Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.

so that the gas undergoes a throttling process (Sec. 4.10) as it expands from 1 to 2. Accord-
ingly, the exit state fixed by p
2
and T
2
has the same value for the specific enthalpy as
at the inlet, h
2
5 h
1
. By progressively lowering the outlet pressure, a finite sequence
of such exit states can be visited, as indicated on Fig. 11.3b. A curve may be drawn
through the set of data points. Such a curve is called an isenthalpic (constant enthalpy)
curve. An isenthalpic curve is the locus of all points representing equilibrium states
of the same specific enthalpy.
The slope of an isenthalpic curve at any state is the Joule–Thomson coefficient at
that state. The slope may be positive, negative, or zero in value. States where the coef-
ficient has a zero value are called inversion states. Notice that not all lines of constant
h have an inversion state. The uppermost curve of Fig. 11.3b, for example, always has
a negative slope. Throttling a gas from an initial state on this curve would result in an
increase in temperature. However, for isenthalpic curves having an inversion state, the
temperature at the exit of the apparatus may be greater than, equal to, or less than the
initial temperature, depending on the exit pressure specified. For states to the right of
an inversion state, the value of the Joule–Thomson coefficient is negative. For these
states, the temperature increases as the pressure at the exit of the apparatus decreases.
At states to the left of an inversion state, the value of the Joule–Thomson coefficient
is positive. For these states, the temperature decreases as the pressure at the exit of the
device decreases. This can be used to advantage in systems designed to liquefy gases.
An innovation in power systems moving from con-
cept to reality promises to help keep computer networks
humming, hospital operating rooms lit, and shopping cen-
ters thriving. Called distributed generation systems, compact
power plants provide electricity for small loads or are linked for
larger applications. With distributed generation, consumers hope
to avoid unpredictable price swings and brownouts.
Distributed generation includes a broad range of technologies
that provide relatively small levels of power at sites close to users,
including but not limited to internal combustion engines, micro-
turbines, fuel cells, and photovoltaic systems.
Although the cost per kilowatt-hour may be higher with dis-
tributed generation, some customers are willing to pay more
to gain control over their electric supply. Computer networks
and hospitals need high reliability, since even short disrup-
tions can be disastrous. Businesses such as shopping centers
also must avoid costly service interruptions. With distributed
generation, the needed reliability is provided by modular units
that can be combined with power management and energy
storage systems to ensure quality power is available when
needed.
Small Power Plants Pack Punch
inversion states
11.6 Constructing Tables of
Thermodynamic Properties
The objective of this section is to utilize the thermodynamic relations introduced thus
far to describe how tables of thermodynamic properties can be constructed. The
characteristics of the tables under consideration are embodied in the tables for water
and the refrigerants presented in the Appendix. The methods introduced in this sec-
tion are extended in Chap. 13 for the analysis of reactive systems, such as gas turbine
and vapor power systems involving combustion. The methods of this section also
provide the basis for computer retrieval of thermodynamic property data.
Two different approaches for constructing property tables are considered:
c The presentation of Sec. 11.6.1 employs the methods introduced in Sec. 11.4 for
assigning specific enthalpy, specific internal energy, and specific entropy to states of
pure, simple compressible substances using p–y–T data, together with a limited
amount of specific heat data. The principal mathematical operation of this approach
is integration.
11.6 Constructing Tables of Thermodynamic Properties 663
