Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.

for gases, but some describe the p–y–T behavior of the liquid phase, at least qual-
itatively. Every equation of state is restricted to particular states. This realm of
applicability is often indicated by giving an interval of pressure, or density, where
the equation can be expected to represent the p–y–T behavior faithfully. When it
is not stated, the realm of applicability of a given equation can be approximated
by expressing the equation in terms of the compressibility factor Z and the
reduced properties p
R
, T
R
, y9
R
and superimposing the result on a generalized com-
pressibility chart or comparing with tabulated compressibility data obtained from
the literature.
11.1.2
Two-Constant Equations of State
Equations of state can be classified by the number of adjustable constants they
include. Let us consider some of the more commonly used equations of state in order
of increasing complexity, beginning with two-constant equations of state.
van der Waals Equation
An improvement over the ideal gas equation of state based on elementary molecular
arguments was suggested in 1873 by van der Waals, who noted that gas molecules
actually occupy more than the negligibly small volume presumed by the ideal gas
model and also exert long-range attractive forces on one another. Thus, not all of the
volume of a container would be available to the gas molecules, and the force they
exert on the container wall would be reduced because of the attractive forces that
exist between molecules. Based on these elementary molecular arguments, the van der
Waals equation of state
is
p 5
RT
y
2 b
2
a
y
2
(11.2)
The constant b is intended to account for the finite volume occupied by the molecules,
the term a
/
y
2
accounts for the forces of attraction between molecules, and
R
is the
universal gas constant. Note than when a and b are set to zero, the ideal gas equation
of state results.
The van der Waals equation gives pressure as a function of temperature and spe-
cific volume and thus is explicit in pressure. Since the equation can be solved for
temperature as a function of pressure and specific volume, it is also explicit in tem-
perature. However, the equation is cubic in specific volume, so it cannot gen-
erally be solved for specific volume in terms of temperature and pressure. The
