowing through the compressor
(a) the work required.
(b) the heat transfer.
c11ThermodynamicRelations.indd698 Page 698 6/21/10 9:37:32 PM user-s146 c11ThermodynamicRelations.indd698 Page 698 6/21/10 9:37:32 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

11.85 Methane at 278C, 10 MPa enters a turbine operating at
steady state, expands adiabatically through a 5 : 1 pressure
ratio, and exits at 2488C. Kinetic and potential energy effects
are negligible. If
c
p
o
5 35 kJ
/
kmol ? K, determine the work
developed per kg of methane flowing through the turbine.
Compare with the value obtained using the ideal gas
model.
11.86 Nitrogen (N
2
) enters a compressor operating at steady
state at 1.5 MPa, 300 K and exits at 8 MPa, 500 K. If the
work input is 240 kJ per kg of nitrogen flowing, determine
the heat transfer, in kJ per kg of nitrogen flowing. Ignore
kinetic and potential energy effects.
11.87 Oxygen (O
2
) enters a control volume operating at steady
state with a mass flow rate of 9 kg/min at 100 bar, 287 K and
is compressed adiabatically to 150 bar, 400 K. Determine the
power required, in kW, and the rate of entropy production, in
kW/K. Ignore kinetic and potential energy effects.
11.88 Argon gas enters a turbine operating at steady state at 100
bar, 325 K and expands adiabatically to 40 bar, 235 K with no
significant changes in kinetic or potential energy. Determine
(a) the work developed, in kJ per kg of argon flowing
through the turbine.
(b) the amount of entropy produced, in kJ/K per kg of argon
flowing.
11.89 Oxygen (O
2
) undergoes a throttling process from 100
bar, 300 K to 20 bar. Determine the temperature after
throttling, in K, and compare with the value obtained using
the ideal gas model.
11.90 Water vapor enters a turbine operating at steady state at
30 MPa, 6008C and expands adiabatically to 6 MPa with no
significant change in kinetic or potential energy. If the isentropic
turbine efficiency is 80%, determine the work developed, in
kJ per kg of steam flowing, using the generalized property
charts. Compare with the result obtained using steam table
data. Discuss.
11.91 Oxygen (O
2
) enters a nozzle operating at steady state at
60 bar, 300 K, 1 m/s and expands isentropically to 30 bar.
Determine the velocity at the nozzle exit, in m/s.
11.92 A quantity of nitrogen gas in a piston–cylinder assembly
undergoes a process at a constant pressure of 80 bar from
220 to 300 K. Determine the work and heat transfer for the
process, each in kJ per kmol of nitrogen.
11.93 A closed, rigid, insulated vessel having a volume of
0.142 m
3
contains oxygen (O
2
) initially at 100 bar, 78C. The
oxygen is stirred by a paddle wheel until the pressure
becomes 150 bar. Determine the
(a) final temperature, in 8C.
(b) work, in kJ.
(c) amount of exergy destroyed in the process, in kJ.
Let T
0
5 78C.
Evaluating p–y–T for Gas Mixtures
11.94 A preliminary design calls for a 1 kmol mixture of CO
2
and C
2
H
6
(ethane) to occupy a volume of 0.15 m
3
at a
temperature of 400 K. The mole fraction of CO
2
is 0.3.
Owing to safety requirements, the pressure should not
exceed 180 bar. Check the pressure using
(a) the ideal gas equation of state.
(b) Kay’s rule together with the generalized compressibility
chart.
(c) the additive pressure rule together with the generalized
compressibility chart.
Compare and discuss these results.
11.95 A gaseous mixture with a molar composition of 60% CO
and 40% H
2
enters a turbine operating at steady state at
3008F, 2000 lbf/in.
2
and exits at 2128F, 1 atm with a volumetric
flow rate of 20,000 ft
3
/min. Estimate the volumetric flow rate
at the turbine inlet, in ft
3
/min, using Kay’s rule. What value
would result from using the ideal gas model? Discuss.