c11ThermodynamicRelations.indd Page 663 6/21/10 9:34:50 PM user-s146c11ThermodynamicRelations.indd Page 663 6/21/10 9:34:50 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
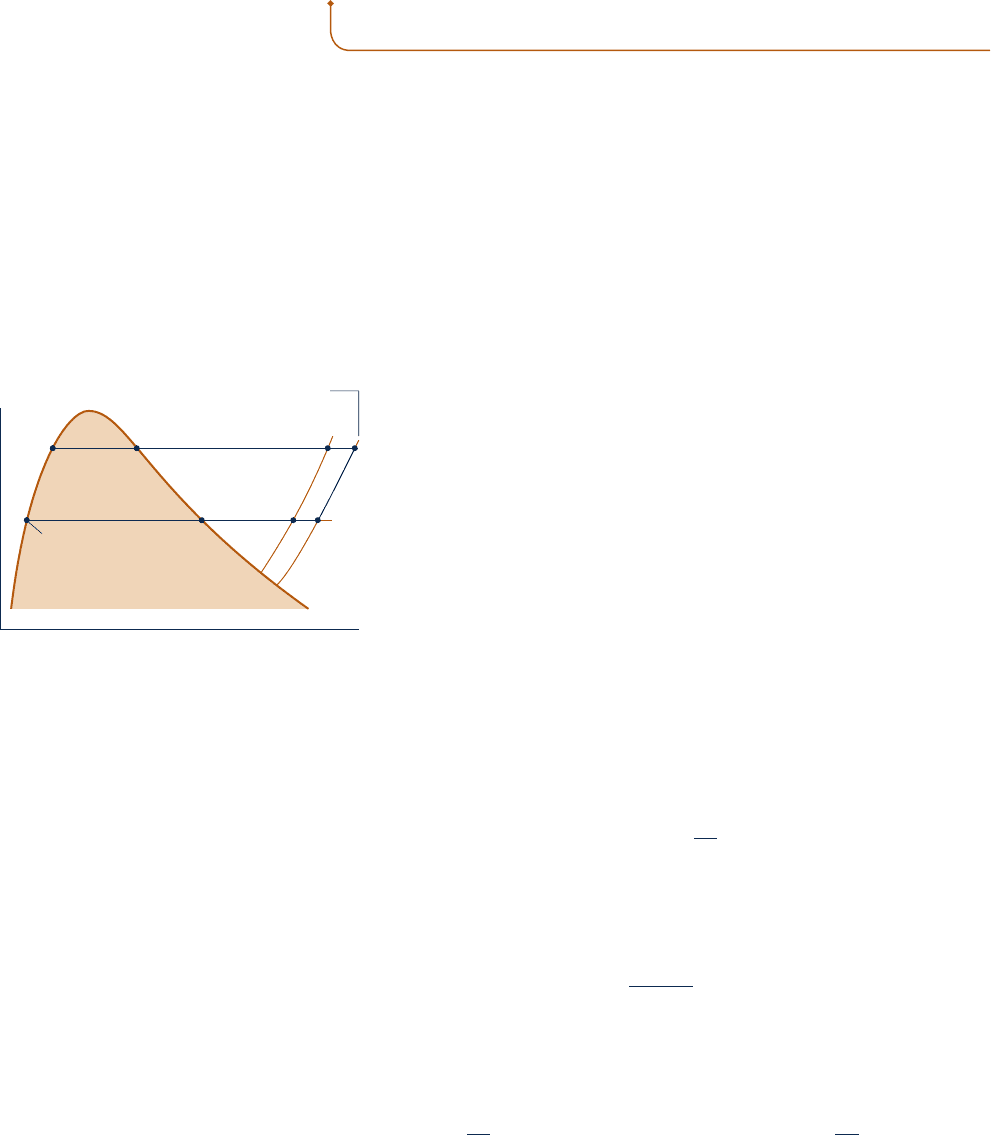
664 Chapter 11
Thermodynamic Relations
c The approach of Sec. 11.6.2 utilizes the fundamental thermodynamic function con-
cept introduced in Sec. 11.3.3. Once such a function has been constructed, the
principal mathematical operation required to determine all other properties is
differentiation.
11.6.1
Developing Tables by Integration Using p–y–T and
Specific Heat Data
In principle, all properties of present interest can be determined using
c
p
5 c
p0
1T2 (11.78)
p 5 p1y, T2, y 5 y1p, T2
In Eqs. 11.78, c
p0
(T) is the specific heat c
p
for the substance under consideration
extrapolated to zero pressure. This function might be determined from data obtained
calorimetrically or from spectroscopic data, using equations supplied by statistical mechan-
ics. Specific heat expressions for several gases are given in Tables A-21. The expressions
p(y, T) and y(p, T) represent functions that describe the saturation pressure–temperature
curves, as well as the p–y–T relations for the single-phase regions.
These functions may be tabular, graphical, or analytical in character.
Whatever their forms, however, the functions must not only represent
the p–y–T data accurately but also yield accurate values for deriva-
tives such as 10y
/
0T2
p
and 1dp
/
dT2
sat
.
Figure 11.4 shows eight states of a substance. Let us consider how
values can be assigned to specific enthalpy and specific entropy at
these states. The same procedures can be used to assign property
values at other states of interest. Note that when h has been assigned
to a state, the specific internal energy at that state can be found from
u 5 h 2 py.
c Let the state denoted by 1 on Fig. 11.4 be selected as the datum
state for enthalpy and entropy. Any value can be assigned to h and
s at this state, but a value of zero would be usual. It should be
noted that the use of an arbitrary datum state and arbitrary refer-
ence values for specific enthalpy and specific entropy suffices only
for evaluations involving differences in property values between
states of the same composition, for then datums cancel.
c Once a value is assigned to enthalpy at state 1, the enthalpy at the saturated vapor
state, state 2, can be determined using the Clapeyron equation, Eq. 11.40
h
2
2 h
1
5 T
1
1y
2
2 y
1
2a
dp
dT
b
sat
where the derivative (dp/dT)
sat
and the specific volumes y
1
and y
2
are obtained
from appropriate representations of the p–y–T data for the substance under con-
sideration. The specific entropy at state 2 is found using Eq. 11.38 in the form
s
2
2 s
1
5
h
2
2 h
1
T
1
c Proceeding at constant temperature from state 2 to state 3, the entropy and enthalpy
are found by means of Eqs. 11.59 and 11.60, respectively. Since temperature is fixed,
these equations reduce to give
s
3
2 s
2
52
#
p
3
p
2
a
0
y
0T
b
p
dp
and
h
3
2 h
2
5
#
p
3
p
2
cy 2 T a
0y
0T
b
p
d dp
With the same procedure, s
4
and h
4
can be determined.
Arbitrary datum
state h
1
= s
1
= 0
1
234
8
765
Isobar–
reduced pressure
p
R
low enough
for the ideal gas
model to be
appropriate
v
T
Fig. 11.4 T–y diagram used to discuss how h
and s can be assigned to liquid and vapor
states.
c11ThermodynamicRelations.indd Page 664 6/21/10 9:34:50 PM user-s146c11ThermodynamicRelations.indd Page 664 6/21/10 9:34:50 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

c The isobar (constant-pressure line) passing through state 4 is assumed to be at a
low enough pressure for the ideal gas model to be appropriate. Accordingly, to
evaluate s and h at states such as 5 on this isobar, the only required information
would be c
p0
(T) and the temperatures at these states. Thus, since pressure is fixed,
Eqs. 11.59 and 11.60 give, respectively
s
5
2 s
4
5
#
T
5
T
4
c
p0
dT
T
and
h
5
2 h
4
5
#
T
5
T
4
c
p0
dT
c Specific entropy and enthalpy values at states 6 and 7 are found from those at state
5 by the same procedure used in assigning values at states 3 and 4 from those at
state 2. Finally, s
8
and h
8
are obtained from the values at state 7 using the Clapeyron
equation.