van der Waals equation is not explicit in specific volume.
EVALUATING a AND b. The van der Waals equation is a two-constant
equation of state. For a specified substance, values for the constants a and b
can be found by fitting the equation to p–y–T data. With this approach sev-
eral sets of constants might be required to cover all states of interest. Alter-
natively, a single set of constants for the van der Waals equation can be
determined by noting that the critical isotherm passes through a point of
inflection at the critical point, and the slope is zero there. Expressed math-
ematically, these conditions are, respectively
a
0
2
p
0
y
2
b
T
5 0,
a
0
p
0y
b
T
5 0
1critical point2
(11.3)
Although less overall accuracy normally results when the constants a and b
are determined using critical point behavior than when they are determined
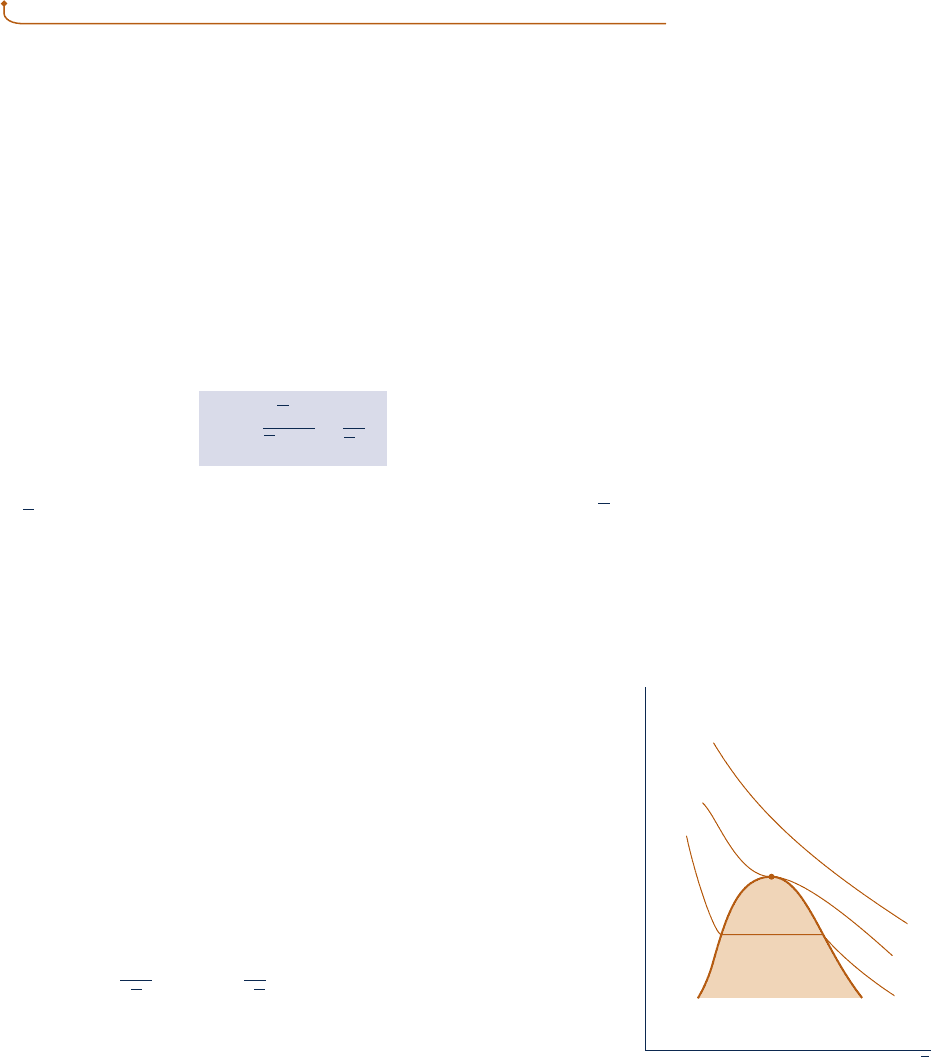
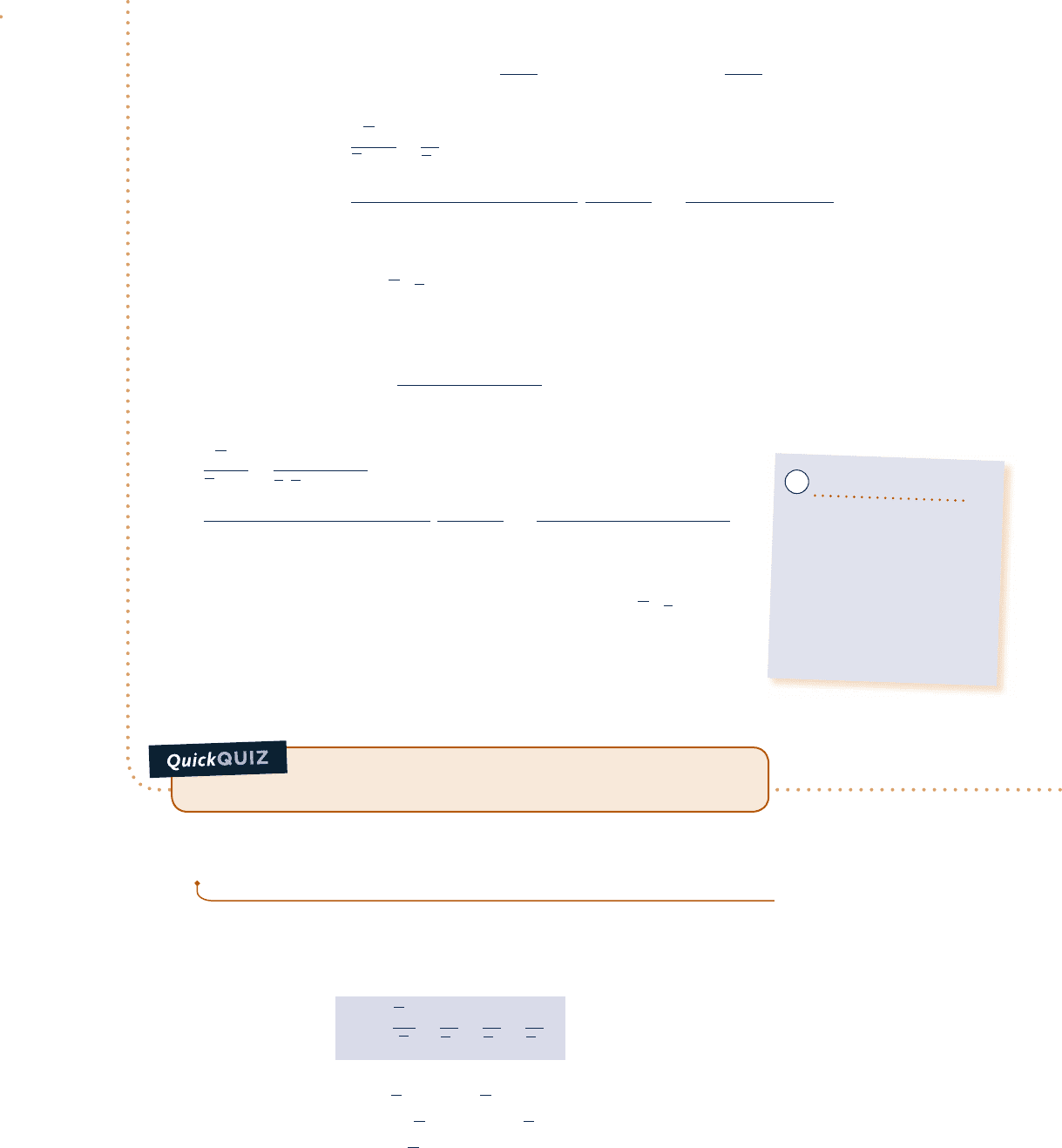
van der Waals equation
p
v
∂p
–––
∂v
(
(
T
= 0
T < T
c
T = T
c
T > T
c
Critical
point
_
11.1 Using Equations of State 633
c11ThermodynamicRelations.indd Page 633 6/21/10 9:33:41 PM user-s146c11ThermodynamicRelations.indd Page 633 6/21/10 9:33:41 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
634 Chapter 11
Thermodynamic Relations
by fitting p–y–T data in a particular region of interest, the advantage of this approach
is that the van der Waals constants can be expressed in terms of the critical pressure
p
c
and critical temperature T
c
, as demonstrated next.
For the van der Waals equation at the critical point
p
c
5
RT
c
y
c
2 b
2
a
y
2
c
Applying Eqs. 11.3 with the van der Waals equation gives
a
0
2
p
0
y
2
b
T
5
2
RT
c
1y
c
2 b2
3
2
6a
y
4
c
5 0
a
0p
0y
b
T
5 2
RT
c
1
y
c
2 b
2
2
1
2a
y
3
c
5 0
Solving the foregoing three equations for a, b, and y
c
in terms of the critical pressure
and critical temperature
a 5
27
64
R
2
T
2
c
p
c
(11.4a)
b 5
RT
c
8p
c
(11.4b)
y
c
5
3
8
RT
c
p
c
(11.4c)
Values of the van der Waals constants a and b determined from Eqs. 11.4a and 11.4b
for several common substances are given in Table A-24 for pressure in bar, specific
volume in m
3
/kmol, and temperature in K. Values of a and b for the same substances
are given in Table A-24E for pressure in atm, specific volume in ft
3
/lbmol, and tem-
perature in 8R.