11.96 A 0.1-m
3
cylinder contains a gaseous mixture with a molar
composition of 97% CO and 3% CO
2
initially at 138 bar. Due
to a leak, the pressure of the mixture drops to 129 bar while the
temperature remains constant at 308C. Using Kay’s rule, estimate
the amount of mixture, in kmol, that leaks from the cylinder.
11.97 A gaseous mixture consisting of 0.75 kmol of hydrogen
(H
2
) and 0.25 kmol of nitrogen (N
2
) occupies 0.085 m
3
at
258C. Estimate the pressure, in bar, using
(a) the ideal gas equation of state.
(b) Kay’s rule together with the generalized compressibility
chart.
(c) the van der Waals equation together with mixture values
for the constants a and b.
(d) the rule of additive pressure together with the generalized
compressibility chart.
11.98 A gaseous mixture of 0.5 lbmol of methane and 0.5 lbmol
of propane occupies a volume of 7.65 ft
3
at a temperature of
1948F. Estimate the pressure using the following procedures
and compare each estimate with the measured value of
pressure, 50 atm:
(a) the ideal gas equation of state.
(b) Kay’s rule together with the generalized compressibility
chart.
(c) the van der Waals equation together with mixture values
for the constants a and b.
(d) the rule of additive pressures together with the van der
Waals equation.
(e) the rule of additive pressures together with the generalized
compressibility chart.
(f) the rule of additive volumes together with the van der
Waals equation.
11.99 One lbmol of a gaseous mixture occupies a volume of
1.78 ft
3
at 2128F. The mixture consists of 69.5% carbon
dioxide and 30.5% ethylene (C
2
H
4
) on a molar basis. Estimate
the mixture pressure, in atm, using
(a) the ideal gas equation of state.
(b) Kay’s rule together with the generalized compressibility
chart.
(c) the additive pressure rule together with the generalized
compressibility chart.
(d) the van der Waals equation together with mixture values
for the constants a and b.
Problems: Developing Engineering Skills 699
c11ThermodynamicRelations.indd699 Page 699 6/22/10 7:21:05 PM user-s146 c11ThermodynamicRelations.indd699 Page 699 6/22/10 7:21:05 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

700 Chapter 11
Thermodynamic Relations
11.100 Air having an approximate molar composition of 79%
N
2
and 21% O
2
fills a 0.36-m
3
vessel. The mass of mixture is
100 kg. The measured pressure and temperature are 101 bar
and 180 K, respectively. Compare the measured pressure
with the pressure predicted using
(a) the ideal gas equation of state.
(b) Kay’s rule.
(c) the additive pressure rule with the Redlich–Kwong
equation.
(d) the additive volume rule with the Redlich–Kwong
equation.
11.101 A gaseous mixture consisting of 50% argon and 50%
nitrogen (molar basis) is contained in a closed tank at 20 atm,
21408F. Estimate the specific volume, in ft
3
/lb, using
(a) the ideal gas equation of state.
(b) Kay’s rule together with the generalized compressibility
chart.
(c) the Redlich–Kwong equation with mixture values for a
and b.
(d) the additive volume rule together with the generalized
compressibility chart.
11.102 Using the Carnahan–Starling–DeSantis equation of
state introduced in Problem 11.19, together with the following
expressions for the mixture values of a and b:
a 5 y
2
1
a
1
1 2y
1
y
2
1
1 2 f
12
21
a
1
a
2
2
1
/
2
1 y
2
2
a
2
b 5 y
1
b
1
1 y
2
b
2
where f
12
is an empirical interaction parameter, determine
the pressure, in kPa, at y = 0.005 m
3
/kg, T 5 1808C for a
mixture of Refrigerants 12 and 13, in which Refrigerant 12
is 40% by mass. For a mixture of Refrigerants 12 and 13,
f
12
5 0.035.
11.103 A rigid vessel initially contains carbon dioxide gas at
328C and pressure p. Ethylene gas is allowed to flow into the
tank until a mixture consisting of 20% carbon dioxide and
80% ethylene (molar basis) exists within the tank at a
temperature of 438C and a pressure of 110 bar. Determine
the pressure p, in bar, using Kay’s rule together with the
generalized compressibility chart.