11.6.2
Developing Tables by Differentiating a Fundamental
Thermodynamic Function
Property tables also can be developed using a fundamental thermodynamic function.
It is convenient for this purpose to select the independent variables of the fundamen-
tal function from among pressure, specific volume (density), and temperature. This
indicates the use of the Helmholtz function c(T, y) or the Gibbs function g(T, p). The
properties of water tabulated in Tables A-2 through A-6 have been calculated using the
Helmholtz function. Fundamental thermodynamic functions also have been employed
successfully to evaluate the properties of other substances in the Appendix tables.
The development of a fundamental thermodynamic function requires considerable
mathematical manipulation and numerical evaluation. Prior to the advent of high-
speed computers, the evaluation of properties by this means was not feasible, and the
approach described in Sec. 11.6.1 was used exclusively. The fundamental function
approach involves three steps:
The first step is the selection of a functional form in terms of the appropriate pair
of independent properties and a set of adjustable coefficients, which may number
50 or more. The functional form is specified on the basis of both theoretical and
practical considerations.
Next, the coefficients in the fundamental function are determined by requiring
that a set of carefully selected property values and/or observed conditions be
satisfied in a least-squares sense. This generally involves the use of property data
requiring the assumed functional form to be differentiated one or more times, such
as p–y–T and specific heat data.
When all coefficients have been evaluated, the function is carefully tested for
accuracy by using it to evaluate properties for which accepted values are known.
These may include properties requiring differentiation of the fundamental function
two or more times. For example, velocity of sound and Joule–Thomson data might
be used.
This procedure for developing a fundamental thermodynamic function is not routine
and can be accomplished only with a computer. However, once a suitable fundamen-
tal function is established, extreme accuracy in and consistency among the thermo-
dynamic properties is possible.
The form of the Helmholtz function used in constructing the steam tables from
which Tables A-2 through A-6 have been extracted is
c1r, T25 c
0
1T21 RT 3ln r 1 rQ1r,t24 (11.79)
where c
0
and Q are given as the sums listed in Table 11.3. The independent variables
are density and temperature. The variable t denotes 1000/T. Values for pressure, specific
1.
2.
3.
11.6 Constructing Tables of Thermodynamic Properties 665
c11ThermodynamicRelations.indd Page 665 6/21/10 9:34:53 PM user-s146c11ThermodynamicRelations.indd Page 665 6/21/10 9:34:53 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
666 Chapter 11 Thermodynamic Relations
internal energy, and specific entropy can be determined by differentiation of Eq. 11.79.
Values for the specific enthalpy and Gibbs function are found from h 5 u 1 py and
g 5 c 1 py, respectively. The specific heat c
y
is evaluated by further differentiation,
c
y
5 10u
/
0T2
y
. With similar operations, other properties can be evaluated. Property
values for water calculated from Eq. 11.79 are in excellent agreement with experimental
data over a wide range of conditions.
Fundamental Equation Used to Construct the Steam Tables
a,b
c 5 c
0
1T21 RT 3In r 1 rQ1r, t24 (1)
where
c
0
5
a
6
i5 1
C
i
/
t
i21
1 C
7
In T 1 C
8
In T
/
t
(2)
and
Q 5 1t 2 t
c
2
a
7
j5 1
1t 2 t
aj
2
j2 2
c
a
8
i5 1
A
ij
1r 2 r
aj
2
i2 1
1 e
2 Er
a
10
i5 9
A
ij
r
i2 9
d
(3)
In (1), (2), and (3), T denotes temperature on the Kelvin scale, t denotes 1000/T, r denotes density
in g/cm
3
, R 5 4.6151 bar ? cm
3
/g ? K or 0.46151 J/g ? K, t
c
5 1000/T
c
5 1.544912, E 5 4.8, and
t
aj
5 t
c
1j 5 12 r
aj
5 0.6341j 5 12
5 2.51j . 12 5 1.01j . 12
The coefficients for c
0
in J/g are given as follows:
C
1
5 1857.065 C
4
5 36.6649 C
7
5 46.0
C
2
5 3229.12 C
5
5 220.5516 C
8
5 21011.249
C
3
5 2419.465 C
6
5 4.85233
Values for the coefficients A
ij
are listed in the original source.
a
TABLE 11.3
a
J. H. Keenan, F. G. Keyes, P. G. Hill, and J. G. Moore, Steam Tables, Wiley, New York, 1969.
b
Also see L. Haar, J. S. Gallagher, and G. S. Kell, NBS/NRC Steam Tables, Hemisphere, Washington, D.C., 1984.
The properties of water are determined in this reference using a different functional form for the Helmholtz
function than given by Eqs. (1)–(3).
ENERGY & ENVIRONMENT Due to the phase-out of chlorine-containing
CFC refrigerants because of ozone layer and global climate change concerns, new
substances and mixtures with no chlorine have been developed in recent years as
possible alternatives (see Sec. 10.3). This has led to significant research efforts to provide the
necessary thermodynamic property data for analysis and design.