GENERALIZED FORM. Introducing the compressibility factor Z 5
p
y
/
RT, the
reduced temperature T
R
5 T/T
c
, the pseudoreduced specific volume y¿
R
5 p
c
y
/
RT
c
,
and the foregoing expressions for a and b, the van der Waals equation can be written
in terms of Z, y9
R
, and T
R
as
Z 5
y
¿
R
y¿
R
2 1
/
8
2
27
/
64
T
R
y¿
R
(11.5)
or alternatively in terms of Z, T
R
, and p
R
as
Z
3
2 a
p
R
8T
R
1 1bZ
2
1 a
27p
R
64T
2
R
bZ 2
27p
2
R
512T
3
R
5 0
(11.6)
The details of these developments are left as exercises. Equation 11.5 can be evalu-
ated for specified values of y9
R
and T
R
and the resultant Z values located on a gen-
eralized compressibility chart to show approximately where the equation performs
satisfactorily. A similar approach can be taken with Eq. 11.6.
The compressibility factor at the critical point yielded by the van der Waals equa-
tion is determined from Eq. 11.4c as
Z
c
5
p
c
y
c
RT
c
5 0.375
Actually, Z
c
varies from about 0.23 to 0.33 for most substances (see Tables A-1).
Accordingly, with the set of constants given by Eqs. 11.4, the van der Waals equation
is inaccurate in the vicinity of the critical point. Further study would show inaccuracy
in other regions as well, so this equation is not suitable for many thermodynamic
evaluations. The van der Waals equation is of interest to us primarily because it is
c11ThermodynamicRelations.indd Page 634 7/25/10 5:15:21 PM user-f391c11ThermodynamicRelations.indd Page 634 7/25/10 5:15:21 PM user-f391 /Users/user-f391/Desktop/Ramakant_25.07.10/Moran_JWCL314/New/Users/user-f391/Desktop/Ramakant_25.07.10/Moran_JWCL314/New
the simplest model that accounts for the departure of actual gas behavior from the
ideal gas equation of state.
Redlich–Kwong Equation
Three other two-constant equations of state that have been widely used are the Berthelot,
Dieterici, and Redlich–Kwong equations. The Redlich–Kwong equation, considered by
many to be the best of the two-constant equations of state, is
p 5
RT
y 2 b
2
a
y
1
y 1 b
2
T
1
y
2
(11.7)
This equation, proposed in 1949, is mainly empirical in nature, with no rigorous jus-
tification in terms of molecular arguments. The Redlich–Kwong equation is explicit
in pressure but not in specific volume or temperature. Like the van der Waals equa-
tion, the Redlich–Kwong equation is cubic in specific volume.
Although the Redlich–Kwong equation is somewhat more difficult to manipulate
mathematically than the van der Waals equation, it is more accurate, particularly at
higher pressures. The two-constant Redlich–Kwong equation performs better than
some equations of state having several adjustable constants; still, two-constant equa-
tions of state tend to be limited in accuracy as pressure (or density) increases. Increased
accuracy at such states normally requires equations with a greater number of adjust-
able constants. Modified forms of the Redlich–Kwong equation have been proposed
to achieve improved accuracy.
EVALUATING a AND b. As for the van der Waals equation, the constants a and
b in Eq. 11.7 can be determined for a specified substance by fitting the equation to
p–y–T data, with several sets of constants required to represent accurately all states
of interest. Alternatively, a single set of constants in terms of the critical pressure and
critical temperature can be evaluated using Eqs. 11.3, as for the van der Waals equa-
tion. The result is
a 5 a¿
R
2
T
c
5
y
2
p
c
and
b 5 b¿
RT
c
p
c
(11.8)
where a9 5 0.42748 and b9 5 0.08664. Evaluation of these constants is left as an
exercise. Values of the Redlich–Kwong constants a and b determined from Eqs. 11.8
for several common substances are given in Table A-24 for pressure in bar, specific
volume in m
3
/kmol, and temperature in K. Values of a and b for the same substances
are given in Table A-24E for pressure in atm, specific volume in ft
3
/lbmol, and tem-
perature in 8R.
GENERALIZED FORM. Introducing the compressibility factor Z, the reduced
temperature T
R
, the pseudoreduced specific volume y9
R
, and the foregoing expressions
for a and b, the Redlich–Kwong equation can be written as
Z 5
y
¿
R
y¿
R
2 b¿
2
a¿
1
y¿
R
1 b¿
2
T
3
/
2
R
(11.9)
Equation 11.9 can be evaluated at specified values of y9
R
and T
R
and the resultant Z
values located on a generalized compressibility chart to show the regions where the
equation performs satisfactorily. With the constants given by Eqs. 11.8, the com-
pressibility factor at the critical point yielded by the Redlich–Kwong equation is
Z
c
5 0.333, which is at the high end of the range of values for most substances,
indicating that inaccuracy in the vicinity of the critical point should be expected.
In Example 11.1, the pressure of a gas is determined using three equations of state
and the generalized compressibility chart. The results are compared.