11.104 Two tanks having equal volumes are connected by a
valve. One tank contains carbon dioxide gas at 1008F and
pressure p. The other tank contains ethylene gas at 1008F
and 1480 lbf/in.
2
The valve is opened and the gases mix,
eventually attaining equilibrium at 1008F and pressure p9
with a composition of 20% carbon dioxide and 80% ethylene
(molar basis). Using Kay’s rule and the generalized
compressibility chart, determine in lbf/in.
2
(a) the initial pressure of the carbon dioxide, p.
(b) the final pressure of the mixture, p9.
Analyzing Multicomponent Systems
11.105 A binary solution at 258C consists of 59 kg of ethyl
alcohol (C
2
H
5
OH) and 41 kg of water. The respective partial
molal volumes are 0.0573 and 0.0172 m
3
/kmol. Determine the
total volume, in m
3
. Compare with the volume calculated using
the molar specific volumes of the pure components, each a
liquid at 258C, in the place of the partial molal volumes.
11.106 The following data are for a binary solution of ethane
(C
2
H
6
) and pentane (C
5
H
12
) at a certain temperature and
pressure:
mole fraction of 0.2 0.3 0.4 0.5 0.6 0.7 0.8
ethane
volume (in m
3
) per 0.119 0.116 0.112 0.109 0.107 0.107 0.11
kmol of solution
Estimate
(a) the specific volumes of pure ethane and pure pentane,
each in m
3
/kmol.
(b) the partial molal volumes of ethane and pentane for an
equimolar solution, each in m
3
/kmol.
11.107 The following data are for a binary mixture of carbon
dioxide and methane at a certain temperature and pressure:
mole fraction of 0.000 0.204 0.406 0.606 0.847 1.000
methane
volume (in ft
3
) per 1.506 3.011 3.540 3.892 4.149 4.277
lbmol of mixture
Estimate
(a) the specific volumes of pure carbon dioxide and pure
methane, each in ft
3
/lbmol.
(b) the partial molal volumes of carbon dioxide and methane
for an equimolar mixture, each in ft
3
/lbmol.
11.108 Using p–y–T data from the steam tables, determine the
fugacity of water as a saturated vapor at (a) 2808C, (b) 5008F.
Compare with the values obtained from the generalized
fugacity chart.
11.109 Determine the fugacity, in atm, for
(a) butane at 555 K, 150 bar.
(b) methane at 1208F, 800 lbf/in.
2
(c) benzene at 8908R, 135 atm.
11.110 Using the equation of state of Problem 11.14 (c),
evaluate the fugacity of ammonia at 750 K, 100 atm and
compare with the value obtained from Fig. A-6.
11.111 Using tabulated compressibility factor data from the
literature, evaluate f/p at T
R
5 1.40 and p
R
5 2.0. Compare
with the value obtained from Fig. A-6.
11.112 Consider the truncated virial expansion
Z 5 1 1 B
ˆ
1T
R
2p
R
1 C
ˆ
1T
R
2p
2
R
1 D
ˆ
1T
R
2p
3
R
(a) Using tabulated compressibility factor data from the
literature, evaluate the coefficients B
ˆ
,
C
ˆ
, and D
ˆ
for 0 , p
R
, 1.0
and each of T
R
5 1.0, 1.2, 1.4, 1.6, 1.8, 2.0.
(b) Obtain an expression for ln (f/p) in terms of T
R
and p
R
.
Using the coefficients of part (a), evaluate f/p at selected
states and compare with tabulated values from the literature.
11.113 Derive the following approximation for the fugacity of
a liquid at temperature T and pressure p:
f1T, p2< f
L
sat
1T2exp e
y
f
1T2
RT
3p 2 p
sat
1T24f
where
f
L
sat
1
T
2
is the fugacity of the saturated liquid at temperature
T. For what range of pressures might the approximation
f
1
T, p
2
< f
L
sat
1
T
2
apply?
c11ThermodynamicRelations.indd700 Page 700 6/21/10 9:37:34 PM user-s146 c11ThermodynamicRelations.indd700 Page 700 6/21/10 9:37:34 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

11.114 Beginning with Eq. 11.122,
(a) evaluate ln f for a gas obeying the Redlich–Kwong
equation of state.