The National Institute of Standards and Technology (NIST) has led governmental efforts
to provide accurate data. Specifically, data have been developed for high-accuracy p–y–T
equations of state from which fundamental thermodynamic functions can be obtained. The
equations are carefully validated using data for velocity of sound, Joule–Thomson coeffi-
cient, saturation pressure-temperature relations, and specific heats. Such data were used
to calculate the property values in Tables A-7 to A-18 in the Appendix. NIST has developed
REFPROP, a computer data base that is the current standard for refrigerant and refrigerant
mixture properties.
Example 11.7 illustrates the use of a fundamental function to determine thermo-
dynamic properties for computer evaluation and to develop tables.
c11ThermodynamicRelations.indd Page 666 7/25/10 5:16:49 PM user-f391c11ThermodynamicRelations.indd Page 666 7/25/10 5:16:49 PM user-f391 /Users/user-f391/Desktop/Ramakant_25.07.10/Moran_JWCL314/New/Users/user-f391/Desktop/Ramakant_25.07.10/Moran_JWCL314/New
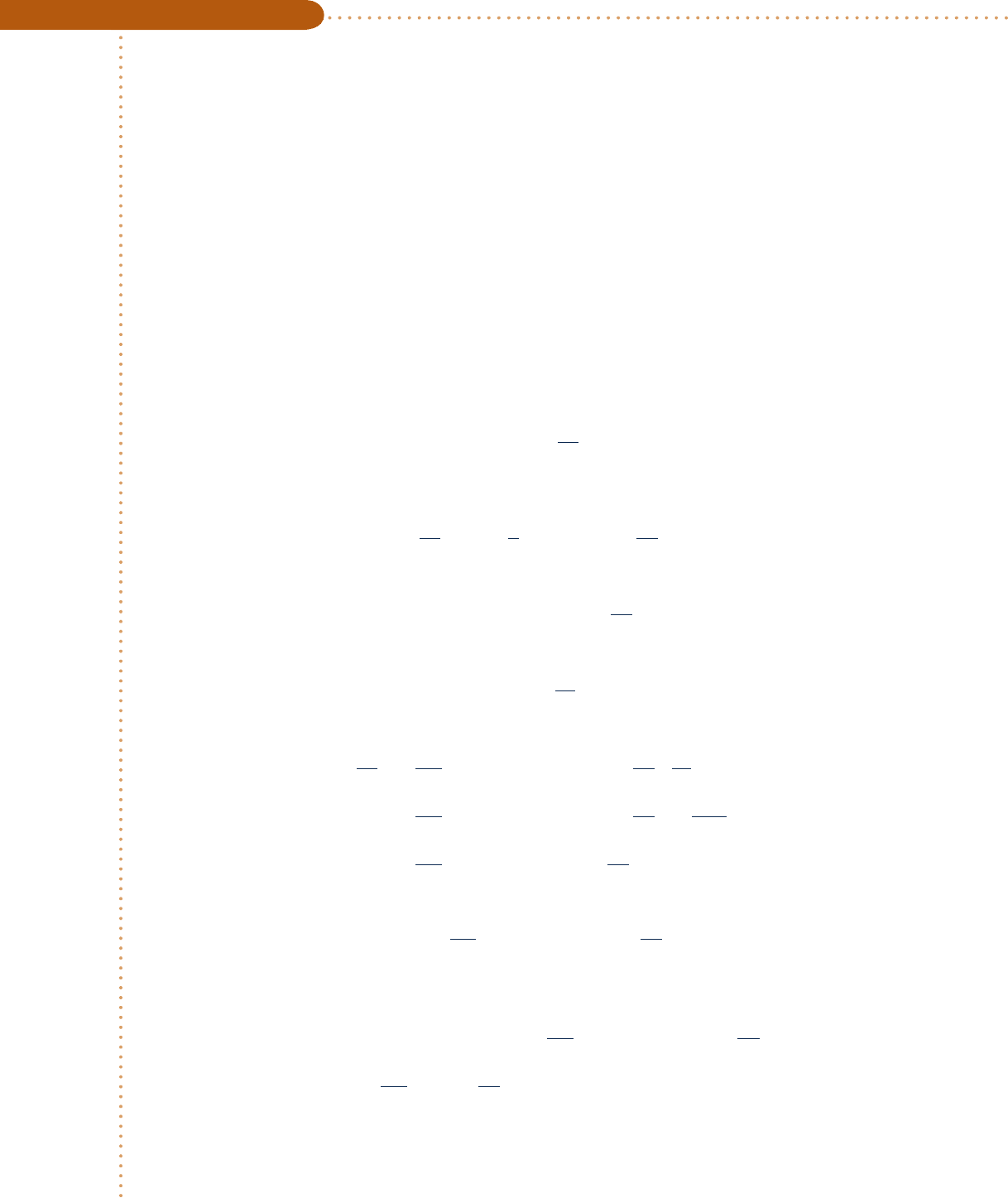
Determining Properties Using a Fundamental Function
c c c c EXAMPLE 11.7 c
The following expression for the Helmholtz function has been used to determine the properties of water:
c1r, T25 c
0
1T21 RT 3ln r 1 rQ1r, t24
where r denotes density and t denotes 1000/T. The functions c
0
and Q are sums involving the indicated inde-
pendent variables and a number of adjustable constants (see Table 11.3). Obtain expressions for (a) pressure,
(b) specific entropy, and (c) specific internal energy resulting from this fundamental thermodynamic function.
SOLUTION
Known:
An expression for the Helmholtz function c is given.
Find: Determine the expressions for pressure, specific entropy, and specific internal energy that result from this
fundamental thermodynamic function.