Redlich–Kwong equation
11.1 Using Equations of State 635
c11ThermodynamicRelations.indd Page 635 6/21/10 9:33:46 PM user-s146c11ThermodynamicRelations.indd Page 635 6/21/10 9:33:46 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
636 Chapter 11
Thermodynamic Relations
Comparing Equations of State
c c c c EXAMPLE 11.1 c
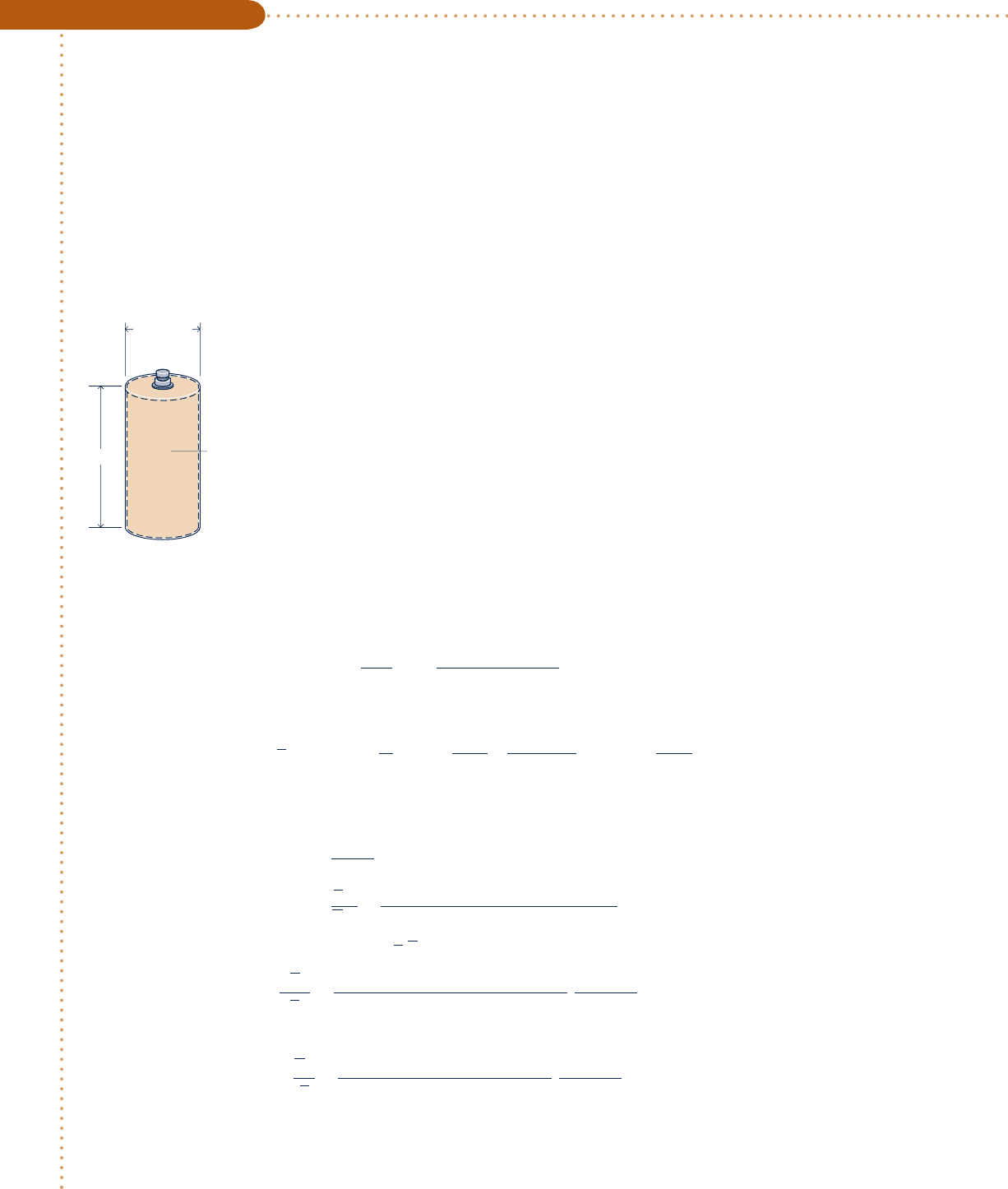
A cylindrical tank containing 4.0 kg of carbon monoxide gas at 2508C has an inner diameter of 0.2 m and a
length of 1 m. Determine the pressure, in bar, exerted by the gas using (a) the generalized compressibility chart,
(b) the ideal gas equation of state, (c) the van der Waals equation of state, (d) the Redlich–Kwong equation of
state. Compare the results obtained.
SOLUTION
Known:
A cylindrical tank of known dimensions contains 4.0 kg of CO gas at 2508C.
Find: Determine the pressure exerted by the gas using four alternative methods.
Schematic and Given Data:
Analysis: The molar specific volume of the gas is required in each part of the solution. Let us begin by evaluat-
ing it. The volume occupied by the gas is
V 5
a
pD
2
4
b
L 5
p10.2 m2
2
11.0 m2
4
5 0.0314 m
3
The molar specific volume is then
y 5 My 5 M a
V
m
b5 a28
kg
kmol
b a
0.0314 m
3
4.0 kg
b5 0.2198
m
3
kmol
(a) From Table A-1 for CO, T
c
5 133 K, p
c
5 35 bar. Thus, the reduced temperature T
R
and pseudoreduced
specific volume y9
R
are, respectively
T
R
5
223 K
133 K
5 1.68
y¿
R
5
yp
c
RT
c
5
10.2198 m
3
/
kmol2135 3 10
5
N
/
m
2
2
18314 N ? m
/
kmol ? K21133 K2
5 0.696
Turning to Fig. A-2, Z < 0.9. Solving Z 5 py
/
RT for pressure and inserting known values
p 5
ZRT
y
5
0.918314 N ? m
/
kmol ? K21223 K2
10.2198 m
3
/
kmol2
`
1 bar
10
5
N
/
m
2
`5 75.9 bar
(b) The ideal gas equation of state gives
p 5
RT
y
5
18314 N ? m
/
kmol ? K21223 K2
10.2198 m
3
/
kmol2
`
1 bar
10
5
N
/
m
2
`5 84.4 bar
Fig. E11.1
D = 0.2 m
L
= 1 m
4 kg CO gas
at –50°C
Engineering Model:
1.