(b) Using the result of part (a), evaluate the fugacity, in bar,
for Refrigerant 134a at 908C, 10 bar. Compare with the
fugacity value obtained from the generalized fugacity chart.
11.115 Consider a one-inlet, one-exit control volume at steady
state through which the flow is internally reversible and
isothermal. Show that the work per unit of mass flowing can
be expressed in terms of the fugacity f as
a
W
#
cv
m
#
b
int
rev
52RT lna
f
2
f
1
b1
V
1
2
2 V
2
2
2
1 g1z
1
2 z
2
2
11.116 Methane expands isothermally and without
irreversibilities through a turbine operating at steady state,
entering at 60 atm, 778F and exiting at 1 atm. Using data
from the generalized fugacity chart, determine the work
developed, in Btu per lb of methane flowing. Ignore kinetic
and potential energy effects.
11.117 Propane (C
3
H
8
) enters a turbine operating at steady
state at 100 bar, 400 K and expands isothermally without
irreversibilities to 10 bar. There are no significant changes in
kinetic or potential energy. Using data from the generalized
fugacity chart, determine the power developed, in kW, for a
mass flow rate of 50 kg/min.
11.118 Ethane (C
2
H
6
) is compressed isothermally without
irreversibilities at a temperature of 320 K from 5 to 40 bar.
Using data from the generalized fugacity and enthalpy departure
charts, determine the work of compression and the heat transfer,
each in kJ per kg of ethane flowing. Assume steady-state
operation and neglect kinetic and potential energy effects.
11.119 Methane enters a turbine operating at steady state at
100 bar, 275 K and expands isothermally without irreversibilities
to 15 bar. There are no significant changes in kinetic or
potential energy. Using data from the generalized fugacity
and enthalpy departure charts, determine the power
developed and heat transfer, each in kW, for a mass flow
rate of 0.5 kg/s.
11.120 Methane flows isothermally and without irreversibilities
through a horizontal pipe operating at steady state, entering
at 50 bar, 300 K, 10 m/s and exiting at 40 bar. Using data
from the generalized fugacity chart, determine the velocity
at the exit, in m/s.
11.121 Determine the fugacity, in atm, for pure ethane at 310 K,
20.4 atm and as a component with a mole fraction of 0.35 in
an ideal solution at the same temperature and pressure.
11.122 Denoting the solvent and solute in a dilute binary liquid
solution at temperature T and pressure p by the subscripts
1 and 2, respectively, show that if the fugacity of the solute
is proportional to its mole fraction in the solution:
f
2
5 ky
2
,
where k is a constant (Henry’s rule), then the fugacity of the
solvent is
f
1
5 y
1
f
1
, where y
1
is the solvent mole fraction and
f
1
is the fugacity of pure 1 at T, p.
11.123 A tank contains 310 kg of a gaseous mixture of 70%
ethane and 30% nitrogen (molar basis) at 311 K and 170
atm. Determine the volume of the tank, in m
3
, using data
from the generalized compressibility chart together with (a)
Kay’s rule, (b) the ideal solution model. Compare with the
measured tank volume of 1 m
3
.
11.124 A tank contains a mixture of 75% argon and 25% ethylene
on a molar basis at 778F, 81.42 atm. For 157 lb of mixture,
estimate the tank volume, in ft
3
, using
(a) the ideal gas equation of state.
(b) Kay’s rule together with data from the generalized
compressibility chart.
(c) the ideal solution model together with data from the
generalized compressibility chart.
11.125 A tank contains a mixture of 70% ethane and 30%
nitrogen (N
2
) on a molar basis at 400 K, 200 atm. For 2130
kg of mixture, estimate the tank volume, in m
3
, using
(a) the ideal gas equation of state.
(b) Kay’s rule together with data from the generalized
compressibility chart.