Analysis: The expressions developed below for p, s, and u require only the functions c
0
(T) an Q(r, t). Once these
functions are determined, p, s, and u can each be determined as a function of density and temperature using
elementary mathematical operations.
(a) When expressed in terms of density instead of specific volume, Eq. 11.28 becomes
p 5 r
2
a
0
c
0r
b
T
as can easily be verified. When T is held constant t is also constant. Accordingly, the following is obtained on
differentiation of the given function:
a
0
c
0r
b
T
5 RT c
1
r
1 Q1r, t21 ra
0Q
0r
b
t
d
Combining these equations gives an expression for pressure
p 5 rRT c1 1 rQ 1 r
2
a
0Q
0r
b
t
d
(a)
(b) From Eq. 11.29
s 52a
0c
0T
b
r
Differentiation of the given expression for c yields
a
0c
0T
b
r
5
dc
0
dT
1 cR1ln r 1 rQ21 RTr a
0
Q
0
t
b
r
dt
dT
d
5
dc
0
dT
1 cR1ln r 1 rQ21 RTr a
0Q
0t
b
r
a2
1000
T
2
bd
5
dc
0
dT
1 R cln r 1 rQ 2 rta
0Q
0t
b
r
d
Combining results gives
s 52
dc
0
dT
2 R cln r 1 rQ 2 rta
0Q
0t
b
r
d
(b)
(c) By definition, c 5 u 2 Ts. Thus, u 5 c 1 Ts. Introducing the given expression for c together with the
expression for s from part (b) results in
u 5 3c
0
1 RT1ln r 1 rQ241 T e2
dc
0
dT
2 R cln r 1 rQ 2 rta
0
Q
0t
b
r
df
5 c
0
2 T
d
c
0
dT
1 RTrt a
0
Q
0t
b
r
11.6 Constructing Tables of Thermodynamic Properties 667
c11ThermodynamicRelations.indd Page 667 6/21/10 9:35:52 PM user-s146 c11ThermodynamicRelations.indd Page 667 6/21/10 9:35:52 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
668 Chapter 11
Thermodynamic Relations
This can be written more compactly by noting that
T
dc
0
dT
5 T
dc
0
dt
dt
dT
5 T
dc
0
dt
a2
1000
T
2
b52t
dc
0
dt
Thus,
c
0
2 T
dc
0
dT
5 c
0
1 t
dc
0
dt
5
d1c
0
t2
dt
Finally, the expression for u becomes
u 5
d1c
0
t2
dt
1 RTrt a
0
Q
0t
b
r
(c)
Using results obtained, how can an expression be developed
for h? Ans. h 5 u 1 p/r. Substitute Eq. (c) for u and Eq. (a) for p and
collect terms.
TAKE NOTE...
Generalized compressibility
charts are provided in
Appendix figures A-1, A-2,
and A-3. See Example 3.7
for an application.
Ability to…
❑
derive expressions for pres-
sure, specific entropy, and
specific internal energy
based on a fundamental
thermodynamic function.
✓
Skills Developed
11.7 Generalized Charts for Enthalpy
and Entropy
Generalized charts giving the compressibility factor Z in terms of the reduced
properties p
R
, T
R
, and y9
R
are introduced in Sec. 3.11. With such charts, estimates
of p–y–T data can be obtained rapidly knowing only the critical pressure and
critical temperature for the substance of interest. The objective of the present sec-
tion is to introduce generalized charts that allow changes in enthalpy and entropy
to be estimated.
Generalized Enthalpy Departure Chart
The change in specific enthalpy of a gas (or liquid) between two states fixed by tem-
perature and pressure can be evaluated using the identity
h1T
2
, p
2
22 h1T
1
, p
1
25 3h
*
1T
2
22 h
*
1T
1
24
1 53h1T
2
, p
2
22 h
*
1T
2
242 3h1T
1
, p
1
22 h
*
1T
1
246 (11.80)
The term [h(T, p) 2 h
*
(T)] denotes the specific enthalpy of the substance relative to
that of its ideal gas model when both are at the same temperature. The superscript
* is used in this section to identify ideal gas property values. Thus, Eq. 11.80 indicates
that the change in specific enthalpy between the two states equals the enthalpy
change determined using the ideal gas model plus a correction that accounts for the
departure from ideal gas behavior. The correction is shown underlined in Eq. 11.80.
The ideal gas term can be evaluated using methods introduced in Chap. 3. Next, we
show how the correction term is evaluated in terms of the enthalpy departure.
DEVELOPING THE ENTHALPY DEPARTURE. The variation of enthalpy with
pressure at fixed temperature is given by Eq. 11.56 as
a
0h
0p
b
T
5 y 2 T a
0
y
0T
b
p
Integrating from pressure p9 to pressure p at fixed temperature T
h1T, p22 h1T, p¿25
#
p
p¿
cy 2 T a
0
y
0T
b
p
d dp
c11ThermodynamicRelations.indd Page 668 6/21/10 9:35:54 PM user-s146 c11ThermodynamicRelations.indd Page 668 6/21/10 9:35:54 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
This equation is not altered fundamentally by adding and subtracting h*(T) on the
left side. That is
3h1T, p22 h
*
1T242 3h1T, p¿22 h
*
1T245
#
p
p¿
cy 2 T a
0y
0T
b
p
d dp
(11.81)
As pressure tends to zero at fixed temperature, the enthalpy of the substance
approaches that of its ideal gas model. Accordingly, as p9 tends to zero
lim
p¿S0
3h1T, p¿22 h
*
1T245 0
In this limit, the following expression is obtained from Eq. 11.81 for the specific
enthalpy of a substance relative to that of its ideal gas model when both are at the
same temperature:
h1T, p22 h
*
1T25
#
p
0
cy 2 T a
0y
0T
b
p
d dp
(11.82)
This also can be thought of as the change in enthalpy as the pressure is increased from
zero to the given pressure while temperature is held constant. Using p–y–T data only,
Eq. 11.82 can be evaluated at states 1 and 2 and thus the correction term of Eq. 11.80
evaluated. Let us consider next how this procedure can be conducted in terms of
compressibility factor data and the reduced properties T
R
and p
R
.