As shown in the accompanying figure, the closed system is taken as the gas.
2. The system is at equilibrium.
c11ThermodynamicRelations.indd Page 636 6/21/10 9:33:47 PM user-s146c11ThermodynamicRelations.indd Page 636 6/21/10 9:33:47 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
11.1.3
Multiconstant Equations of State
To fit the p–y–T data of gases over a wide range of states, Beattie and Bridgeman pro-
posed in 1928 a pressure-explicit equation involving five constants in addition to the gas
constant. The Beattie–Bridgeman equation can be expressed in a truncated virial form as
p 5
RT
y
1
b
y
2
1
g
y
3
1
d
y
4
(11.10)
where
b 5 BRT 2 A 2 cR
/
T
2
g 52BbRT 1 Aa 2 BcR
/
T
2
d 5 BbcR
/
T
2
(11.11)
Beattie–Bridgeman
equation
(c) For carbon monoxide, the van der Waals constants a and b given by Eqs. 11.4 can be read directly from
Table A-24. Thus
a 5 1.474 bara
m
3
kmol
b
2
and
b 5 0.0395
m
3
kmol
Substituting into Eq. 11.2
p
5
RT
y 2 b
2
a
y
2
5
18314 N ? m
/
kmol ? K21223 K2
10.2198 2 0.039521m
3
/
kmol2
`
1 bar
10
5
N
/
m
2
`2
1.474 bar1m
3
/
kmol2
2
10.2198 m
3
/
kmol2
2
5 72.3 bar
Alternatively, the values for y9
R
and T
R
obtained in the solution of part (a) can be substituted into Eq. 11.5, giv-
ing Z 5 0.86. Then, with p 5 ZRT
/
y, p 5 72.5 bar. The slight difference is attributed to roundoff.
(d) For carbon monoxide, the Redlich–Kwong constants given by Eqs. 11.8 can be read directly from Table
A-24. Thus
a 5
17.22 bar1m
6
21K2
1
/
2
1kmol2
2
and
b 5 0.02737 m
3
/
kmol
Substituting into Eq. 11.7
p
5
RT
y 2 b
2
a
y
1
y 1 b
2
T
1
/
2
5
18314 N ? m
/
kmol ? K21223 K2
10.2198 2 0.027372 m
3
/
kmol
`
1 bar
10
5
N
/
m
2
`2
17.22 bar
10.2198210.24717212232
1
/
2
5 75.1 bar
Alternatively, the values for y9
R
and T
R
obtained in the solution of part (a) can
be substituted into Eq. 11.9, giving Z 5 0.89. Then, with p 5 ZRT
/
y, p 5 75.1
bar. In comparison to the value of part (a), the ideal gas equation of state
predicts a pressure that is 11% higher and the van der Waals equation gives a
value that is 5% lower. The Redlich–Kwong value is about 1% less than the
value obtained using the compressibility chart.
Using the given temperature and the pressure value deter-
mined in part (a), check the value of Z using Fig. A-2. Ans. Z
<
0.9.
Ability to…
❑
determine pressure using
the compressibility chart,
ideal gas model, and the
van der Waals and Redlich–
Kwong equations of state.
❑
perform unit conversions
correctly.
✓
Skills Developed
11.1 Using Equations of State 637
c11ThermodynamicRelations.indd Page 637 6/22/10 7:29:51 PM user-s146c11ThermodynamicRelations.indd Page 637 6/22/10 7:29:51 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
638 Chapter 11
Thermodynamic Relations
The five constants a, b, c, A, and B appearing in these equations are determined by
curve fitting to experimental data.
Benedict, Webb, and Rubin extended the Beattie–Bridgeman equation of state to
cover a broader range of states. The resulting equation, involving eight constants in
addition to the gas constant, has been particularly successful in predicting the p–y–T
behavior of light hydrocarbons. The Benedict–Webb–Rubin equation is
p 5
RT
y
1 aBRT 2 A 2
C
T
2
b
1
y
2
1
1b
RT 2 a2
y
3
1
aa
y
6
1
c
y
3
T
2
a1 1
g
y
2
bexp a2
g
y
2
b
(11.12)
Values of the constants appearing in Eq. 11.12 for five common substances are given in
Table A-24 for pressure in bar, specific volume in m
3
/kmol, and temperature in K. Values
of the constants for the same substances are given in Table A-24E for pressure in atm,
specific volume in ft
3
/lbmol, and temperature in 8R. Because Eq. 11.12 has been so suc-
cessful, its realm of applicability has been extended by introducing additional constants.
Equations 11.10 and 11.12 are merely representative of multiconstant equations of
state. Many other multiconstant equations have been proposed. With high-speed com-
puters, equations having 50 or more constants have been developed for representing
the p–y–T behavior of different substances.
11.2 Important Mathematical Relations
Values of two independent intensive properties are sufficient to fix the intensive state
of a simple compressible system of specified mass and composition—for instance, tem-
perature and specific volume (see Sec. 3.1). All other intensive properties can be deter-
mined as functions of the two independent properties: p 5 p(T, y), u 5 u(T, y), h 5
h(T, y), and so on. These are all functions of two independent variables of the form
z 5 z (x, y), with x and y being the independent variables. It might also be recalled
that the differential of every property is exact (Sec. 2.2.1). The differentials of nonprop-
erties such as work and heat are inexact. Let us review briefly some concepts from
calculus about functions of two independent variables and their differentials.