(c) the ideal solution model together with data from the
generalized compressibility chart.
11.126 An equimolar mixture of O
2
and N
2
enters a compressor
operating at steady state at 10 bar, 220 K with a mass flow rate
of 1 kg/s. The mixture exits at 60 bar, 400 K with no significant
change in kinetic or potential energy. Stray heat transfer from
the compressor can be ignored. Determine for the compressor
(a) the power required, in kW.
(b) the rate of entropy production, in kW/K.
Assume the mixture is modeled as an ideal solution. For the
pure components:
10 bar, 220 K 60 bar, 400 K
h (kJ/kg) s (kJ/kg ? K) h (kJ/kg) s (kJ/kg ? K)
Oxygen 195.6 5.521 358.2 5.601
Nitrogen 224.1 5.826 409.8 5.911
11.127 A gaseous mixture with a molar analysis of 70% CH
4
and 30% N
2
, enters a compressor operating at steady state at
10 bar, 250 K and a molar flow rate of 6 kmol/h. The mixture
exits the compressor at 100 bar. During compression, the
temperature of the mixture departs from 250 K by no more
than 0.1 K. The power required by the compressor is reported
to be 6 kW. Can this value be correct? Explain. Ignore kinetic
and potential energy effects. Assume the mixture is modeled
as an ideal solution. For the pure components at 250 K:
h (kJ/kg) s (kJ/kg ? K)
10 bar 100 bar 10 bar 100 bar
Methane 506.0 358.6 10.003 8.3716
Nitrogen 256.18 229.68 5.962 5.188
11.128 The departure of a binary solution from ideal solution
behavior is gauged by the activity coefficient, g
i
5 a
i
/y
i
, where a
i
is the activity of component i and y
i
is its mole fraction in the
solution (i 5 1, 2). Introducing Eq. 11.140, the activity coefficient
can be expressed alternatively as g
i
5 f
i
/
y
i
f 8
i
. Using this
expression together with the Gibbs–Duhem equation, derive
the following relation among the activity coefficients and the
mole fractions for a solution at temperature T and pressure p:
ay
1
0 ln g
1
0y
1
b
p, T
5 ay
2
0 ln
g
2
0y
2
b
p, T
How might this expression be used?
Problems: Developing Engineering Skills 701
c11ThermodynamicRelations.indd701 Page 701 6/21/10 9:37:35 PM user-s146 c11ThermodynamicRelations.indd701 Page 701 6/21/10 9:37:35 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

702 Chapter 11
Thermodynamic Relations
c DESIGN & OPEN-ENDED PROBLEMS: EXPLORING ENGINEERING PRACTICE
11.1D Design a laboratory flask for containing up to 10 kmol
of mercury vapor at pressures up to 3 MPa, and temperatures
from 900 to 1000 K. Consider the health and safety of the
technicians who would be working with such a mercury
vapor-filled container. Use a p-y-T relation for mercury vapor
obtained from the literature, including appropriate property
software. Write a report including at least three references.
11.2D The p–h diagram (Sec. 10.2.4) used in the refrigeration
engineering field has specific enthalpy and the natural
logarithm of pressure as coordinates. Inspection of such a
diagram suggests that in portions of the vapor region constant-
entropy lines are nearly linear, and thus the relation between h,
ln p and s might be expressible there as
h1s, p25 1A s 1 B2 ln p 1 1C s 1 D2
Investigate the viability of this expression for pressure
ranging up to 10 bar, using data for Refrigerant 134a.
Summarize your conclusions in a memorandum.
11.3D Compressed natural gas (CNG) is being used as a fuel to
replace gasoline for automobile engines. Aluminum cylinders
wrapped in a fibrous composite can provide lightweight,
economical, and safe on-board storage. The storage vessels
should hold enough CNG for 100 to 125 miles of urban travel,
at storage pressures up to 3000 lbf/in.
2
, and with a maximum
total mass of 150 lb. Adhering to applicable U.S. Department
of Transportation standards, specify both the size and number
of cylinders that would meet the above design constraints.