The integral of Eq. 11.82 can be expressed in terms of the compressibility factor
Z and the reduced properties T
R
and p
R
as follows. Solving Z 5 py/RT gives
y 5
ZRT
p
On differentiation
a
0y
0T
b
p
5
RZ
p
1
RT
p
a
0Z
0T
b
p
With the previous two expressions, the integrand of Eq. 11.82 becomes
y 2 T a
0y
0T
b
p
5
ZRT
p
2 T c
RZ
p
1
RT
p
a
0
Z
0T
b
p
d52
RT
2
p
a
0Z
0T
b
p
(11.83)
Equation 11.83 can be written in terms of reduced properties as
y 2 T a
0y
0T
b
p
52
RT
c
p
c
?
T
2
R
p
R
a
0Z
0T
R
b
p
R
Introducing this into Eq. 11.82 gives on rearrangement
h
*
1T22 h1T, p
2
RT
c
5 T
2
R
#
p
R
0
a
0Z
0T
R
b
p
R
dp
R
p
R
Or, on a per mole basis, the enthalpy departure is
h
*
1T22 h1T, p2
RT
c
5 T
2
R
#
p
R
0
a
0
Z
0T
R
b
p
R
dp
R
p
R
(11.84)
The right side of Eq. 11.84 depends only on the reduced temperature T
R
and
reduced pressure p
R
. Accordingly, the quantity 1h
*
2 h2
/
RT
c
, the enthalpy depar-
ture, is a function only of these two reduced properties. Using a generalized equa-
tion of state giving Z as a function of T
R
and p
R
, the enthalpy departure can
readily be evaluated with a computer. Tabular representations are also found in
the literature. Alternatively, the graphical representation provided in Fig. A-4 can
be employed.
enthalpy departure
11.7 Generalized Charts for Enthalpy and Entropy 669
c11ThermodynamicRelations.indd Page 669 6/21/10 9:35:57 PM user-s146 c11ThermodynamicRelations.indd Page 669 6/21/10 9:35:57 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
670 Chapter 11
Thermodynamic Relations
Using the Generalized Enthalpy Departure Chart
c c c c EXAMPLE 11.8 c
Nitrogen enters a turbine operating at steady state at 100 bar and 300 K and exits at 40 bar and 245 K. Using
the enthalpy departure chart, determine the work developed, in kJ per kg of nitrogen flowing, if heat transfer
with the surroundings can be ignored. Changes in kinetic and potential energy from inlet to exit also can be
neglected.
SOLUTION
Known:
A turbine operating at steady state has nitrogen entering at 100 bar and 300 K and exiting at 40 bar
and 245 K.
Find: Using the enthalpy departure chart, determine the work developed.
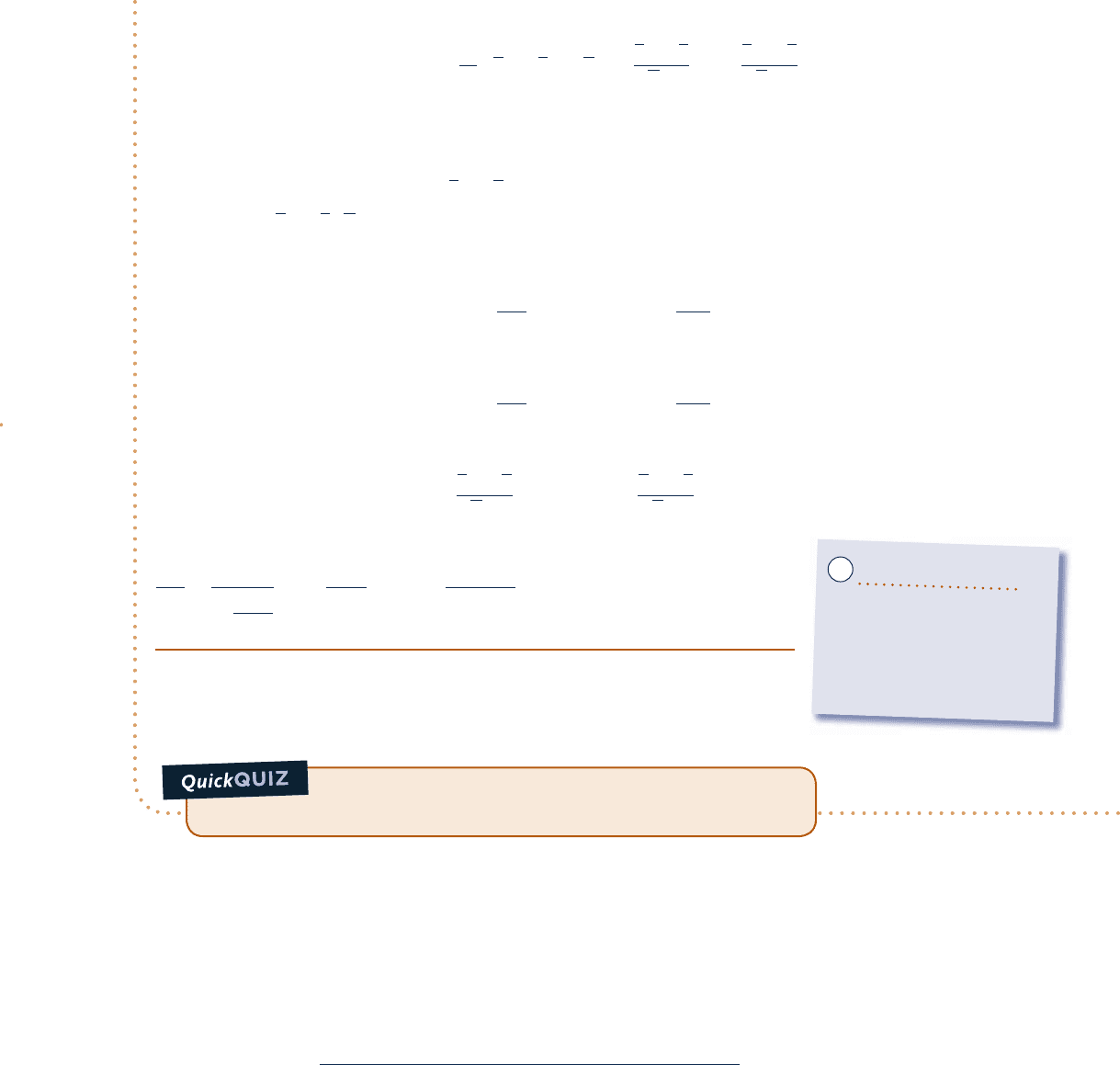
Schematic and Given Data:
1
2
2s
T
s
12
N
2
p
1
= 100 bar
T
1
= 300 K
p
2
= 40 bar
T
2
= 245 K
W
cv
–––
m
·
·
Fig. E11.8
Engineering Model:
1.
The control volume shown on the accom-
panying figure operates at steady state.
2. There is no significant heat transfer between
the control volume and its surroundings.
3. Changes in kinetic and potential energy
between inlet and exit can be neglected.
4. Equilibrium property relations apply at the
inlet and exit.
Analysis: The mass and energy rate balances reduce at steady state to give
0 5
Q
#
cv
m
#
2
W
#
cv
m
#
1 ch
1
2 h
2
1
V
2
1
2 V
2
2
2
1 g1z
1
2 z
2