The exact differential of a function z, continuous in the variables x and y, is
dz 5 a
0z
0x
b
y
dx 1 a
0z
0y
b
x
dy
(11.13a)
This can be expressed alternatively as
dz 5 M dx 1 N dy (11.13b)
where M 5 10z
/
0x2
y
and N 5 10z
/
0y2
x
. The coefficient M is the partial derivative of z
with respect to x (the variable y being held constant). Similarly, N is the partial
derivative of z with respect to y (the variable x being held constant).
If the coefficients M and N have continuous first partial derivatives, the order in
which a second partial derivative of the function z is taken is immaterial. That is
0
0y
ca
0z
0x
b
y
d
x
5
0
0x
ca
0z
0y
b
x
d
y
(11.14a)
or
a
0M
0y
b
x
5 a
0
N
0x
b
y
(11.14b)
which can be called the test for exactness, as discussed next.
Benedict–Webb–Rubin
equation
exact differential
TAKE NOTE...
The state principle for
simple systems is intro-
duced in Sec. 3.1.
test for exactness
c11ThermodynamicRelations.indd Page 638 6/21/10 9:33:57 PM user-s146c11ThermodynamicRelations.indd Page 638 6/21/10 9:33:57 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
In words, Eqs. 11.14 indicate that the mixed second partial derivatives of the func-
tion z are equal. The relationship in Eqs. 11.14 is both a necessary and sufficient
condition for the exactness of a differential expression, and it may therefore be used
as a test for exactness. When an expression such as M dx 1 N dy does not meet this
test, no function z exists whose differential is equal to this expression. In thermody-
namics, Eq. 11.14 is not generally used to test exactness but rather to develop addi-
tional property relations. This is illustrated in Sec. 11.3 to follow.
Two other relations among partial derivatives are listed next for which applications
are found in subsequent sections of this chapter. These are
a
0x
0y
b
z
a
0
y
0x
b
z
5 1
(11.15)
and
a
0
y
0z
b
x
a
0z
0x
b
y
a
0x
0y
b
z
521
(11.16)
consider the three quantities x, y, and z, any two of which may
be selected as the independent variables. Thus, we can write x 5 x(y, z) and y 5 y(x, z).
The differentials of these functions are, respectively
dx 5 a
0x
0y
b
z
dy 1 a
0x
0z
b
y
dz and dy 5 a
0y
0x
b
z
dx 1 a
0y
0z
b
x
dz
Eliminating dy between these two equations results in
c1 2 a
0x
0y
b
z
a
0
y
0x
b
z
ddx 5 ca
0x
0y
b
z
a
0
y
0z
b
x
1 a
0x
0z
b
y
ddz
(11.17)
Since x and z can be varied independently, let us hold z constant and vary x. That is,
let dz 5 0 and dx ? 0. It then follows from Eq. 11.17 that the coefficient of dx must
vanish, so Eq. 11.15 must be satisfied. Similarly, when dx 5 0 and
dz ? 0
, the coeffi-
cient of dz in Eq. 11.17 must vanish. Introducing Eq. 11.15 into the resulting expression
and rearranging gives Eq. 11.16. The details are left as an exercise. b b b b b
APPLICATION. An equation of state p 5 p(T, y) provides a specific example of
a function of two independent variables. The partial derivatives
1
0
p
/
0T
2
y
and
1
0
p
/
0y
2
T
of p(T, y) are important for subsequent discussions. The quantity
1
0
p
/
0T
2
y
is the partial
derivative of p with respect to T (the variable y being held constant). This partial
derivative represents the slope at a point on a line of constant specific volume (isomet-
ric) projected onto the p–T plane. Similarly, the partial derivative
1
0
p
/
0y
2
T
is the partial
derivative of p with respect to y (the variable T being held constant). This partial
derivative represents the slope at a point on a line of constant temperature (isotherm)
projected on the p–y plane. The partial derivatives
1
0
p
/
0T
2
y
and
1
0
p
/
0y
2
T
are themselves
intensive properties because they have unique values at each state.
The p–y–T surfaces given in Figs. 3.1 and 3.2 are graphical representations of func-
tions of the form p 5 p(y, T). Figure 11.1 shows the liquid, vapor, and two-phase
regions of a p–y–T surface projected onto the p–y and p–T planes. Referring first to
Fig. 11.1a, note that several isotherms are sketched. In the single-phase regions, the
partial derivative
1
0
p
/
0y
2
T
giving the slope is negative at each state along an isotherm
except at the critical point, where the partial derivative vanishes. Since the isotherms
are horizontal in the two-phase liquid–vapor region, the partial derivative
1
0
p
/
0y
2
T
vanishes there as well. For these states, pressure is independent of specific volume
and is a function of temperature only: p 5 p
sat
(T ).