11.4D A portable refrigeration machine requiring no external
power supply and using carbon dioxide at its triple point is
described in U.S. Patent No. 4,096,707. Estimate the cost of
the initial carbon dioxide charge required by such a machine
to maintain a 6 ft by 8 ft by 15 ft cargo container at 358F for
up to 24 hours, if the container is fabricated from sheet metal
covered with a 1-in. layer of polystyrene. Would you
recommend the use of such a refrigeration machine? Report
your findings in a PowerPoint presentation.
11.5D A power plant located at a river’s mouth where
freshwater river currents meet saltwater ocean tides can
generate electricity by exploiting the difference in composition
of the freshwater and saltwater. The technology for generating
power is called reverse electrodialysis. While only small-scale
demonstration power plants using reverse electrodialysis
have been developed thus far, some observers have high
expectations for the approach. Investigate the technical
readiness and economic feasibility of this renewable power
source for providing 3%, or more, of annual U.S. electricity
by 2030. Present your conclusions in a report, including a
discussion of potential adverse environmental effects of such
power plants and at least three references.
11.6D During a phase change from liquid to vapor at fixed
pressure, the temperature of a binary nonazeotropic solution
such as an ammonia–water solution increases rather than
remains constant as for a pure substance. This attribute is
exploited in both the Kalina power cycle and in the Lorenz
refrigeration cycle. Write a report assessing the status of
technologies based on these cycles. Discuss the principal
advantages of using binary nonazeotropic solutions. What
are some of the main design issues related to their use in
power and refrigeration systems?
11.7D The following data are known for a 100-ton ammonia–
water absorption system like the one shown in Fig. 10.12.
The pump is to handle 570 lb of strong solution per minute.
The generator conditions are 175 lbf/in.
2
, 2208F. The absorber
is at 29 lbf/in.
2
with strong solution exiting at 808F. For the
evaporator, the pressure is 30 lbf/in.
2
and the exit temperature
is 108F. Specify the type and size, in horsepower, of the pump
required. Present your findings in a memorandum.
11.8D The Servel refrigerator works on an absorption principle
and requires no moving parts. An energy input by heat
transfer is used to drive the cycle, and the refrigerant circulates
due to its natural buoyancy. This type of refrigerator is
commonly employed in mobile applications, such as
recreational vehicles. Liquid propane is burned to provide
the required energy input during mobile operation, and
electric power is used when the vehicle is parked and can be
connected to an electrical outlet. Investigate the principles of
operation of commercially available Servel-type systems, and
study their feasibility for solar-activated operation. Consider
applications in remote locations where electricity or gas is
not available. Write a report summarizing your findings.
11.9D In the experiment for the regelation of ice, a small-diameter
wire weighted at each end is draped over a block of ice. The
loaded wire is observed to cut slowly through the ice without
leaving a trace. In one such set of experiments, a weighted
1.00-mm-diameter wire is reported to have passed through 08C
ice at a rate of 54 mm/h. Perform the regelation experiment
and propose a plausible explanation for this phenomenon.
11.10D Figure P11.10D shows the schematic of a hydraulic
accumulator in the form of a cylindrical pressure vessel with
a piston separating a hydraulic fluid from a charge of nitrogen
gas. The device has been proposed as a means for storing
some of the exergy of a decelerating vehicle as it comes to
rest. The exergy is stored by compressing the nitrogen. When
the vehicle accelerates again, the gas expands and returns
some exergy to the hydraulic fluid which is in communication
with the vehicle’s drive train, thereby assisting the vehicle to
accelerate. In a proposal for one such device, the nitrogen
operates in the range 50–150 bar and 200–350 K. Develop a
thermodynamic model of the accumulator and use the model
to assess its suitability for vehicle deceleration/acceleration.
Hydraulic
fluid
Nitrogen
gas
Piston
Fig. P11.10D
c11ThermodynamicRelations.indd702 Page 702 6/22/10 7:21:08 PM user-s146 c11ThermodynamicRelations.indd702 Page 702 6/22/10 7:21:08 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