2d
where m
#
is the mass flow rate. Dropping the heat transfer term by assumption 2 and the kinetic and potential
energy terms by assumption 3 gives on rearrangement
W
#
cv
m
#
5 h
1
2 h
2
EVALUATING ENTHALPY CHANGE. The change in specific enthalpy between
two states can be evaluated by expressing Eq. 11.80 in terms of the enthalpy departure as
h
2
2 h
1
5 h
2
*
2 h
1
*
2 RT
c
ca
h
*
2 h
RT
c
b
2
2 a
h
*
2 h
RT
c
b
1
d
(11.85)
The first underlined term in Eq. 11.85 represents the change in specific enthalpy
between the two states assuming ideal gas behavior. The second underlined term is
the correction that must be applied to the ideal gas value for the enthalpy change to
obtain the actual value for the enthalpy change. Referring to the engineering litera-
ture, the quantity 1h
*
2 h2
/
RT
c
at states 1 and 2 can be calculated with an equation
giving Z(T
R
, p
R
) or obtained from tables. This quantity also can be evaluated at state
1 from the generalized enthalpy departure chart, Fig. A-4, using the reduced tem-
perature T
R1
and reduced pressure p
R1
corresponding to the temperature T
1
and
pressure p
1
at the initial state, respectively. Similarly, 1h
*
2 h2
/
RT
c
at state 2 can be
evaluated from Fig. A-4 using T
R2
and p
R2
. The use of Eq. 11.85 is illustrated in the
next example.
c11ThermodynamicRelations.indd Page 670 6/21/10 9:35:59 PM user-s146 c11ThermodynamicRelations.indd Page 670 6/21/10 9:35:59 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
Generalized Entropy Departure Chart
A generalized chart that allows changes in specific entropy to be evaluated can be
developed in a similar manner to the generalized enthalpy departure chart introduced
earlier in this section. The difference in specific entropy between states 1 and 2 of a
gas (or liquid) can be expressed as the identity
s1T
2
, p
2
22 s1T
1
, p
1
25 s
*
1T
2
, p
2
22 s
*
1T
1
, p
1
2
1 53s1T
2
, p
2
22 s
*
1T
2
, p
2
242 3s1T
1
, p
1
22 s
*
1T
1
, p
1
246 (11.86)
where
3
s
1
T, p
2
2 s
*
1
T, p
24
denotes the specific entropy of the substance relative to
that of its ideal gas model when both are at the same temperature and pressure.
Equation 11.86 indicates that the change in specific entropy between the two states
equals the entropy change determined using the ideal gas model plus a correction
(shown underlined) that accounts for the departure from ideal gas behavior. The ideal
gas term can be evaluated using methods introduced in Sec. 6.5. Let us consider next
how the correction term is evaluated in terms of the entropy departure.
The term h
1
2 h
2
can be evaluated as follows:
h
1
2 h
2
5
1
M
eh
1
*
2
h
2
*
2
RT
c
ca
h
*
2 h
RT
c
b
1
2 a
h
*
2 h
RT
c
b
2
df
In this expression, M is the molecular weight of nitrogen and the other terms have the same significance as in
Eq. 11.85.