Figure 11.1b shows the liquid and vapor regions with several isometrics (constant
specific volume lines) superimposed. In the single-phase regions, the isometrics are
11.2 Important Mathematical Relations 639
c11ThermodynamicRelations.indd Page 639 6/21/10 9:33:58 PM user-s146c11ThermodynamicRelations.indd Page 639 6/21/10 9:33:58 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
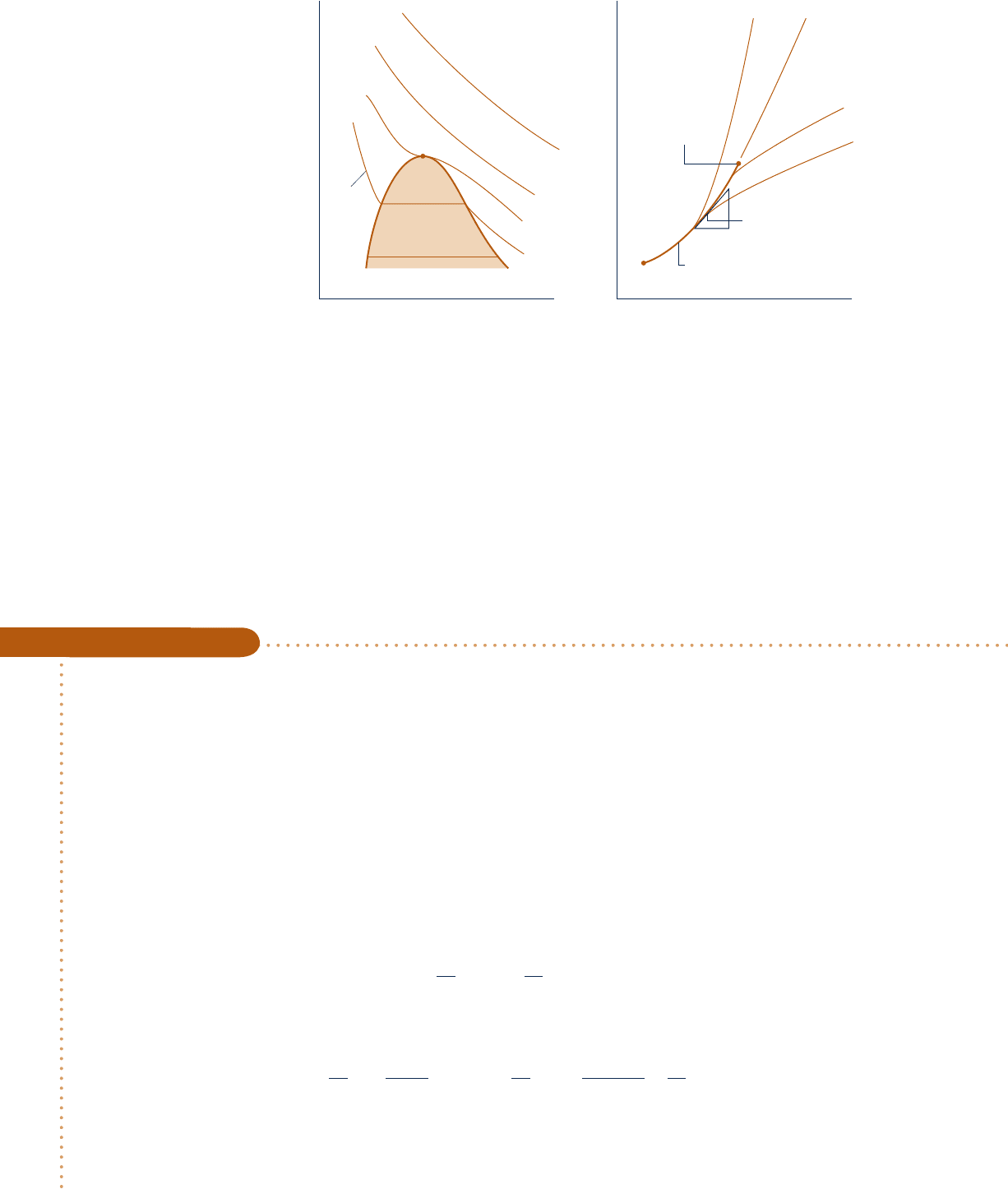
640 Chapter 11
Thermodynamic Relations
nearly straight or are slightly curved and the partial derivative 10p
/
0T2
y
is positive at
each state along the curves. For the two-phase liquid–vapor states corresponding to
a specified value of temperature, the pressure is independent of specific volume and
is determined by the temperature only. Hence, the slopes of isometrics passing through
the two-phase states corresponding to a specified temperature are all equal, being
given by the slope of the saturation curve at that temperature, denoted simply as
(dp/dT)
sat
. For these two-phase states, 10p
/
0T2
y
5 1dp
/
dT2
sat
.
In this section, important aspects of functions of two variables have been intro-
duced. The following example illustrates some of these ideas using the van der Waals
equation of state.
p
v
(a
)
p
T
(b)
Critical
point
Liquid
Triple
point
Locus of saturation states
Vapor
Isometric
Isometric
v < v
c
v = v
c
v > v
c
∂p
–––
∂T
(
(
v
> 0
∂p
–––
∂v
(
(
T
< 0
∂p
–––
∂v
(
(
T
= 0
∂p
–––
∂v
(
(
T
< 0
∂p
–––
∂T
(
(
v
> 0
dp
–––
dT
(
(
sat
T < T
c
T = T
c
T > T
c
Isotherm
Isotherm
Critical
point
(∂p/∂v)
T
= 0
Liquid-vapor
Fig. 11.1 Diagrams used to discuss (≠p/≠
y
)
T
and (≠p/≠T)
y
. (a) p–
y
diagram. (b) Phase diagram.
Applying Mathematical Relations
c c c c EXAMPLE 11.2 c
For the van der Waals equation of state, (a) determine an expression for the exact differential dp, (b) show that
the mixed second partial derivatives of the result obtained in part (a) are equal, and (c) develop an expression
for the partial derivative 10y
/
0T2
p
.