With specific enthalpy values from Table A-23 at T
1
5 300 K and T
2
5 245 K, respectively
h
1
*
2 h
2
*
5 8723 2 7121 5 1602 kJ
/
kmol
The terms
1
h
*
2 h
2
/
RT
c
at states 1 and 2 required by the above expression for h
1
2 h
2
can be determined
from Fig. A-4. First, the reduced temperature and reduced pressure at the inlet and exit must be determined.
From Tables A-1, T
c
5 126 K, p
c
5 33.9 bar. Thus, at the inlet
T
R1
5
300
12
6
5 2.38,
p
R1
5
100
33.9
5 2.95
At the exit
T
R2
5
245
12
6
5 1.94,
p
R2
5
40
33.9
5 1.18
By inspection of Fig. A-4
➊
a
h
*
2 h
RT
c
b
1
< 0.5,
a
h
*
2 h
RT
c
b
2
< 0.31
Substituting values
W
#
cv
m
#
5
1
28
kg
kmol
c1602
kJ
kmol
2 a8.314
kJ
kmol ? K
b 1126 K210.5 2 0.312d5 50.1 kJ
/
kg
➊ Due to inaccuracy in reading values from a graph such as Fig. A-4, we cannot
expect extreme accuracy in the final calculated result.
Ability to…
❑
use data from the generalized
enthalpy departure chart to
calculate the change in
enthalpy of nitrogen.
✓
Skills Developed
Determine the work developed, in kJ per kg of nitrogen flow-
ing, assuming the ideal gas model. Ans. 57.2 kJ/kg.
11.7 Generalized Charts for Enthalpy and Entropy 671
c11ThermodynamicRelations.indd Page 671 6/21/10 9:36:03 PM user-s146 c11ThermodynamicRelations.indd Page 671 6/21/10 9:36:03 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
672 Chapter 11 Thermodynamic Relations
DEVELOPING THE ENTROPY DEPARTURE. The following Maxwell relation
gives the variation of entropy with pressure at fixed temperature:
a
0s
0p
b
T
52
a
0y
0T
b
p
(11.35)
Integrating from pressure p9 to pressure p at fixed temperature T gives
s1T, p22 s1T, p¿252
#
p
p¿
a
0y
0T
b
p
dp
(11.87)
For an ideal gas, y 5 RT/p, so
1
0y
/
0T
2
p
5 R
/
p. Using this in Eq. 11.87, the change
in specific entropy assuming ideal gas behavior is
s
*
1T, p22 s
*
1T, p¿252
#
p
p¿
R
p
dp
(11.88)
Subtracting Eq. 11.88 from Eq. 11.87 gives
3s1T, p22 s
*
1T, p242 3s1T, p¿22 s
*
1T, p¿245
#
p
p¿
c
R
p
2 a
0y
0T
b
p
d
dp
(11.89)
Since the properties of a substance tend to merge into those of its ideal gas model
as pressure tends to zero at fixed temperature, we have
lim
p
¿S0
3s1T, p¿22 s
*
1T, p¿245 0
Thus, in the limit as p9 tends to zero, Eq. 11.89 becomes
s1T, p22 s
*
1T, p25
#
p
0
c
R
p
2 a
0y
0T
b
p
d dp
(11.90)
Using p–y–T data only, Eq. 11.90 can be evaluated at states 1 and 2 and thus the
correction term of Eq. 11.86 evaluated.
Equation 11.90 can be expressed in terms of the compressibility factor Z and the
reduced properties T
R
and p
R
. The result, on a per mole basis, is the entropy departure
s
*
1T, p22 s1T, p2
R
5
h
*
1T22 h1T, p2
RT
R
T
c
1
#
p
R
0
1Z 2 12
dp
R
p
R
(11.91)
The right side of Eq. 11.91 depends only on the reduced temperature T
R
and
reduced pressure p
R
. Accordingly, the quantity
1
s
*
2 s
2
/
R
,
the entropy departure, is
a function only of these two reduced properties. As for the enthalpy departure, the
entropy departure can be evaluated with a computer using a generalized equation of
state giving Z as a function of T
R
and p
R
. Alternatively, tabular data from the litera-
ture or the graphical representation provided in Fig. A-5 can be employed.
EVALUATING ENTROPY CHANGE. The change in specific entropy between two
states can be evaluated by expressing Eq. 11.86 in terms of the entropy departure as
s
2
2 s
1
5 s
2
*
2 s
1
*
2 R ca
s
*
2 s
R
b
2
2 a
s
*
2 s
R
b
1
d
(11.92)
The first underlined term in Eq. 11.92 represents the change in specific entropy
between the two states assuming ideal gas behavior. The second underlined term is
the correction that must be applied to the ideal gas value for entropy change to
obtain the actual value for the entropy change. The quantity
1
s
*
2 s
2
1
/
R appearing in
Eq. 11.92 can be evaluated from the generalized entropy departure chart, Fig. A-5,
using the reduced temperature T
R1
and reduced pressure p
R1
corresponding to the
temperature T
1
and pressure p
1
at the initial state, respectively. Similarly,
1
s
*
2 s
2
2
/
R
entropy departure
c11ThermodynamicRelations.indd Page 672 6/21/10 9:36:06 PM user-s146 c11ThermodynamicRelations.indd Page 672 6/21/10 9:36:06 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