SOLUTION
Known:
The equation of state is the van der Waals equation.
Find: Determine the differential dp, show that the mixed second partial derivatives of dp are equal, and develop
an expression for 10y
/
0T2
p
.
Analysis:
(a)
By definition, the differential of a function p 5 p(T, y) is
dp 5 a
0
p
0T
b
y
dT 1 a
0p
0y
b
T
dy
The partial derivatives appearing in this expression obtained from the van der Waals equation expressed as p 5
RT/(y 2 b) 2 a/y
2
are
M 5 a
0p
0T
b
y
5
R
y 2 b
,
N 5 a
0p
0y
b
T
52
RT
1y 2 b2
2
1
2a
y
3
c11ThermodynamicRelations.indd Page 640 6/21/10 9:33:59 PM user-s146c11ThermodynamicRelations.indd Page 640 6/21/10 9:33:59 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
11.3 Developing Property Relations
In this section, several important property relations are developed, including the
expressions known as the Maxwell relations. The concept of a fundamental thermo-
dynamic function is also introduced. These results, which are important for subsequent
discussions, are obtained for simple compressible systems of fixed chemical composi-
tion using the concept of an exact differential.
Accordingly, the differential takes the form
dp 5 a
R
y 2 b
bdT 1 c
2RT
1y 2 b2
2
1
2a
y
3
ddy
(b) Calculating the mixed second partial derivatives
a
0M
0y
b
T
5
0
0y
ca
0
p
0T
b
y
d
T
52
R
1y 2 b2
2
a
0N
0T
b
y
5
0
0T
ca
0
p
0y
b
T
d
y
52
R
1y 2 b2
2
Thus, the mixed second partial derivatives are equal, as expected.
(c) An expression for 10y
/
0T2
p
can be derived using Eqs. 11.15 and 11.16. Thus, with x 5 p, y 5 y, and z 5
T, Eq. 11.16 gives
a
0y
0T
b
p
a
0
p
0y
b
T
a
0T
0p
b
y
521
or
a
0y
0T
b
p
52
1
10p
/
0y2
T
10T
/
0p2
y
Then, with x 5 T, y 5 p, and z 5 y, Eq. 11.15 gives
a
0T
0p
b
y
5
1
10p
/
0T2
y
Combining these results
a
0y
0T
b
p
5
10p
/
0T2
y
10p
/
0y2
T
The numerator and denominator of this expression have been evaluated in part (a), so
➊
a
0y
0T
b
p
52
R
/
1y 2 b2
32RT
/
1y 2 b2
2
1 2a
/
y
3
4
which is the desired result.
➊ Since the van der Waals equation is cubic in specific volume, it can be solved
for y (T, p) at only certain states. Part (c) shows how the partial derivative
10y
/
0T2
p
can be evaluated at states where it exists.
Ability to…
❑
use Eqs. 11.15 and 11.16
together with the van der
Waals equation of state to
develop a thermodynamic
property relation.
✓
Skills Developed
Using the results obtained, develop an expression for
a
0y
0T
b
p
of an ideal gas. Ans. y/T.
11.3 Developing Property Relations 641
c11ThermodynamicRelations.indd Page 641 7/25/10 5:15:50 PM user-f391c11ThermodynamicRelations.indd Page 641 7/25/10 5:15:50 PM user-f391 /Users/user-f391/Desktop/Ramakant_25.07.10/Moran_JWCL314/New/Users/user-f391/Desktop/Ramakant_25.07.10/Moran_JWCL314/New
642 Chapter 11
Thermodynamic Relations
11.3.1
Principal Exact Differentials
The principal results of this section are obtained using Eqs. 11.18, 11.19, 11.22, and
11.23. The first two of these equations are derived in Sec. 6.3, where they are
referred to as the T ds equations. For present purposes, it is convenient to express
them as
du 5 T ds 2 p dy (11.18)
dh 5 T ds 1 y dp (11.19)
The other two equations used to obtain the results of this section involve, respec-
tively, the specific
Helmholtz function c defined by
c 5 u 2 Ts (11.20)
and the specific Gibbs function g defined by
g 5 h 2 Ts (11.21)
The Helmholtz and Gibbs functions are properties because each is defined in terms
of properties. From inspection of Eqs. 11.20 and 11.21, the units of c and g are the
same as those of u and h. These two new properties are introduced solely because they
contribute to the present discussion, and no physical significance need be attached to
them at this point.
Forming the differential dc
dc 5 du 2 d1Ts25 du 2 T ds 2 s dT
Substituting Eq. 11.18 into this gives
dc 52p dy 2 s dT (11.22)
Similarly, forming the differential dg
dg 5 dh 2 d1Ts25 dh 2 T ds 2 s dT
Substituting Eq. 11.19 into this gives
dg 5 y dp 2 s dT (11.23)
11.3.2
Property Relations from Exact Differentials
The four differential equations introduced above, Eqs. 11.18, 11.19, 11.22, and 11.23,
provide the basis for several important property relations. Since only properties are
involved, each is an exact differential exhibiting the general form dz 5 M dx 5 N dy
considered in Sec. 11.2. Underlying these exact differentials are, respectively, functions
of the form u(s, y), h(s, p), c(y, T), and g(T, p). Let us consider these functions in the
order given.
The differential of the function u 5 u(s, y) is
du 5 a
0
u
0s
b
y
ds 1 a
0u
0y
b
s
dy
Helmholtz function
Gibbs function
c11ThermodynamicRelations.indd Page 642 6/21/10 9:34:04 PM user-s146c11ThermodynamicRelations.indd Page 642 6/21/10 9:34:04 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
